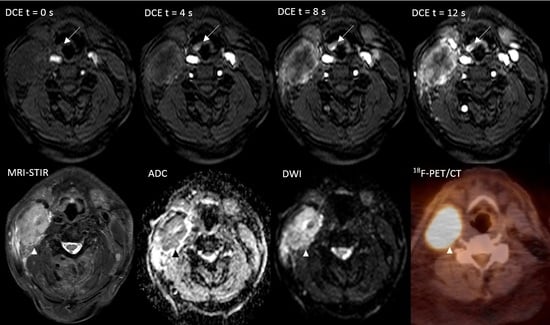
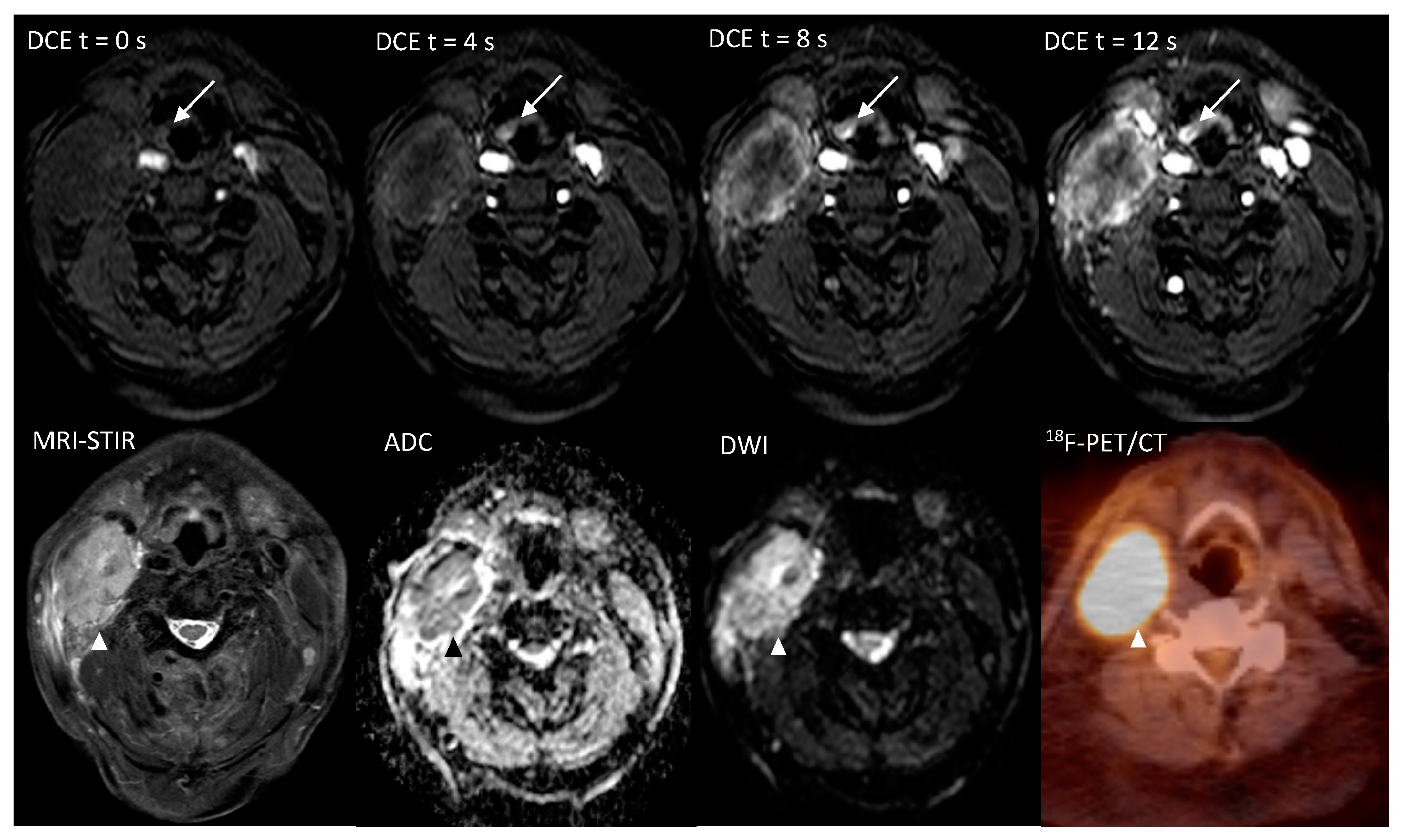
The Additional Value of Ultrafast DCE-MRI to DWI-MRI and 18F-FDG-PET to Detect Occult Primary Head and Neck Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Diagnostic Performance of Single DWI, DCE or 18F-FDG-PET/CT Analysis
2.2. Diagnostic Performance of the Combined Use of DWI, DCE and 18F-FDG-PET/CT
2.3. Interobserver Agreement
3. Discussion
4. Materials and Methods
4.1. Image Acquisition
4.2. Qualitative Image Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Noij, D.P.; Martens, R.M.; Zwezerijnen, B.; Koopman, T.; de Bree, R.; Hoekstra, O.S.; de Graaf, P.; Castelijns, J.A. Diagnostic value of diffusion-weighted imaging and 18F-FDG-PET/CT for the detection of unknown primary head and neck cancer in patients presenting with cervical metastasis. Eur. J. Radiol. 2018, 107, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.J.; Ridge, J.A. Management of Squamous Cancer Metastatic to Cervical Nodes with an Unknown Primary Site. J. Clin. Oncol. 2015, 33, 3328–3337. [Google Scholar] [CrossRef] [PubMed]
- Eskander, A.; Ghanem, T.; Agrawal, A.; The Education Committee of American Head and Neck Society (AHNS). AHNS Series: Do you know your guidelines? Guideline recommendations for head and neck cancer of unknown primary site. Head Neck 2017, 40, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Gődény, M.; Lengyel, Z.; Polony, G.; Nagy, Z.T.; Léránt, G.; Zámbó, O.; Remenár, É.; Tamás, L.; Kásler, M. Impact of 3T multiparametric MRI and FDG-PET-CT in the evaluation of occult primary cancer with cervical node metastasis. Cancer Imaging 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Demiroz, C.; Vainshtein, J.M.; Koukourakis, G.V.; Gutfeld, O.; Prince, M.E.; Bradford, C.R.; Wolf, G.T.; McLean, S.; Worden, F.P.; Chepeha, U.B.; et al. Head and neck squamous cell carcinoma of unknown primary: Neck dissection and radiotherapy or definitive radiotherapy. Head Neck 2013, 36, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, K.E.; Koshy, M.; Paulino, A.C. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 2004, 101, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Grau, C.; Johansen, L.V.; Jakobsen, J.; Geertsen, P.; Andersen, E.; Jensen, B.B. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother. Oncol. 2000, 55, 121–129. [Google Scholar] [CrossRef]
- Bammer, R. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 2003, 45, 169–184. [Google Scholar] [CrossRef]
- Srinivasan, A.; Dvorak, R.; Perni, K.; Rohrer, S.; Mukherji, S. Differentiation of Benign and Malignant Pathology in the Head and Neck Using 3T Apparent Diffusion Coefficient Values: Early Experience. Am. J. Neuroradiol. 2007, 29, 40–44. [Google Scholar] [CrossRef]
