Radiomic Features and Machine Learning for the Discrimination of Renal Tumor Histological Subtypes: A Pragmatic Study Using Clinical-Routine Computed Tomography
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
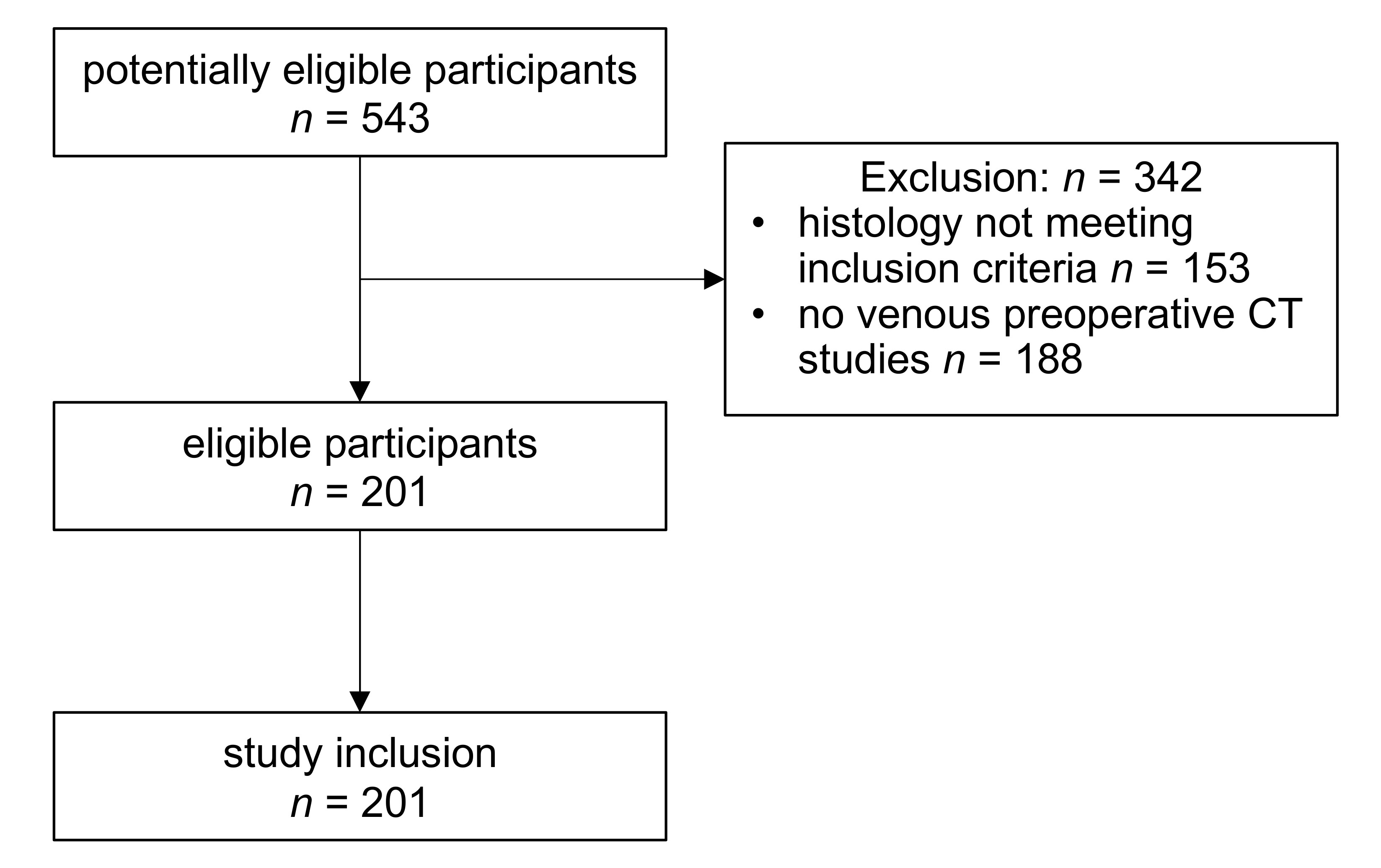
2.1. Study Cohort
2.2. Machine Learning Algorithms
2.3. Subgroup Analyses
2.4. Sensitivity Analyses
3. Discussion
4. Material and Methods
4.1. Study Cohort Selection
4.2. CT Imaging
4.3. Radiomic Feature Analyses
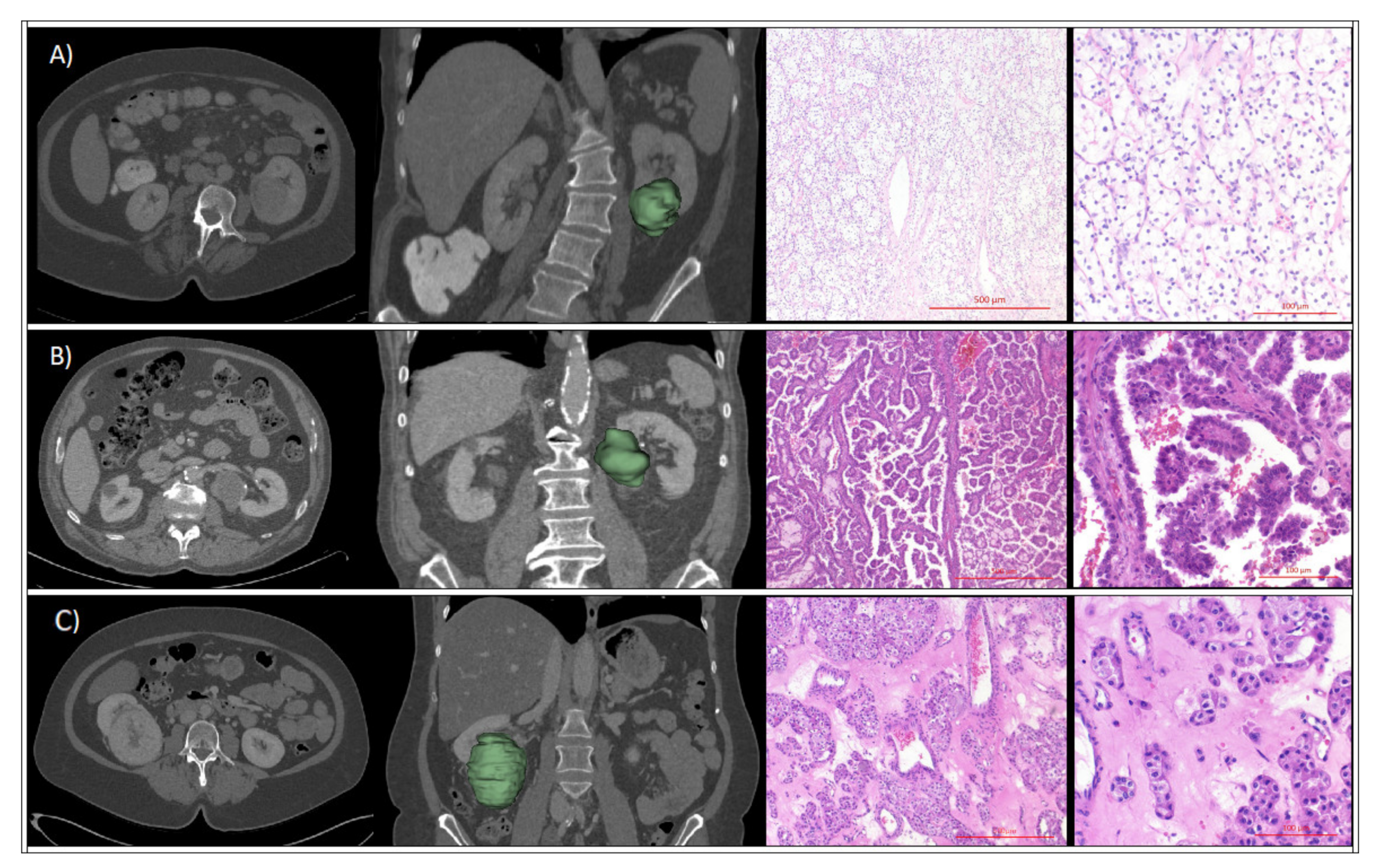
4.4. Renal Tumor Assessment
4.5. Machine Learning
4.6. Statistical Analyses and Diagnostic Performance Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AML | angiomyolipoma |
| AUC | area under the ROC curve |
| CD | cluster of differentiation |
| CK | cytokeratin |
| ccRCC | clear cell renal cell carcinoma |
| CT | computed tomography |
| C5.0 | boosted classification tree |
| glmnet | elastic net penalized multinomial regression |
| HE | hematoxylin-eosin |
| HMB | human melanoma black |
| ICC | interobserver correlation coefficient |
| IQR | inter-quartile range |
| KNN | k-nearest neighbor |
| nnet | neural network |
| POM | probability of malignancy |
| RCC | renal cell carcinoma |
| RF | random forest |
| RFE | recursive feature elimination |
| ROC | receiver operating characteristics |
| ROI | region of interest |
| SMOTE | synthetic minority oversampling technique |
| SVM | support vector machine |
| US | United States |
| xgboost | extreme gradient boosting |
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.M.; Miller, D.C.; Daignault, S.; Hollenbeck, B.K. Rising Incidence of Small Renal Masses: A Need to Reassess Treatment Effect. J. Natl. Cancer Inst. 2006, 98, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.; Mallin, K.; Ritchey, J.; Cooperberg, M.R.; Carroll, P.R. Renal cell cancer stage migration. Cancer 2008, 113, 78–83. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Gill, I.S.; Ellison, L.M. The Evolving Presentation of Renal Carcinoma in the United States: Trends From the Surveillance, Epidemiology, and End Results Program. J. Urol. 2006, 176, 2397–2400. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. Solid Renal Tumors: An Analysis of Pathological Features Related to Tumor Size. J. Urol. 2003, 170, 2217–2220. [Google Scholar] [CrossRef]
