Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery
Simple Summary
Abstract
1. Introduction
2. Transporter-Targeted NDDS for Improved Oral Absorption
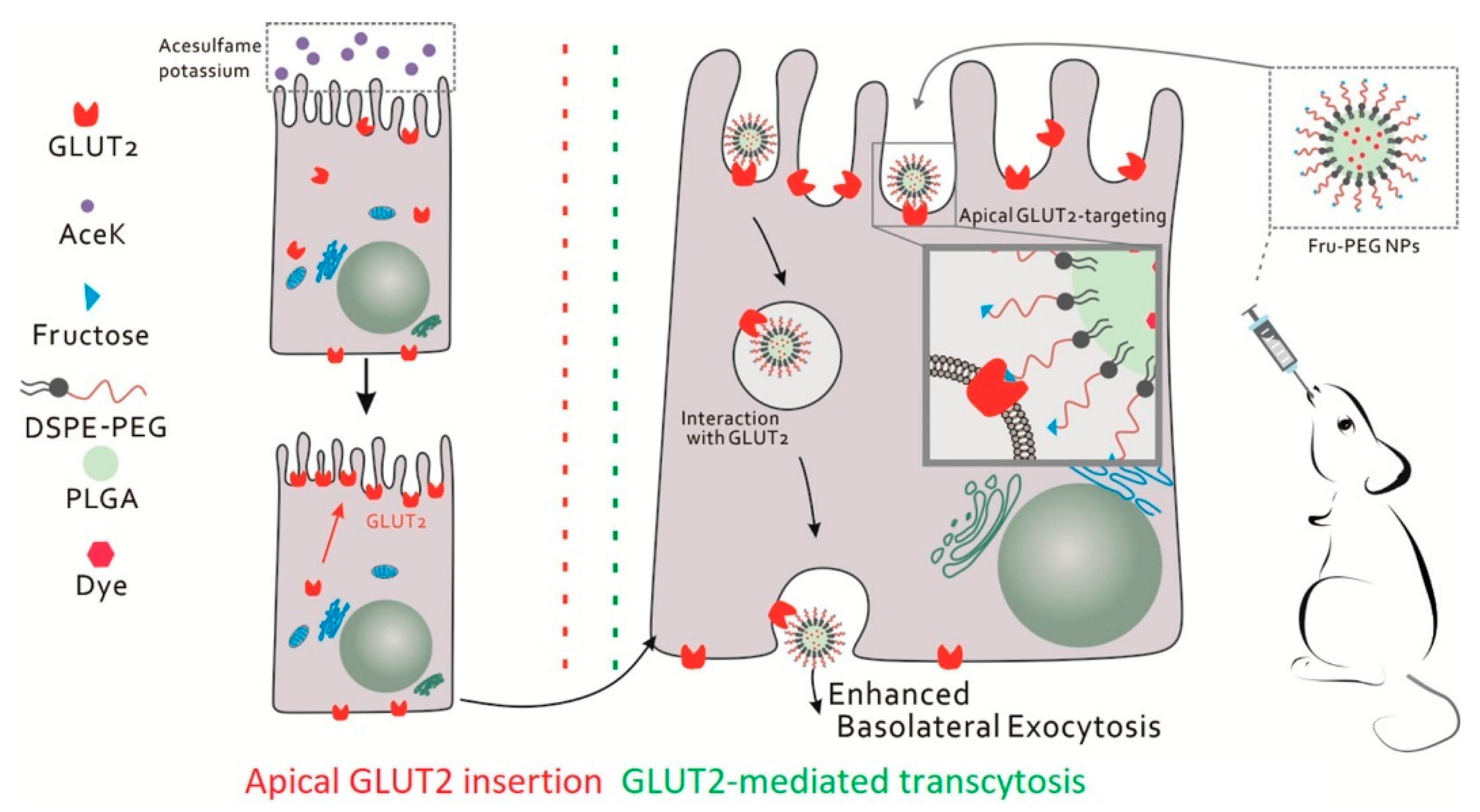
2.1. Increasing the Oral Absorption of Small Molecules
2.2. Increasing the Oral Absorption of Macromolecules
3. Transporter-Targeted NDDS for Enhancing BBB Permeation
4. Transporter-Targeted NDDS for Drug Delivery into Tumor Cells
5. Transporter-Targeted NDDS for Topical Ocular Drug Delivery
6. Transporter-Targeted NDDS for Other Indications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Z.; Cai, A.; Lin, X.; Jiang, X.; Zhou, B.; Wang, J.; Yao, Q.; Chen, R.; Kou, L. Nanoparticle mediated codelivery of nifuratel and doxorubicin for synergistic anticancer therapy through STAT3 inhibition. Colloids Surf. B. Biointerfaces 2020, 193, 111109. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Jiang, X.; Xiao, S.; Zhao, Y.Z.; Yao, Q.; Chen, R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Control. Release 2020, 318, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Jiang, X.; Zhai, Y.Y.; Luo, L.Z.; Xu, H.L.; Xiao, J.; Kou, L.; Zhao, Y.Z. Protective effects and mechanisms of bilirubin nanomedicine against acute pancreatitis. J. Control. Release 2020, 322, 312–325. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Wang, L.F.; Huang, Z.W.; Chen, R.; Yang, Y.; Xu, H.L.; Kou, L.; Zhao, Y.Z. Aloe/poloxamer hydrogel as an injectable beta-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur. J. Pharm. Sci. 2020, 148, 105316. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, Z.W.; Zhai, Y.Y.; Yue, M.; Luo, L.Z.; Xue, P.P.; Han, Y.H.; Xu, H.L.; Kou, L.; Zhao, Y.Z. Localized controlled release of bilirubin from beta-cyclodextrin-conjugated epsilon-polylysine to attenuate oxidative stress and inflammation in transplanted islets. ACS Appl. Mater. Interfaces 2020, 12, 5462–5475. [Google Scholar] [CrossRef]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Kou, L.; Tu, Y.; Tang, X.; Zhu, L. Tumor-targeted drug delivery and sensitization by MMP2-responsive polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 71–80. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface engineered dendrimers in siRNA delivery and gene silencing. Curr. Pharm. Des. 2017, 23, 2952–2975. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- International Transporter Consortium; Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Liu, X. Overview: Role of drug transporters in drug disposition and its clinical significance. Adv. Exp. Med. Biol. 2019, 1141, 1–12. [Google Scholar]
- Kou, L.; Sun, R.; Bhutia, Y.D.; Yao, Q.; Chen, R. Emerging advances in P-glycoprotein inhibitory nanomaterials for drug delivery. Expert Opin. Drug Deliv. 2018, 15, 869–879. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC transporters at the blood-brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2019, 12, 20. [Google Scholar] [CrossRef]
- Ganapathy, M.E.; Huang, W.; Wang, H.; Ganapathy, V.; Leibach, F.H. Valacyclovir: A substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem. Biophys. Res. Commun. 1998, 246, 470–475. [Google Scholar] [CrossRef]
- Sugawara, M.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V.; Ganapathy, M.E. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J. Pharm. Sci. 2000, 89, 781–789. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Kou, L.; He, Z.; Sun, J. Special topic: Emerging role of transporters in drug interaction and delivery. Asian J. Pharm. Sci. 2020, 15, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tian, C.; Wang, M.; Huang, D.; Wei, W.; Liu, Y.; Li, L.; Sun, B.; Kou, L.; Kan, Q.; et al. Dipeptide-modified nanoparticles to facilitate oral docetaxel delivery: New insights into PepT1-mediated targeting strategy. Drug Deliv. 2018, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jiang, M.; Kou, L.; Zhang, L.; Li, G.; Yao, Q.; Shang, L.; Chen, Y. Ascorbate-conjugated nanoparticles for promoted oral delivery of therapeutic drugs via sodium-dependent vitamin C transporter 1 (SVCT1). Artif. Cells Nanomed. Biotechnol. 