- Thoeny, H.C.; de Keyzer, F.; King, A.D. Diffusion-weighted MR Imaging in the Head and Neck. Radiology 2012, 263, 19–32. [Google Scholar] [CrossRef]
- Kwee, T.C.; Basu, S.; Cheng, G.; Alavi, A. FDG PET/CT in carcinoma of unknown primary. Eur. J. Nucl. Med. Mol. Imaging 2009, 37, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.; Buus, S.; Loft, A.; Keiding, S.; Overgaard, M.; Hansen, H.S.; Grau, C.; Bundgaard, T.; Kirkegaard, J.; Overgaard, J. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck 2008, 30, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Kunda, L.D.; Segall, G.M.; Ku, G.H.; Terris, D.J. Uses and limitations of FDG positron emission tomography in patients with head and neck cancer. Laryngoscope 1999, 109, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Cheng, N.M.; Hsieh, C.H.; Ng, S.H.; Lin, C.Y.; Yen, T.C.; Hsu, C.L.; Wan, H.M.; Liao, C.T.; Chang, K.P.; et al. Multiparametric imaging using 18F-FDG PET/CT heterogeneity parameters and functional MRI techniques: Prognostic significance in patients with primary advanced oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiotherapy. Oncotarget 2017, 8, 62606–62621. [Google Scholar] [CrossRef]
- Chatterjee, A.; He, D.; Fan, X.; Wang, S.; Szasz, T.; Yousuf, A.; Pineda, F.; Antic, T.; Mathew, M.; Karczmar, G.S.; et al. Performance of Ultrafast DCE-MRI for Diagnosis of Prostate Cancer. Acad. Radiol. 2017, 25, 349–358. [Google Scholar] [CrossRef]
- Cover, K.S.; Duvivier, K.M.; de Graaf, P.; Wittenberg, R.; Smit, R.; Kuijer, J.P.A.; Hofman, M.B.; Slotman, B.J.; Verdaasdonk, R.M. Summarizing the 4D image stack of ultrafast dynamic contrast enhancement MRI of breast cancer in 3D using color intensity projections. J. Magn. Reson. Imaging 2018, 49, 1391–1399. [Google Scholar] [CrossRef]
- Van Zelst, J.C.; Vreemann, S.; Witt, H.J.; Gubern-Merida, A.; Dorrius, M.D.; Duvivier, K.; Lardenoije-Broker, S.; Lobbes, M.B.; Loo, C.; Veldhuis, W.; et al. Multireader Study on the Diagnostic Accuracy of Ultrafast Breast Magnetic Resonance Imaging for Breast Cancer Screening. Investig. Radiol. 2018, 53, 579–586. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Gawlitza, M.; Höhn, A.K.; Boehm, A.; Kahn, T.; Stumpp, P. Correlations Between DCE MRI and Histopathological Parameters in Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2016, 10, 17–21. [Google Scholar] [CrossRef]
- Gaddikeri, S.; Tailor, T.; Anzai, Y.; Gaddikeri, R. Dynamic Contrast-Enhanced MR Imaging in Head and Neck Cancer: Techniques and Clinical Applications. Am. J. Neuroradiol. 2015, 37, 588–595. [Google Scholar] [CrossRef]