- Johnson, D.C.; Vukina, J.; Smith, A.B.; Meyer, A.-M.; Wheeler, S.B.; Kuo, T.-M.; Tan, H.-J.; Woods, M.E.; Raynor, M.C.; Wallen, E.M.; et al. Preoperatively Misclassified, Surgically Removed Benign Renal Masses: A Systematic Review of Surgical Series and United States Population Level Burden Estimate. J. Urol. 2015, 193, 30–35. [Google Scholar] [CrossRef]
- Juntu, J.; Sijbers, J.; De Backer, S.; Rajan, J.; Van Dyck, D. Machine learning study of several classifiers trained with texture analysis features to differentiate benign from malignant soft-tissue tumors in T1-MRI images. J. Magn. Reson. Imaging 2010, 31, 680–689. [Google Scholar] [CrossRef]
- Yu, H.; Scalera, J.; Khalid, M.; Touret, A.-S.; Bloch, N.; Li, B.; Qureshi, M.M.; Soto, J.A.; Anderson, S.W. Texture analysis as a radiomic marker for differentiating renal tumors. Abdom. Radiol. 2017, 42, 2470–2478. [Google Scholar] [CrossRef]
- Coy, H.; Young, J.R.; Douek, M.L.; Brown, M.S.; Sayre, J.; Raman, S.S. Quantitative computer-aided diagnostic algorithm for automated detection of peak lesion attenuation in differentiating clear cell from papillary and chromophobe renal cell carcinoma, oncocytoma, and fat-poor angiomyolipoma on multiphasic multidetector computed tomography. Abdom. Radiol. 2017, 7, 110–1928. [Google Scholar] [CrossRef]
- Feng, Z.; Rong, P.; Cao, P.; Zhou, Q.; Zhu, W.; Yan, Z.; Liu, Q.; Wang, W. Machine learning-based quantitative texture analysis of CT images of small renal masses: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur. Radiol. 2017, 28, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Yardimci, A.H.; Bektas, C.T.; Turkcanoglu, M.H.; Erdim, C.; Yucetas, U.; Koca, S.B.; Kilickesmez, O. Textural differences between renal cell carcinoma subtypes: Machine learning-based quantitative computed tomography texture analysis with independent external validation. Eur. J. Radiol. 2018, 107, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hand, D.J.; Till, R.J. A Simple Generalisation of the Area Under the ROC Curve for Multiple Class Classification Problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]