2018, 46, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, C.; Zhou, W.; Chen, X.; Zhang, Y.; Lu, Y.; Zhang, Y.; Chen, Q.; Liang, D.; Sun, T.; et al. GLUT1-mediated effective anti-miRNA21 pompon for cancer therapy. Acta Pharmaceut. Sinica B 2019, 9, 832–842. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Zhang, Y.; Jiang, X.; Guo, Y.; An, S.; Ma, H.; He, X.; Jiang, C. Choline derivate-modified doxorubicin loaded micelle for glioma therapy. ACS Appl. Mater. Interfaces 2015, 7, 21589–21601. [Google Scholar] [CrossRef]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 2016, 1863, 2531–2539. [Google Scholar] [CrossRef]
- Borner, V.; Fei, Y.J.; Hartrodt, B.; Ganapathy, V.; Leibach, F.H.; Neubert, K.; Brandsch, M. Transport of amino acid aryl amides by the intestinal H+/peptide cotransport system, PEPT1. Eur. J. Biochem. 1998, 255, 698–702. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Leibach, F.H.; Ganapathy, V.; Thwaites, D.T. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Plugers Arch. 2002, 4452, 139–146. [Google Scholar]
- Gourdon, B.; Chemin, C.; Moreau, A.; Arnauld, T.; Baumy, P.; Cisternino, S.; Péan, J.-M.; Declèves, X. Functionalized PLA-PEG nanoparticles targeting intestinal transporter PepT1 for oral delivery of acyclovir. Int. J. Pharmaceut. 2017, 529, 357–370. [Google Scholar] [CrossRef]
- Gourdon, B.; Chemin, C.; Moreau, A.; Arnauld, T.; Delbos, J.-M.; Péan, J.-M.; Declèves, X. Influence of PLA-PEG nanoparticles manufacturing process on intestinal transporter PepT1 targeting and oxytocin transport. Eur. J. Pharmaceut. Biopharmaceut. 2018, 129, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.F.; Butler, R.N.; Brooks, D.A. Intestinal fructose transport and malabsorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G202–G206. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Affleck, J.; Patel, N.; Kellett, G.L. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol. 2007, 582, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Bai, Y.; Wang, L.; Liu, X.; Zhou, R.; Li, L.; Wu, R.; Zhang, Z.; Zhu, X.; Huang, Y. Promoting apical-to-basolateral unidirectional transport of nanoformulations by manipulating the nutrient-absorption pathway. J. Control. Release 2020, 323, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H.; Zhao, D.; Ding, D.; Sun, M.; Kou, L.; Luo, C.; Zhang, D.; Yi, X.; Dong, J.; et al. Combination of l-carnitine with lipophilic linkage-donating gemcitabine derivatives as intestinal novel organic cation transporter 2-targeting oral prodrugs. J. Med. Chem. 2017, 60, 2552–2561. [Google Scholar] [CrossRef]
- Kou, L.; Sun, R.; Ganapathy, V.; Yao, Q.; Chen, R. Recent advances in drug delivery via the organic cation/carnitine transporter 2 (OCTN2/SLC22A5). Expert Opin. Ther. Targets 2018, 22, 715–726. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, L.; Jiang, Q.; Sun, Y.; Zhao, D.; Sun, M.; He, Z.; Sun, J.; Wang, Y. Intestinal OCTN2- and MCT1-targeted drug delivery to improve oral bioavailability. Asian J. Pharm. Sci. 2020, 15, 158–173. [Google Scholar] [CrossRef]
- Kou, L.; Yao, Q.; Sun, M.; Wu, C.; Wang, J.; Luo, Q.; Wang, G.; Du, Y.; Fu, Q.; Wang, J.; et al. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: A case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv. Healthc. Mat. 2017, 6, 1700165. [Google Scholar] [CrossRef]
- Kou, L.; Sun, R.; Xiao, S.; Cui, X.; Sun, J.; Ganapathy, V.; Yao, Q.; Chen, R. OCTN2-targeted nanoparticles for oral delivery of paclitaxel: Differential impact of the polyethylene glycol linker size on drug delivery in vitro, in situ, and in vivo. Drug Deliv. 2020, 27, 170–179. [Google Scholar] [CrossRef]
