Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Caryophyllane Sesquiterpenes
2.1. Natural Occurrence
2.2. Chemical Features
2.3. General Pharmacological Activities
2.4. Safety Profile
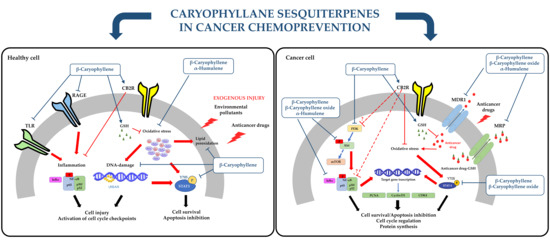
3. Caryophyllane Sesquiterpenes in Cancer Chemoprevention
3.1. Blocking/Protective Properties
3.1.1. Antimutagenicity and Genoprotection
3.1.2. Cytoprotection against Anticancer Drug Toxicity
3.2. Suppressing Properties
3.2.1. Antiproliferative Activity
3.2.2. In Vivo Anticancer Activity
3.2.3. Modulation of Pro-Apoptotic Intracellular Signalings in Cancer Cells
3.3. Chemosensitizing Properties
3.3.1. Potentiation of Anticancer Drug Activity
3.3.2. Inhibition of ATP-Binding Cassette (ABC) Transporters
4. Open Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. Mol. Mech. Mutagen. 2005, 591, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G. Current paradigms of cancer chemoprevention. Turk. J. Boil. 2014, 38, 839–847. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Yang, C.-C.; Shyur, L.-F. Phytomedicine—Modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol. Res. 2016, 114, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Kong, A.-N.T. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer Chemoprevention by Natural Products: How Far Have We Come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- William, W.N.; Heymach, J.V.; Kim, E.S.; Lippman, S.M. Molecular targets for cancer chemoprevention. Nat. Rev. Drug Discov. 2009, 8, 213–225. [Google Scholar] [CrossRef]
- Marin, J.J.; Lozano, E.; Herraez, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Briz, O.; Serrano, M.A.; Efferth, T.; Macias, R. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1444–1453. [Google Scholar] [CrossRef]
- Marin, J.J.; Briz, O.; Herraez, E.; Lozano, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Osorio-Padilla, L.M.; Santos-Llamas, A.I.; Serrano, M.A.; et al. Molecular bases of the poor response of liver cancer to chemotherapy. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 182–192. [Google Scholar] [CrossRef]
- Patterson, S.L.; Maresso, K.C.; Hawk, E. Cancer Chemoprevention: Successes and Failures. Clin. Chem. 2013, 59, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetology 2017, 60, 1639–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, B.K.; Umar, A.; Richmond, E. Introduction: Cancer chemoprevention and its context. Semin. Oncol. 2015, 43, 19–21. [Google Scholar] [CrossRef] [Green Version]
- Ball, S.; Arevalo, M.; Juarez, E.; Payne, J.D.; Jones, C. Breast cancer chemoprevention: An update on current practice and opportunities for primary care physicians. Prev. Med. 2019, 129, 105834. [Google Scholar] [CrossRef]
- Mocellin, S.; Pilati, P.; Briarava, M.; Nitti, N. Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials. J. Natl. Cancer Inst. 2015, 108, 318. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.; Nichols, H.B.; House, M.; Sandler, D.P. Risk versus Benefit of Chemoprevention among Raloxifene and Tamoxifen Users with a Family History of Breast Cancer. Cancer Prev. Res. 2019, 12, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Azzouni, F.; Mohler, J. Role of 5α-Reductase Inhibitors in Prostate Cancer Prevention and Treatment. Urology 2012, 79, 1197–1205. [Google Scholar] [CrossRef]
- Wang, L.; Lei, Y.; Gao, Y.; Cui, D.; Tang, Q.; Li, R.; Wang, D.; Chen, Y.; Zhang, B.; Wang, H. Association of finasteride with prostate cancer. Medicine 2020, 99, e19486. [Google Scholar] [CrossRef]
- De, A.; Kuppusamy, G. Metformin in breast cancer: Preclinical and clinical evidence. Curr. Probl. Cancer 2020, 44, 100488. [Google Scholar] [CrossRef]
- Zell, J.A.; McLaren, C.E.; Morgan, T.R.; Lawson, M.J.; Rezk, S.; Albers, C.G.; Chen, W.-P.; Carmichael, J.C.; Chung, J.; Richmond, E.; et al. A Phase IIa Trial of Metformin for Colorectal Cancer Risk Reduction among Individuals with History of Colorectal Adenomas and Elevated Body Mass Index. Cancer Prev. Res. 2019, 13, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Sato, Y.; Kiyokawa, T.; Koshizaka, M.; Hanaoka, H.; Shozu, M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann. Oncol. 2016, 27, 262–266. [Google Scholar] [CrossRef]
- Wan, G.; Yu, X.; Chen, P.; Wang, X.; Pan, D.; Wang, X.; Li, L.; Cai, X.; Cao, F. Metformin therapy associated with survival benefit in lung cancer patients with diabetes. Oncotarget 2016, 7, 35437–35445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, A.D.; Thakker, D.; Vyas, A.; Salkini, M.; Madhavan, S.; Sambamoorthi, U. Impact of metformin on clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2015, 18, 110–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Li, Q.; Wang, J.; Liu, M.; Wang, Y.; Chen, Z.; Ye, Y.; Guan, Q.; Zhou, Y. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients. Medicine 2019, 98, e16234. [Google Scholar] [CrossRef]