- Kutikov, A.; Fossett, L.K.; Ramchandani, P.; Tomaszewski, J.E.; Siegelman, E.S.; Banner, M.P.; Van Arsdalen, K.N.; Wein, A.J.; Malkowicz, S.B. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 2006, 68, 737–740. [Google Scholar] [CrossRef]
- Amin, J.; Xu, B.; Badkhshan, S.; Abbotoy, D.; Murekeyisoni, C.; Attwood, K.M.; Schwaab, T.; Hendler, C.; Petroziello, M.; Creighton, T.T.; et al. Identification and Validation of Radiographic Enhancement for Reliable Differentiation of CD117(+) Benign Renal Oncocytoma and Chromophobe Renal Cell Carcinoma. Clin. Cancer Res. 2018, 24, 3898–3907. [Google Scholar] [CrossRef]
- Uhlig, J.; Biggemann, L.; Nietert, M.M.; Beißbarth, T.; Lotz, J.; Kim, H.S.; Trojan, L.; Uhlig, A. Discriminating malignant and benign clinical T1 renal masses on computed tomography. Medicine 2020, 99, e19725. [Google Scholar] [CrossRef]
- Hodgdon, T.; McInnes, M.D.; Schieda, N.; Flood, T.A.; Lamb, L.; Thornhill, R. Can Quantitative CT Texture Analysis be Used to Differentiate Fat-poor Renal Angiomyolipoma from Renal Cell Carcinoma on Unenhanced CT Images? Radiology 2015, 276, 787–796. [Google Scholar] [CrossRef]
- Lee, H.S.; Hong, H.; Jung, D.C.; Park, S.; Kim, J. Differentiation of fat-poor angiomyolipoma from clear cell renal cell carcinoma in contrast-enhanced MDCT images using quantitative feature classification. Med Phys. 2017, 44, 3604–3614. [Google Scholar] [CrossRef]
- Erdim, C.; Yardimci, A.H.; Bektas, C.T.; Kocak, B.; Koca, S.B.; Demir, H.; Kilickesmez, O. Prediction of Benign and Malignant Solid Renal Masses: Machine Learning-Based CT Texture Analysis. Acad. Radiol. 2020, 27, 1422–1429. [Google Scholar] [CrossRef]