- Xie, F.; Yao, N.; Qin, Y.; Zhang, Q.; Chen, H.; Yuan, M.; Tang, J.; Li, X.; Fan, W.; Zhang, Q.; et al. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int. J. Nanomed. 2012, 7, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, J.; Zhang, Q.; Wu, W.; Mo, H.; Huang, L.; Zhang, W. l-Carnitine conjugated chitosan-stearic acid polymeric micelles for improving the oral bioavailability of paclitaxel. Drug Deliv. 2020, 27, 575–584. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jin, Y.; Deng, Y.; Zou, Y.; Han, S.; Zhou, C.; Zhou, Y.; Liu, Y. Efficient oral delivery of poorly water-soluble drugs using carnitine/organic cation transporter 2-mediated polymeric micelles. ACS Biomat. Sci. Eng. 2020, 6, 2146–2158. [Google Scholar] [CrossRef]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Reeck, G.R.; Cho, K.J.; Lee, Y.-k. Oral delivery of taurocholic acid linked heparin–docetaxel conjugates for cancer therapy. J. Control. Release 2013, 170, 74–82. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Song, X.; Wang, P.; Li, Y. Cholate-modified polymer-lipid hybrid nanoparticles for oral delivery of quercetin to potentiate the antileukemic effect. Int. J. Nanomed. 2019, 14, 4045–4057. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, D.; Xu, J.; Wang, J.; Wei, Q.; Xiong, S. Bile acid transporter mediated STC/Soluplus self-assembled hybrid nanoparticles for enhancing the oral drug bioavailability. Int. J. Pharm. 2020, 579, 119120. [Google Scholar] [CrossRef]
- Kim, K.S.; Suzuki, K.; Cho, H.; Youn, Y.S.; Bae, Y.H. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano 2018, 12, 8893–8900. [Google Scholar] [CrossRef]
- Kim, K.S.; Youn, Y.S.; Bae, Y.H. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. J. Control. Release 2019, 311–312, 85–95. [Google Scholar] [CrossRef]
- Prasad, P.D.; Wang, H.; Kekuda, R.; Fujita, T.; Fei, Y.J.; Devoe, L.D.; Leibach, F.H.; Ganapathy, V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenase, biotin, and lipoate. J. Biol. Chem. 1998, 273, 7501–7506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, W.; Fei, Y.J.; Xia, H.; Yang-Feng, T.L.; Leibach, F.H.; Devoe, L.D.; Ganapathy, V.; Prasad, P.D. Human placental Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J. Biol. Chem. 1999, 274, 14875–14883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Ye, Y.; Zhang, T.; Wang, H.; Ma, Z.; Wu, B. Nanostructured lipid carriers used for oral delivery of oridonin: An effect of ligand modification on absorption. Int. J. Pharm. 2015, 479, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Burzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clemencon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family SLC23. Mol. Asp. Med. 2013, 34, 436–454. [Google Scholar] [CrossRef]
- Lee, Y.; Nam, J.H.; Shin, H.C.; Byun, Y. Conjugation of low-molecular-weight heparin and deoxycholic acid for the development of a new oral anticoagulant agent. Circulation 2001, 104, 3116–3120. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.Y.; Lee, E.; Lee, Y.-K.; Kim, C.Y.; Moon, H.T.; Byun, Y. Absorption study of deoxycholic acid-heparin conjugate as a new form of oral anti-coagulant. J. Control. Release 2007, 120, 4–10. [Google Scholar] [CrossRef]
- Lee, Y.-k.; Kim, S.K.; Lee, D.Y.; Lee, S.; Kim, C.-Y.; Shin, H.-C.; Moon, H.T.; Byun, Y. Efficacy of orally active chemical conjugate of low molecular weight heparin and deoxycholic acid in rats, mice and monkeys. J. Control. Release 2006, 111, 290–298. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Park, J.; Alam, F.; Chung, S.W.; Park, J.W.; Kim, K.; Kwon, I.C.; Kim, I.-S.; Kim, S.Y.; Byun, Y. Oligomeric bile acid-mediated oral delivery of low molecular weight heparin. J. Control. Release 2014, 175, 17–24. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Chung, S.W.; Alam, F.; Park, J.; Lee, K.E.; Jeon, H.; Kim, K.; Kwon, I.C.; Kim, I.-S.; Kim, S.Y.; et al. Functional transformations of bile acid transporters induced by high-affinity macromolecules. Sci. Rep. 2014, 4, 4163. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Alam, F.; Park, J.W.; Kim, K.; Kwon, I.C.; Ryu, G.H.; Byun, Y. Prevention effect of orally active heparin conjugate on cancer-associated thrombosis. J. Control. Release 2014, 195, 155–161. [Google Scholar] [CrossRef]