- Furukawa, M.; Parvathaneni, U.; Maravilla, K.; Richards, T.L.; Anzai, Y. Dynamic contrast-enhanced MR perfusion imaging of head and neck tumors at 3 Tesla. Head Neck 2012, 35, 923–929. [Google Scholar] [CrossRef]
- Shoushtari, A.; Saylor, D.; Kerr, K.L.; Sheng, K.; Thomas, C.; Jameson, M.J.; Reibel, J.; Shonka, D.; Levine, P.; Read, P. Outcomes of Patients with Head-and-Neck Cancer of Unknown Primary Origin Treated with Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. 2011, 81, e83–e91. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Bolton, R.C.D.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2014, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- De Vet, H.C.; Mokkink, L.B.; Terwee, C.B.; Hoekstra, O.S.; Knol, D.L. Clinicians are right not to like Cohen’s κ. BMJ 2013, 346, f2125. [Google Scholar] [CrossRef] [PubMed]

| Clinically Occult Primaries n = 29 (%) | |
|---|---|
| Age, median, range | 60, 45–76 |
| Gender, n (%) | |
| Male | 20 (69) |
| Female | 9 (31) |
| Primary tumor location, n (%) | |
| Tonsil | 9 (31) |
| Base of tongue | 3 (10) |
| Hypopharynx | 5 (17) |
| Other | 3 (10) |
| Retromolar trigone | 1 (3) |
| Piriform sinus/Epiglottis | 2 (7) |
| Unknown | 9 (31) |
| T-stage **, n (%) | |
| Tx | 9 (31) |
| T1 | 13 (45) |
| T2 | 5 (17) |
| T3 | 1 (3) |
| T4 * | 1 (3) |
| N-stage **, n (%) | |
| N1 | 6 (21) |
| N2a | 6 (21) |
| N2b | 11 (38) |
| N2c | 4 (14) |
| N3 | 2 (7) |
| AJCC-Stage, n (%) | |
| III | 5 (17) |
| IV | 24 (83) |
| HPV, n (%) | |
| Positive | 17 (59) |
| Negative | 9 (31) |
| Unknown | 3 (10) |
| Final | PT location | DWI | DCE | PET | ||||
|---|---|---|---|---|---|---|---|---|
| Patient | PT Location Score | False Positive | PT Location Score | False Positive | PT Location Score | False Positive | ||
| 1 | UP | - | + Left Tonsil | +++ Left Tonsil | ||||
| 2 | T1 | Right Tonsil | +++ | +++ | +++ | |||
| 3 | T1 | Left Tonsil | +++ | +++ | +++ | |||
| 4 | T1 | Left Tonsil | + | + | + | |||
| 5 | T1 | Right Tonsil | + | +++ | +++ | |||
| 6 | T3 | Right Epiglottis/Piriform sinus | - | + | +++ | |||
| 7 | T2 | Right base of tongue | + | + | +++ | |||
| 8 | UP | - | - | - | ||||
| 9 | UP | - | + Left Tonsil | - | - | + Left Tonsil | ||
| 10 | UP | - | + Right Tonsil | - | + Right Tonsil | - | +++ Right Tonsil | |
| 11 | UP | - | + Right Tonsil | - | + Right Tonsil | - | + Right Tonsil | |
| 12 | UP | + Left Tonsil | - | - | + Left Tonsil + Right tonsil + Left Hypopharynx | |||
| 13 | T1 | Left Hypopharynx | + | + | + | |||
| 14 | T1 | Right piriform sinus | +++ | + | +++ | + Right Tonsil | ||
| 15 | T1 | Right base of tongue | +++ | + | +++ | |||
| 16 | UP | + Right Tonsil | + Right Tonsil | - | ||||
| 17 | T1 | Right Tonsil | +++ | +++ | +++ | |||
| 18 | T1 | Left Tonsil | +++ | +++ | +++ | |||