- You, M.-W.; Kim, N.; Choi, H. The value of quantitative CT texture analysis in differentiation of angiomyolipoma without visible fat from clear cell renal cell carcinoma on four-phase contrast-enhanced CT images. Clin. Radiol. 2019, 74, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Min, P.Q.; Wang, P.J.; Cheng, W.X.; Zhang, X.H.; Wang, Y.; Zhao, X.H.; Mao, X.Q. Dynamic CT Evaluation of Tumor Vascularity in Renal Cell Carcinoma. Am. J. Roentgenol. 2006, 186, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Meregalli, C.; Bombelli, S.; Di Stefano, V.; Salerno, F.; Torsello, B.; De Marco, S.; Bovo, G.; Cifola, I.; Mangano, E.; et al. The glucose and lipid metabolism reprogramming is grade-dependent in clear cell renal cell carcinoma primary cultures and is targetable to modulate cell viability and proliferation. Oncotarget 2017, 8, 113502–113515. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Loizzo, D.; Franzin, R.; Battaglia, S.; Ferro, M.; Cantiello, F.; Castellano, G.; Bettocchi, C.; Ditonno, P.; Battaglia, M. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev. Mol. Diagn. 2019, 19, 397–407. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Sallustio, F.; Ribatti, D.; Giglio, A.; Signorile, M.L.; Grossi, V.; Sanese, P.; Napoli, A.; Maiorano, E.; et al. Integrated multi-omics characterization reveals a distinctive metabolic signature and the role of NDUFA4L2 in promoting angiogenesis, chemoresistance, and mitochondrial dysfunction in clear cell renal cell carcinoma. Aging 2018, 10, 3957–3985. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, M.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Chawla, N.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Ambroise, C.; McLachlan, G.J. Selection bias in gene extraction on the basis of microarray gene-expression data. Proc. Natl. Acad. Sci. USA 2002, 99, 6562–6566. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models inRUsing thecaretPackage. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]

| Cohort | Parameter | Level | Total | ccRCC | Papillary | Oncocytoma | AML | Chromophobe | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Full cohort without SMOTE | n | 201 | 131 | 29 | 16 | 14 | 11 | ||