- Alam, F.; Al-Hilal, T.A.; Chung, S.W.; Seo, D.; Mahmud, F.; Kim, H.S.; Kim, S.Y.; Byun, Y. Oral delivery of a potent anti-angiogenic heparin conjugate by chemical conjugation and physical complexation using deoxycholic acid. Biomaterials 2014, 35, 6543–6552. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, K.; Kumar, T.S.; Lee, J.; Kim, S.K.; Lee, D.Y.; Lee, Y.-k.; Byun, Y. Synthesis and biological properties of insulin−deoxycholic acid chemical conjugates. Bioconjug. Chem. 2005, 16, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xia, D.; Zhu, Q.; Li, X.; He, S.; Zhu, C.; Guo, S.; Hovgaard, L.; Yang, M.; Gan, Y. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials 2018, 151, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Xu, G.; Yao, P. Liver-targeted delivery of insulin-loaded nanoparticles via enterohepatic circulation of bile acids. Drug Deliv. 2018, 25, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate transporters (SLC16): Function, regulation, and role in health and disease. Pharmacol. Rev. 2010, 72, 466–485. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-chain fatty acid transporters: Role in colonic homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar]
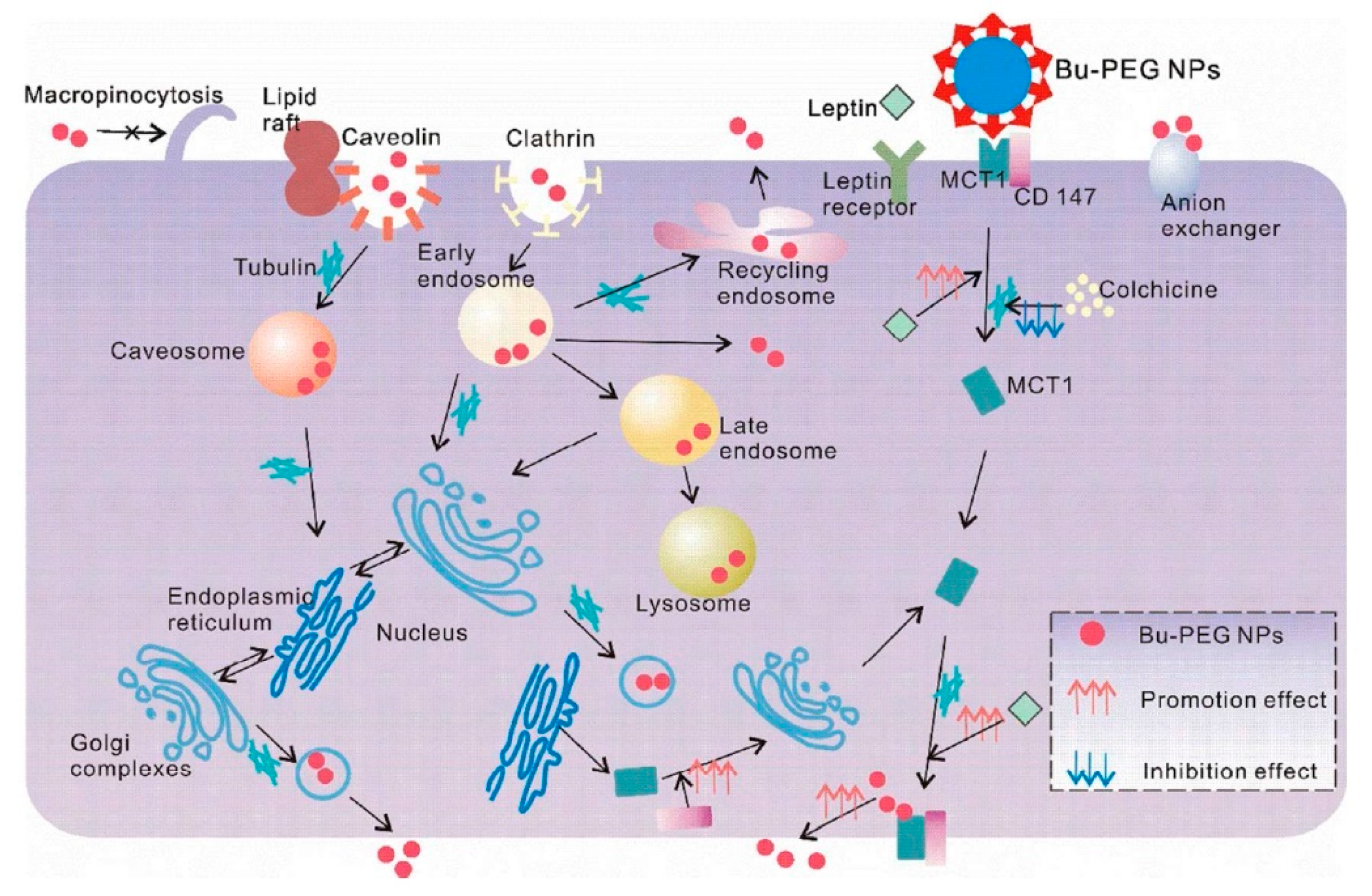
- Wu, L.; Liu, M.; Shan, W.; Zhu, X.; Li, L.; Zhang, Z.; Huang, Y. Bioinspired butyrate-functionalized nanovehicles for targeted oral delivery of biomacromolecular drugs. J. Control. Release 2017, 262, 273–283. [Google Scholar] [CrossRef]
- Wu, L.; Bai, Y.; Liu, M.; Li, L.; Shan, W.; Zhang, Z.; Huang, Y. Transport mechanisms of butyrate modified nanoparticles: Insight into “easy entry, hard transcytosis” of active targeting system in oral administration. Mol. Pharm. 2018, 15, 4273–4283. [Google Scholar] [CrossRef]
- Venishetty, V.K.; Samala, R.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. beta-Hydroxybutyric acid grafted solid lipid nanoparticles: A novel strategy to improve drug delivery to brain. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 388–397. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, J.; Lu, Y.; He, W.; Li, X.; Wu, W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 167–176. [Google Scholar] [CrossRef]
- Hu, C.; Tao, L.; Cao, X.; Chen, L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Anraku, Y.; Kuwahara, H.; Fukusato, Y.; Mizoguchi, A.; Ishii, T.; Nitta, K.; Matsumoto, Y.; Toh, K.; Miyata, K.; Uchida, S.; et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef]
- Jiang, X.; Xin, H.; Gu, J.; Du, F.; Feng, C.; Xie, Y.; Fang, X. Enhanced antitumor efficacy by d-glucosamine-functionalized and paclitaxel-loaded poly(ethylene glycol)-co-poly(trimethylene carbonate) polymer nanoparticles. J. Pharm. Sci. 2014, 103, 1487–1496. [Google Scholar] [CrossRef]
- Shao, K.; Ding, N.; Huang, S.; Ren, S.; Zhang, Y.; Kuang, Y.; Guo, Y.; Ma, H.; An, S.; Li, Y.; et al. Smart nanodevice combined tumor-specific vector with cellular microenvironment-triggered property for highly effective antiglioma therapy. ACS Nano 2014, 8, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Lockman, P.R.; Nguyen, T.H.; Van der Schyf, C.J.; Crooks, P.A.; Dwoskin, L.P.; Allen, D.D. 3D-QSAR study of bis-azaaromatic quaternary ammonium analogs at the blood–brain barrier choline transporter. Bioorg. Med. Chem. 2005, 13, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, L.; Ye, D.; Huang, S.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Liu, S.; Ye, L.; et al. Choline-derivate-modified nanoparticles for brain-targeting gene delivery. Adv. Mater. 2011, 23, 4516–4520. [Google Scholar] [CrossRef]
- Huse, J.T.; Holland, E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 2010, 10, 319–331. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Shao, K.; Liu, Y.; An, S.; Kuang, Y.; Guo, Y.; Ma, H.; Wang, X.; Jiang, C. A choline derivate-modified nanoprobe for glioma diagnosis using MRI. Sci. Rep. 2013, 3, 1623. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Kuang, Y.; An, S.; Ma, H.; Jiang, C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials 2013, 34, 9142–9148. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacin, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Kharya, P.; Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Majumdar, S.; Jain, S.K. Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting. J. Nanopart. Res. 2013, 15, 2022. [Google Scholar] [CrossRef]