| 19 | T2 | Left Hypopharynx | - | + Right Tonsil | + | + Right Tonsil | - | + Left Tonsil + Right Tonsil |
| 20 | T1 | Left Tonsil | + | + | + Right Tonsil | + | + Right Tonsil | |
| 21 | T1 | Right Tonsil | + | + | + | |||
| 22 | T1 | Left Hypopharynx | +++ | +++ | +++ | |||
| 23 | T1 | Left Hypopharynx | + | + | +++ | |||
| 24 | T1 | Right base of tongue | + | +++ | +++ | |||
| 25 | UP | + Left Hypopharynx | + Left Tonsil + Left Hypopharynx | +++ Left Tonsil | ||||
| 26 | UP | + Left Tonsil | + Left Hypopharynx | +++ Left Hypopharynx | ||||
| 27 | T1 | Left hypopharynx | - | + Left Tonsil | - | + | ||
| 28 | T4 | Left Retromolar trigone | +++ | +++ | +++ | |||
| 29 | T2 | Left Tonsil | - | - | - | +++ Right base of tongue | ||
| Per-Location (n = 174) | AUC (95%CI) | Sensitivity (%, 95%CI, Ratio) | Specificity (%, 95%CI, Ratio) | YI | |
|---|---|---|---|---|---|
| I | DWI | 0.92 (0.87–0.96) | 80 (56.3–94.3, 16/20) | 93.5 (88.4–96.8, 144/154) | 0.735 |
| DCE | 0.93 (0.88–0.96) | 90 (68.3–98.8, 18/20) | 92.9 (87.6–96.4, 143/154) | 0.829 | |
| 18F-FDG-PET/CT | 0.90 (84.1–93.8) | 90 (68.3–98.8, 18/20) | 89.6 (83.7–94, 138/154) | 0.796 | |
| II | Co-detection on DWI and DCE | 0.94 (0.90–0.97) | 80 (56.3–97.3, 16/20) | 96.1 (91.7–98.6, 148/154) | 0.761 |
| Either DWI or DCE | 0.90 (0.84–0.94) | 90 (68.3–98.8, 18/20) | 90.3 (84.4–94.5, 139/154) | 0.803 | |
| Co-detection on PET + DWI | 0.94 (0.90–0.97) | 80 (56.3–94.3, 16/20) | 96.1 (91.7–98.6, 148/154) | 0.761 | |
| Either PET or DWI | 0.87 (0.81–0.92) | 90 (68.3–98.8, 18/20) | 87 (80.7–91.9, 134/154) | 0.770 | |
| III | Co-detection on DWI and DCE and PET | 0.95 (0.91–0.98) | 80 (56.3–94.3, 16/20) | 97.4 (93.5–99.3, 150/154) | 0.774 |
| Either DWI or DCE or PET | 0.87 (0.81–0.92) | 95 (75.1–99.9, 19/20) | 86.4 (79.9–99.9, 133/154) | 0.814 |
| Per-Patient (n = 29) | AUC (95%CI) | Sensitivity (%, 95%CI, Ratio) | Specificity (%, 95%CI, Ratio) | YI | |
|---|---|---|---|---|---|
| I | DWI | 0.72 (0.53–0.87) | 95 (75.1–99.9, 19/20) | 22.2 (2.8–60, 2/9) | 0.117 |
| DCE | 0.72 (0.53–0.87) | 90 (68.3–98.8, 18/20) | 33.3 (7.5–70.1, 3/9) | 0.233 | |
| 18F-FDG-PET/CT | 0.76 (0.56–0.90) | 100 (100, 20/20) | 22.2 (2.8–60, 2/9) | 0.222 | |
| II | Co-detection on DWI and DCE | 0.76 (0.56–0.90) | 90 (68.3–98.8, 18/20) | 44.4 (13.7–78.8, 4/9) | 0.344 |
| Either DWI or DCE | 0.69 (0.49–0.85) | 95 (75.1–99.9, 19/20) | 11.1 (0.3–48.3, 1/9) | 0.061 | |
| Co-detection on DWI and PET | 0.76 (0.56–0.90) | 95 (75.1–99.9, 19/20) | 33.3 (7.5–70.1, 3/9) | 0.283 | |
| Either DWI or PET | 0.72 (0.53–0.87) | 100 (100, 20/20) | 11.1 (0.3–48.3, 1/9) | 0.111 | |
| III | Co-detection on DWI and DCE and PET | 0.79 (60.3–92.0) | 90 (68.3–98.8, 18/20) | 55.6 (21.2–86.3, 5/9) | 0.456 |