| age | mean ± sd | 64 ± 11 | 65 ± 11 | 67 ± 11 | 63 ± 8.4 | 56 ± 14 | 60 ± 9.2 | 0.02 | |
| median (min; max) | 66 (31; 85) | 68 (31; 85) | 68 (40; 81) | 62 (51; 77) | 56 (31; 76) | 59 (47; 78) | |||
| gender | female | 73 (36.3%) | 43 (32.8%) | 5 (17.2%) | 9 (56.2%) | 9 (64.3%) | 7 (63.6%) | <0.01 | |
| male | 128 (63.7%) | 88 (67.2%) | 24 (82.8%) | 7 (43.8%) | 5 (35.7%) | 4 (36.4%) | |||
| Max 3D Diameter | mean ± sd | 58 ± 28 | 57 ± 23 | 57 ± 35 | 58 ± 30 | 51 ± 35 | 72 ± 48 | 0.46 | |
| median (min; max) | 52 (13; 192) | 54 (21; 144) | 47 (22; 183) | 52 (20; 141) | 43 (13; 127) | 59 (16; 192) | |||
| Full cohort with SMOTE | n | 389 | 131 | 58 | 64 | 70 | 66 | ||
| age | mean ± sd | 62 ± 11 | 65 ± 11 | 67 ± 9.7 | 64 ± 6.6 | 55 ± 11 | 59 ± 7.2 | <0.01 | |
| median (min; max) | 63 (31; 85) | 68 (31; 85) | 69 (40; 81) | 65 (51; 77) | 56 (31; 76) | 59 (47; 78) | |||
| gender | female | 176 (45.2%) | 43 (32.8%) | 9 (15.5%) | 38 (59.4%) | 43 (61.4%) | 43 (65.2%) | <0.01 | |
| male | 213 (54.8%) | 88 (67.2%) | 49 (84.5%) | 26 (40.6%) | 27 (38.6%) | 23 (34.8%) | |||
| Max. 3D Diameter | mean ± sd | 57 ± 28 | 57 ± 23 | 56 ± 30 | 54 ± 24 | 51 ± 29 | 70 ± 35 | <0.01 | |
| median (min; max) | 51 (13; 192) | 54 (21; 144) | 47 (22; 183) | 50 (20; 141) | 46 (13; 127) | 64 (16; 192) |
| Parameter | Level | Total | Any Imaging Artifacts | No Imaging Artifacts | p Value |
|---|---|---|---|---|---|
| n | 201 | 60 | 141 | ||
| imaging center | external imaging center | 167 (83.1%) | 58 (96.7%) | 109 (77.3%) | <0.01 |
| tertiary imaging center | 34 (16.9%) | 2 (3.3%) | 32 (22.7%) | ||
| slice thickness | mean ± sd | 2.79 ± 1.81 | 4.3 ± 1.31 | <0.01 | |
| median (IQR) | 2 (1–5) | 5 (4.5–5) | 1.2 (1–3) |
| No Upsampling | SMOTE | |||||
|---|---|---|---|---|---|---|
| Machine Learning Algorithm | No Feature Selection | RFE | PCA | No Feature Selection | RFE | PCA |
| C5.0 | 0.65 | 0.58 | 0.65 | 0.71 | 0.65 | 0.63 |
| glmnet | 0.64 | 0.66 | 0.65 | 0.66 | 0.69 | 0.68 |
| knn | 0.58 | 0.57 | 0.59 | 0.59 | 0.58 | 0.56 |