- Rautio, J.; Gynther, M.; Laine, K. LAT1-mediated prodrug uptake: A way to breach the blood-brain barrier? Ther. Deliv. 2013, 4, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Di, X.; Zhang, S.; Kan, Q.; Liu, H.; Lu, T.; Wang, Y.; Fu, Q.; Sun, J.; He, Z. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf. B. Biointerfaces 2016, 141, 260–267. [Google Scholar] [CrossRef]
- Bhunia, S.; Vangala, V.; Bhattacharya, D.; Ravuri, H.G.; Kuncha, M.; Chakravarty, S.; Sistla, R.; Chaudhuri, A. Large Amino Acid Transporter 1 Selective Liposomes of l-DOPA Functionalized Amphiphile for Combating Glioblastoma. Mol. Pharm. 2017, 14, 3834–3847. [Google Scholar] [CrossRef]
- Salmaso, S.; Pappalardo, J.S.; Sawant, R.R.; Musacchio, T.; Rockwell, K.; Caliceti, P.; Torchilin, V.P. Targeting glioma cells in vitro with ascorbate-conjugated pharmaceutical nanocarriers. Bioconjug. Chem. 2009, 20, 2348–2355. [Google Scholar] [CrossRef]
- Inano, A.; Sai, Y.; Nikaido, H.; Hasimoto, N.; Asano, M.; Tsuji, A.; Tamai, I. Acetyl-L-carnitine permeability across the blood-brain barrier and involvement of carnitine transporter OCTN2. Biopharm. Drug Dispos. 2003, 24, 357–365. [Google Scholar] [CrossRef]
- Virmani, A.; Binienda, Z. Role of carnitine esters in brain neuropathology. Mol. Asp. Med. 2004, 25, 533–549. [Google Scholar] [CrossRef]
- Kou, L.; Hou, Y.; Yao, Q.; Guo, W.; Wang, G.; Wang, M.; Fu, Q.; He, Z.; Ganapathy, V.; Sun, J. L-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1605–1616. [Google Scholar] [CrossRef]
- Kou, L.; Sun, R.; Xiao, S.; Zheng, Y.; Chen, Z.; Cai, A.; Zheng, H.; Yao, Q.; Ganapathy, V.; Chen, R. Ambidextrous approach to disrupt redox balance in tumor cells with increased ROS production and decreased GSH synthesis for cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 26722–26730. [Google Scholar] [CrossRef]
- Shan, X.H.; Hu, H.; Xiong, F.; Gu, N.; Geng, X.D.; Zhu, W.; Lin, J.; Wang, Y.F. Targeting Glut1-overexpressing MDA-MB-231 cells with 2-deoxy-D-g1ucose modified SPIOs. Eur. J. Radiol. 2012, 81, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Zhu, Z.Y.; Xiong, C.; Hua, X.Q.; Shan, X.H.; Zhang, Y.; Gu, N. Preparation, characterization of 2-deoxy-D-glucose functionalized dimercaptosuccinic acid-coated maghemite nanoparticles for targeting tumor cells. Pharm. Res. 2012, 29, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, T.; Gholibegloo, E.; Riyahi Alam, N.; Haghgoo, S.; Musa, A.E.; Khoobi, M. Glucosamine conjugated gadolinium (III) oxide nanoparticles as a novel targeted contrast agent for cancer diagnosis in MRI. J. Biomed. Phys. Eng. 2020, 10, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Zaritski, A.; Castillo-Ecija, H.; Kumarasamy, M.; Peled, E.; Sverdlov Arzi, R.; Carcaboso, A.M.; Sosnik, A. Selective accumulation of galactomannan amphiphilic nanomaterials in pediatric solid tumor xenografts correlates with GLUT1 gene expression. ACS Appl. Mater. Interfaces 2019, 11, 38483–38496. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, H.J.; Kim, D.D. Poly((D,L)lactic-glycolic)acid-star glucose nanoparticles for glucose transporter and hypoglycemia-mediated tumor targeting. Int. J. Nanomed. 2017, 12, 7453–7467. [Google Scholar] [CrossRef]
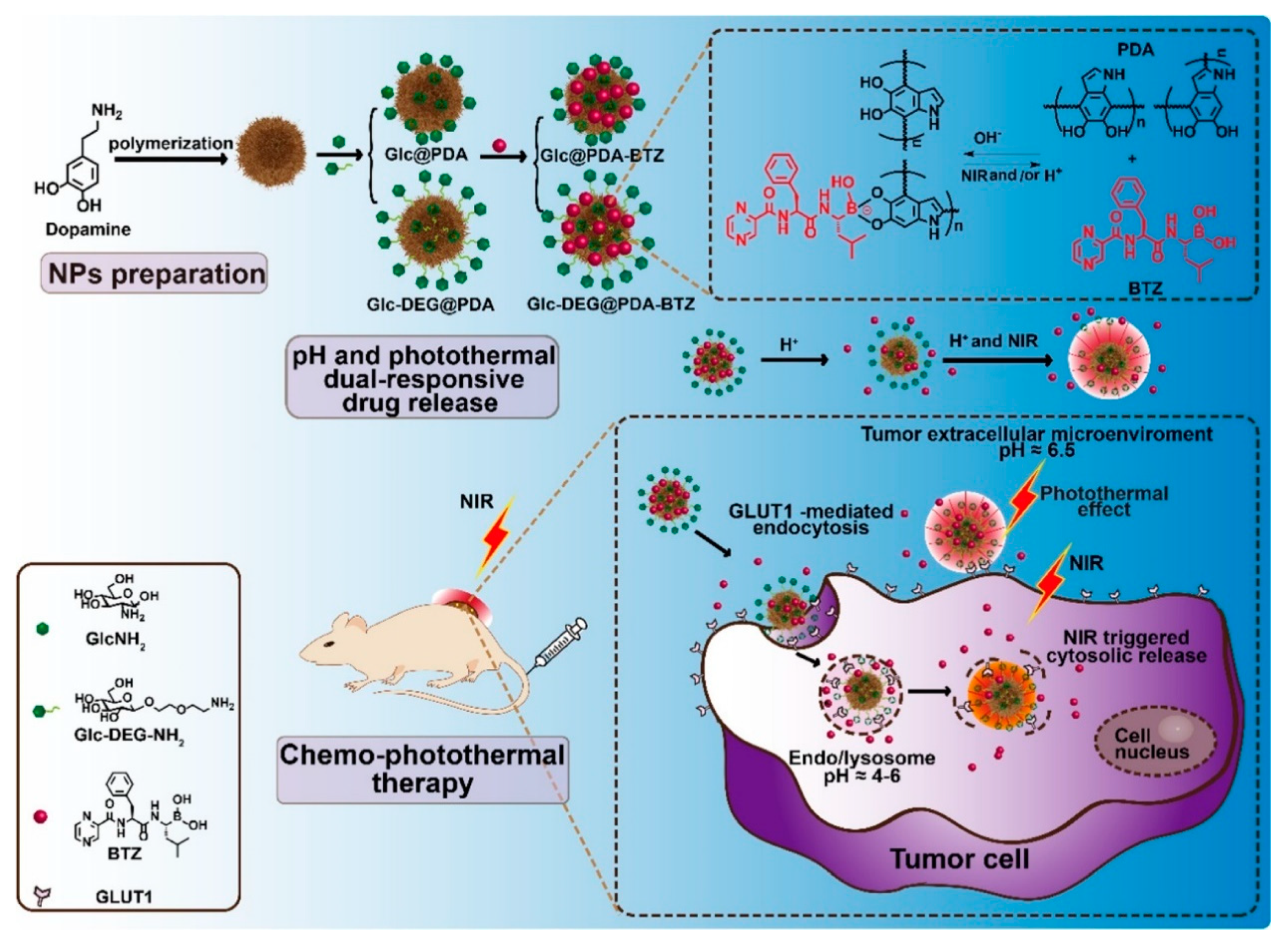
- Li, Y.; Hong, W.; Zhang, H.; Zhang, T.T.; Chen, Z.; Yuan, S.; Peng, P.; Xiao, M.; Xu, L. Photothermally triggered cytosolic drug delivery of glucose functionalized polydopamine nanoparticles in response to tumor microenvironment for the GLUT1-targeting chemo-phototherapy. J. Control. Release 2020, 317, 232–245. [Google Scholar] [CrossRef]