| Either DWI or DCE or PET | 0.72 (52.8–87.3) | 100 (100, 20/20) | 11.1 (0.3–48.3, 1/9) | 0.111 |
| Per-Location | DWI | DCE | 18F-FDG-PET/CT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | n | % (95%CI) | Total | n | % (95%CI) | Total | n | % (95%CI) | ||
| Overall Agreement | Mean | 174 | 153 | 87.9 (82.1–92.4) | 174 | 151 | 86.8 (80.8–91.4) | 174 | 151 | 86.8 (80.8–91.4) |
| Specific Agreement | Total | 174 | 163 | 93.7 (89–96.8) | 174 | 163 | 93.7 (89–96.8) | 174 | 156 | 89.7 (84.1–93.8) |
| Positive | 23.5 | 18 | 76.6 (54.8–91.4) | 24.5 | 19 | 77.6 (56.4–91.8) | 31 | 22 | 71 (52–85.8) | |
| Negative | 150.5 | 145 | 96.3 (92–98.7) | 149.5 | 144 | 96.3 (91.9–98.7) | 143 | 134 | 93.7 (88.4–97.1) | |
| Per-patient | ||||||||||
| Overall Agreement | Mean | 29 | 12 | 41.4 (23.5–61.1) | 29 | 12 | 41.4 (23.5–61.1) | 29 | 19 | 65.5 (45.7–82.1) |
| Specific Agreement | Total | 29 | 24 | 82.8 (64.2–94.2) | 29 | 21 | 72.4 (52.8–87.3) | 29 | 27 | 93.1 (77.2–99.2) |
| Positive | 23.5 | 21 | 89.4 (69.8–98.1) | 23 | 19 | 82.6 (61.8–98.1) | 27 | 26 | 96.3 (81–99.9) | |
| Negative | 5.5 | 3 | 55 (13.2–91.5) | 6 | 2 | 55 (13.2–91.5) | 2 | 1 | 50 (1.26–98.7) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, R.M.; Stappen, R.v.d.; Koopman, T.; Noij, D.P.; Comans, E.F.; Zwezerijnen, G.J.; Vergeer, M.R.; Leemans, C.R.; de Bree, R.; Boellaard, R.; et al. The Additional Value of Ultrafast DCE-MRI to DWI-MRI and 18F-FDG-PET to Detect Occult Primary Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2826. https://doi.org/10.3390/cancers12102826
Martens RM, Stappen Rvd, Koopman T, Noij DP, Comans EF, Zwezerijnen GJ, Vergeer MR, Leemans CR, de Bree R, Boellaard R, et al. The Additional Value of Ultrafast DCE-MRI to DWI-MRI and 18F-FDG-PET to Detect Occult Primary Head and Neck Squamous Cell Carcinoma. Cancers. 2020; 12(10):2826. https://doi.org/10.3390/cancers12102826
Chicago/Turabian StyleMartens, Roland M., Ruud van der Stappen, Thomas Koopman, Daniel P. Noij, Emile F. Comans, Gerben J. Zwezerijnen, Marije R. Vergeer, C. René Leemans, Remco de Bree, Ronald Boellaard, and et al. 2020. "The Additional Value of Ultrafast DCE-MRI to DWI-MRI and 18F-FDG-PET to Detect Occult Primary Head and Neck Squamous Cell Carcinoma" Cancers 12, no. 10: 2826. https://doi.org/10.3390/cancers12102826
APA StyleMartens, R. M., Stappen, R. v. d., Koopman, T., Noij, D. P., Comans, E. F., Zwezerijnen, G. J., Vergeer, M. R., Leemans, C. R., de Bree, R., Boellaard, R., Castelijns, J. A., & de Graaf, P. (2020). The Additional Value of Ultrafast DCE-MRI to DWI-MRI and 18F-FDG-PET to Detect Occult Primary Head and Neck Squamous Cell Carcinoma. Cancers, 12(10), 2826. https://doi.org/10.3390/cancers12102826