| nnet | 0.63 | 0.57 | 0.60 | 0.68 | 0.67 | 0.64 |
| ranger | 0.68 | 0.63 | 0.70 | 0.69 | 0.63 | 0.72 |
| rf | 0.68 | 0.65 | 0.67 | 0.7 | 0.64 | 0.70 |
| svmRadial | 0.65 | 0.58 | 0.66 | 0.65 | 0.62 | 0.67 |
| xgboost | 0.7 | 0.62 | 0.67 | 0.72 | 0.67 | 0.71 |
| Pair | AUC |
|---|---|
| ccRCC/AML | 0.77 |
| ccRCC/chromophobe | 0.71 |
| ccRCC/oncocytoma | 0.67 |
| ccRCC/papillary | 0.67 |
| papillary/AML | 0.75 |
| papillary/chromophobe | 0.62 |
| papillary/oncocytoma | 0.74 |
| oncocytoma/AML | 0.55 |
| oncocytoma/chromophobe | 0.45 |
| AML/chromophobe | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlig, J.; Leha, A.; Delonge, L.M.; Haack, A.-M.; Shuch, B.; Kim, H.S.; Bremmer, F.; Trojan, L.; Lotz, J.; Uhlig, A. Radiomic Features and Machine Learning for the Discrimination of Renal Tumor Histological Subtypes: A Pragmatic Study Using Clinical-Routine Computed Tomography. Cancers 2020, 12, 3010. https://doi.org/10.3390/cancers12103010
Uhlig J, Leha A, Delonge LM, Haack A-M, Shuch B, Kim HS, Bremmer F, Trojan L, Lotz J, Uhlig A. Radiomic Features and Machine Learning for the Discrimination of Renal Tumor Histological Subtypes: A Pragmatic Study Using Clinical-Routine Computed Tomography. Cancers. 2020; 12(10):3010. https://doi.org/10.3390/cancers12103010
Chicago/Turabian StyleUhlig, Johannes, Andreas Leha, Laura M. Delonge, Anna-Maria Haack, Brian Shuch, Hyun S. Kim, Felix Bremmer, Lutz Trojan, Joachim Lotz, and Annemarie Uhlig. 2020. "Radiomic Features and Machine Learning for the Discrimination of Renal Tumor Histological Subtypes: A Pragmatic Study Using Clinical-Routine Computed Tomography" Cancers 12, no. 10: 3010. https://doi.org/10.3390/cancers12103010
APA StyleUhlig, J., Leha, A., Delonge, L. M., Haack, A.-M., Shuch, B., Kim, H. S., Bremmer, F., Trojan, L., Lotz, J., & Uhlig, A. (2020). Radiomic Features and Machine Learning for the Discrimination of Renal Tumor Histological Subtypes: A Pragmatic Study Using Clinical-Routine Computed Tomography. Cancers, 12(10), 3010. https://doi.org/10.3390/cancers12103010