- Sztandera, K.; Dzialak, P.; Marcinkowska, M.; Stanczyk, M.; Gorzkiewicz, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Sugar modification enhances cytotoxic activity of PAMAM-doxorubicin conjugate in glucose-deprived MCF-7 cells—Possible role of GLUT1 transporter. Pharm. Res. 2019, 36, 140. [Google Scholar] [CrossRef]
- Kumar, P.; Paknikar, K.M.; Gajbhiye, V. A robust pH-sensitive unimolecular dendritic nanocarrier that enables targeted anti-cancer drug delivery via GLUT transporters. Colloids Surf. B. Biointerfaces 2018, 171, 437–444. [Google Scholar] [CrossRef]
- Yi, Y.; Kim, H.J.; Zheng, M.; Mi, P.; Naito, M.; Kim, B.S.; Min, H.S.; Hayashi, K.; Perche, F.; Toh, K.; et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J. Control. Release 2019, 295, 268–277. [Google Scholar] [CrossRef]
- Cheng, T.M.; Chu, H.L.; Lee, Y.C.; Wang, D.Y.; Chang, C.C.; Chung, K.L.; Yen, H.C.; Hsiao, C.W.; Pan, X.Y.; Kuo, T.R.; et al. Quantitative analysis of glucose metabolic cleavage in glucose transporters overexpressed cancer cells by target-specific fluorescent gold nanoclusters. Analyt. Chem. 2018, 90, 3974–3980. [Google Scholar] [CrossRef]
- Abolhasani, A.; Biria, D.; Abolhasani, H.; Zarrabi, A.; Komeili, T. Investigation of the role of glucose decorated chitosan and PLGA nanoparticles as blocking agents to glucose transporters of tumor cells. Int. J. Nanomed. 2019, 14, 9535–9546. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Galluccio, M.; Console, L.; Indiveri, C. Glutamine transporters as pharmacological targets: From function to drug design. Asian J. Pharm. Sci. 2020, 15, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J.; Wang, Z.; Yang, Z.; Li, Z.; Deng, H.; Li, L.; Peng, X.; Feng, M. Glutamine addiction activates polyglutamine-based nanocarriers delivering therapeutic siRNAs to orthotopic lung tumor mediated by glutamine transporter SLC1A5. Biomaterials 2018, 183, 77–92. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Babu, E.; Prasad, P.D.; Ganapathy, V. The amino acid transporter SLC6A14 in cancer and its potential use in chemotherapy. Asian J. Pharm. Sci. 2014, 9, 293–303. [Google Scholar] [CrossRef]
- Li, L.; Di, X.; Wu, M.; Sun, Z.; Zhong, L.; Wang, Y.; Fu, Q.; Kan, Q.; Sun, J.; He, Z. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: Toward a novel active targeting strategy in breast cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 987–998. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Chen, S.; Nabavi, E.; Regoutz, A.; Payne, D.J.; Elson, D.S.; Dexter, D.T.; Dunlop, I.E.; Porter, A.E. Multibranched gold nanoparticles with intrinsic LAT-1 targeting capabilities for selective photothermal therapy of breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 39259–39270. [Google Scholar] [CrossRef]
- Coothankansaswamy, V.; Cao, S.; Xu, Y.; Prasad, P.D.; Singh, P.K.; Reynolds, C.P.; Yang, S.; Ogura, J.; Ganapathy, V.; Bhutia, Y.D. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 2016, 1735, 3292–3306. [Google Scholar] [CrossRef]
- Karunakaran, S.; Umapathy, N.S.; Thangaraju, M.; Hatanaka, T.; Itagaki, S.; Munn, D.H.; Prasad, P.D.; Ganapathy, V. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 2008, 414, 343–355. [Google Scholar] [CrossRef]
- Gupta, N.; Miyauchi, S.; Martindale, R.G.; Herdman, A.V.; Podolsky, R.; Miyake, K.; Mager, S.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta 2005, 1741, 215–223. [Google Scholar] [CrossRef]
- Gupta, N.; Prasad, P.D.; Ghamande, S.; Moore-Martin, P.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Mager, S.; Ganapathy, M.E.; Ganapathy, V. Up-regulation of the amino acid transporter ATB0,+ (SLC6A14) in carcinoma of the cervix. Gynecol. Oncol. 2006, 100, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Gong, P.; Sun, M.; Kou, L.; Ganapathy, V.; Jing, Y.; He, Z.; Sun, J. Transporter occluded-state conformation-induced endocytosis: Amino acid transporter ATB0,+-mediated tumor targeting of liposomes for docetaxel delivery for hepatocarcinoma therapy. J. Control. Release 2016, 243, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, B.; Tao, W.; Li, J.; Kou, L.; Lian, H.; Che, X.; He, Z.; Sun, J. ATB0,+ transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes. Biomater. Sci. 2017, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Huang, H.; Lin, X.; Jiang, X.; Wang, Y.; Luo, Q.; Sun, J.; Yao, Q.; Ganapathy, V.; Chen, R. Endocytosis of ATB0,+ (SLC6A14)-targeted liposomes for drug delivery and its therapeutic application for pancreatic cancer. Expert Opin. Drug Deliv. 2020, 17, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharmaceut. Drug Dispos. 2013, 34, 29–44. [Google Scholar] [CrossRef]
- Nakanishi, T.; Hatanaka, T.; Huang, W.; Prasad, P.D.; Leibach, F.H.; Ganapathy, M.E.; Ganapathy, V. Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB0,+ from mouse colon expressed in HRPE cells and Xenopus oocytes. J. Physiol. 2001, 532, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Haramura, M.; Fei, Y.J.; Miyauchi, S.; Bridges, C.C.; Ganapathy, P.S.; Smith, S.B.; Ganapathy, V.; Ganapathy, M.E. Transport of amino acid-based prodrugs by the Na+- and Cl− -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J. Pharmacol. Exp. Ther. 2004, 308, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Yao, Q.; Sivaprakasam, S.; Luo, Q.; Sun, Y.; Fu, Q.; He, Z.; Sun, J.; Ganapathy, V. Dual targeting of l-carnitine-conjugated nanoparticles to OCTN2 and ATB0,+ to deliver chemotherapeutic agents for colon cancer therapy. Drug Deliv. 2017, 24, 1338–1349. [Google Scholar] [CrossRef]
- Uram, Ł.; Filipowicz, A.; Misiorek, M.; Pieńkowska, N.; Markowicz, J.; Wałajtys-Rode, E.; Wołowiec, S. Biotinylated PAMAM G3 dendrimer conjugated with celecoxib and/or Fmoc-l-Leucine and its cytotoxicity for normal and cancer human cell lines. Eur. J. Pharm. Sci. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, Y.; Xu, T.; Wang, X.; Wen, L.-P. Targeting cancer cells with biotin–dendrimer conjugates. Eur. J. Med. Chem. 2009, 44, 862–868. [Google Scholar] [CrossRef]
- Cheng, Y.; Ou, Z.; Li, Q.; Yang, J.; Hu, M.; Zhou, Y.; Zhuang, X.; Zhang, Z.J.; Guan, S. Cabazitaxel liposomes with aptamer modification enhance tumor-targeting efficacy in nude mice. Mol. Med. Rep. 2019, 19, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Sahoo, R.K.; Nakhate, K.T.; Gupta, U. Biotinylated HPMA centered polymeric nanoparticles for Bortezomib delivery. Int. J. Pharm. 2020, 579, 119173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Shi, B.X.; Cheng, H.; Li, C.; Gu, Z.W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Biotinylated thermoresponsive micelle self-assembled from double-hydrophilic block copolymer for drug delivery and tumor target. Biomaterials 2008, 29, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef] [PubMed]
- Morral-Ruíz, G.; Melgar-Lesmes, P.; López-Vicente, A.; Solans, C.; García-Celma, M.J. Biotinylated polyurethane-urea nanoparticles for targeted theranostics in human hepatocellular carcinoma. Nano Res. 2015, 8, 1729–1745. [Google Scholar] [CrossRef]
- Barot, M.; Gokulgandhi, M.R.; Pal, D.; Mitra, A.K. Mitochondrial localization of P-glycoprotein and peptide transporters in corneal epithelial cells—Novel strategies for intracellular drug targeting. Exp. Eye Res. 2013, 106, 47–54. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Xu, S.; Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Transport of L-carnitine in human corneal and conjunctival epithelial cells. Mol. Vis. 2010, 16, 1823–1831. [Google Scholar]
- Garrett, Q.; Xu, S.; Simmons, P.A.; Vehige, J.; Flanagan, J.L.; Willcox, M.D. Expression and localization of carnitine/organic cation transporter OCTN1 and OCTN2 in ocular epithelium. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4844–4849. [Google Scholar] [CrossRef]
- Bongiovi, F.; Fiorica, C.; Palumbo, F.S.; Di Prima, G.; Giammona, G.; Pitarresi, G. Imatinib-loaded micelles of hyaluronic acid derivatives for potential treatment of neovascular ocular diseases. Mol. Pharm. 2018, 15, 5031–5045. [Google Scholar] [CrossRef]
- Shamsi, F.A.; Chaudhry, I.A.; Boulton, M.E.; Al-Rajhi, A.A. L-carnitine protects human retinal pigment epithelial cells from oxidative damage. Curr. Eye Res. 2007, 32, 575–584. [Google Scholar] [CrossRef]
- Charrier, L.; Driss, A.; Yan, Y.; Nduati, V.; Klapproth, J.M.; Sitaraman, S.V.; Merlin, D. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab. Investig. 2006, 86, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Ayyadurai, S.; Charania, M.A.; Xiao, B.; Viennois, E.; Merlin, D. PepT1 expressed in immune cells has an important role in promoting the immune response during experimentally induced colitis. Lab. Investig. 2013, 93, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, M.; Wang, D.; Li, G.; Huang, J.; Tan, S.; Bao, L.; Li, Q.; Li, G.; Si, L. A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis. Biomater. Sci. 2019, 7, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Kim, S.T.; Tang, R.; Yan, B.; Rotello, V.M. Insulin-based regulation of glucose-functionalized nanoparticle uptake in muscle cells. J. Mater. Chem. B 2014, 2, 4610–4614. [Google Scholar] [CrossRef]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012, 1, e00049. [Google Scholar] [CrossRef]
- Witzigmann, D.; Uhl, P.; Sieber, S.; Kaufman, C.; Einfalt, T.; Schoneweis, K.; Grossen, P.; Buck, J.; Ni, Y.; Schenk, S.H.; et al. Optimization-by-design of hepatotropic lipid nanoparticles targeting the sodium-taurocholate cotransporting polypeptide. Elife 2019, 8, e42276. [Google Scholar] [CrossRef]
- Mishra, P.R.; Jain, N.K. Biotinylated methotrexate loaded erythrocytes for enhanced liver uptake. ‘A study on the rat’. Int. J. Pharm. 2002, 231, 145–153. [Google Scholar] [CrossRef]
- Park, J.; Al-Hilal, T.A.; Jeong, J.-H.; Choi, J.U.; Byun, Y. Design, synthesis, and therapeutic evaluation of poly(acrylic acid)–tetraDOCA conjugate as a bile acid transporter inhibitor. Bioconjug. Chem. 2015, 26, 1597–1605. [Google Scholar] [CrossRef]
- Zhu, Y.; Huo, X.; Wang, C.; Meng, Q.; Liu, Z.; Sun, H.; Tan, A.; Ma, X.; Peng, J.; Liu, K. Organic anion transporters also mediate the drug–drug interaction between imipenem and cilastatin. Asian J. Pharm. Sci. 2020, 15, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Katoono, R.; Yui, N.; Sugiura, T.; Kubo, Y.; Kato, Y.; Tsuji, A. Cationic polyrotaxanes effectively inhibit uptake via carnitine/organic cationic transporters without cytotoxicity. Macromol. Biosci. 2008, 8, 665–669. [Google Scholar] [CrossRef] [PubMed]



| Transporter | HUGO Nomenclature | Target for Drug Delivery |
|---|---|---|
| ASCT2 | SLC1A5 | Tumors |
| GLUT1 | SLC2A1 | Blood–brain barrier Glioma |
| GLUT2 | SLC2A2 | Oral absorption |
| GLUT4 | SLC2A4 | Muscle, heart, adipocyte |
| SMVT | SLC5A6 | Oral absorption Tumors, Liver |
| ChT | SLC5A7 | Blood–brain barrier Glioma |
| ATB0,+ | SLC6A14 | Tumors |
| LAT1 | SLC7A5 | Blood–brain barrier Tumors |
| ASBT | SLC10A2 | Oral absorption |
| PepT1 | SLC15A1 | Oral absorption Ocular delivery Colitis |
| MCT1 | SLC16A1 | Oral absorption Blood–brain barrier |
| OCTN2 | SLC22A5 | Oral absorption Blood–brain barrier Glioma Ocular delivery |
| SVCT2 | SLC23A2 | Blood–brain barrier |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, L.; Yao, Q.; Zhang, H.; Chu, M.; Bhutia, Y.D.; Chen, R.; Ganapathy, V. Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery. Cancers 2020, 12, 2837. https://doi.org/10.3390/cancers12102837
Kou L, Yao Q, Zhang H, Chu M, Bhutia YD, Chen R, Ganapathy V. Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery. Cancers. 2020; 12(10):2837. https://doi.org/10.3390/cancers12102837
Chicago/Turabian StyleKou, Longfa, Qing Yao, Hailin Zhang, Maoping Chu, Yangzom D. Bhutia, Ruijie Chen, and Vadivel Ganapathy. 2020. "Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery" Cancers 12, no. 10: 2837. https://doi.org/10.3390/cancers12102837
APA StyleKou, L., Yao, Q., Zhang, H., Chu, M., Bhutia, Y. D., Chen, R., & Ganapathy, V. (2020). Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery. Cancers, 12(10), 2837. https://doi.org/10.3390/cancers12102837