- Wang, J.; Cho, N.L.; Zauber, A.G.; Hsu, M.; Dawson, D.; Srivastava, A.; Mitchell-Richards, K.A.; Markowitz, S.D.; Bertagnolli, M.M. Chemopreventive Efficacy of the Cyclooxygenase-2 (Cox-2) Inhibitor, Celecoxib, Is Predicted by Adenoma Expression of Cox-2 and 15-PGDH. Cancer Epidemiol. Biomark. Prev. 2018, 27, 728–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L.; Christie, D.; Arora, D.; Anoopkumar-Dukie, S. Cyclooxygenase-2 inhibitors delay relapse and reduce Prostate Specific Antigen (PSA) velocity in patients treated with radiotherapy for nonmetastatic prostate cancer: A pilot study. Prostate Int. 2020, 8, 34–40. [Google Scholar] [CrossRef]
- Friis, S.; Riis, A.H.; Erichsen, R.; Baron, J.A.; Sørensen, H.T. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer Risk. Ann. Intern. Med. 2015, 163, 347–355. [Google Scholar] [CrossRef]
- Gaist, D.; A García-Rodríguez, L.; Sørensen, H.T.; Hallas, J.; Friis, S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: A case–control study. Br. J. Cancer 2013, 108, 1189–1194. [Google Scholar] [CrossRef] [Green Version]
- Murakami, R.; Chen, C.; Lyu, S.-Y.; Lin, C.-E.; Tzeng, P.-C.; Wang, T.-F.; Chang, J.-C.; Shieh, Y.-H.; Chen, I.-F.; Huang, S.K.; et al. Lovastatin lowers the risk of breast cancer: A population-based study using logistic regression with a random effects model. SpringerPlus 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzerro, P.; Proto, M.C.; Gangemi, G.; Malfitano, A.M.; Ciaglia, E.; Pisanti, S.; Santoro, A.; Laezza, C.; Bifulco, M. Pharmacological Actions of Statins: A Critical Appraisal in the Management of Cancer. Pharmacol. Rev. 2011, 64, 102–146. [Google Scholar] [CrossRef] [PubMed]
- Yaoyao, Z.; Fu, S.-W. GW27-e0113 Systematic review with network meta-analysis: Statins and risk of hepatocellular carcinoma. J. Am. Coll. Cardiol. 2016, 68, C128. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Alfaras, I.; Planas, J.M. Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol. Res. 2012, 65, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.E.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Garzotto, M.; Davis, E.W.; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2019, 72, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Mokbel, K. Chemoprevention of Breast Cancer with Vitamins and Micronutrients: A Concise Review. In Vivo 2019, 33, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and Risk of Prostate Cancer. Medicine 2015, 94, e1260. [Google Scholar] [CrossRef]
- Meyskens, F.L.; Curt, G.A.; Brenner, D.E.; Gordon, G.; Herberman, R.B.; Finn, O.; Kelloff, G.J.; Khleif, S.N.; Sigman, C.C.; Szabo, E.; et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: Moving what we have learned into the clinic. Cancer Prev. Res. 2011, 4, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Shoemaker, R.H.; Suen, C.S.; Holmes, C.A.; Fay, J.R.; Steele, V.E. The National Cancer Institute’s PREVENT Cancer Preclinical Drug Development Program: Overview, current projects, animal models, agent development strategies, and molecular targets. Semin. Oncol. 2015, 43, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Wink, M.; Gershenzon, J.; A Roberts, J.; Evan, D.; McManus, M.T.; Rose, J.K.C. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. Annu. Plant Rev. Online 2018, 202, 258–303. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Nicoletti, M.; Baldan, V.; Dall’Acqua, S. New Drugs from Old Natural Compounds: Scarcely Investigated Sesquiterpenes as New Possible Therapeutic Agents. Curr. Med. Chem. 2018, 25, 1241–1258. [Google Scholar] [CrossRef]
- Quintana, J. Recent Advances on Cytotoxic Sesquiterpene Lactones. Curr. Pharm. Des. 2019, 24, 4355–4361. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Chuang, L.-F.; Fan, T.-Y.; Li, J.-J.; Sung, P.-J. Kobusone: Occurrence of a norsesquiterpenoid in the gorgonian coral Rumphella antipathies (Gorgoniidae). Biochem. Syst. Ecol. 2007, 35, 470–471. [Google Scholar] [CrossRef]
- Chuang, L.F.; Fan, T.Y.; Li, J.J.; Kuo, J.; Fang, L.S.; Wang, W.H.; Sung, P.-J. Isokobusone, a caryophyllane-type norsesquiterpenoid from the gorgonian coral Rumphella antipathies (Gorgoniidae). Platax 2007, 2007, 61–67. [Google Scholar]
- Ahmed, A.F.; Su, J.H.; Shiue, R.T.; Pan, X.J.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. New beta-caryophyllene-derived terpenoids from the soft coral Sinularia nanolobata. J. Nat. Prod. 2004, 67, 592–597. [Google Scholar] [CrossRef]
- Sung, P.J.; Chuang, L.F.; Kuo, J.; Fan, T.Y.; Hu, W.P. Rumphellatin A, the first chloride-containing caryophyllane-typenorsesquiterpenoid from Rumphella antipathies. Tetrahedron. Lett. 2007, 48, 3987–3989. [Google Scholar] [CrossRef]
- Sung, P.J.; Chuang, L.F.; Hu, W.P. Rumphellatins B and C, two new caryophyllane-type hemiketal norsesquiterpenoids from the Formosangorgonian coral Rumphella antipathies. Bull. Chem. Soc. Jpn. 2007, 80, 2395–2399. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, Y.-D.; Hwang, T.-L.; Chuang, L.-F.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellatin D, a Novel Chlorinated Caryophyllane from Gorgonian Coral Rumphella antipathies. Chem. Lett. 2008, 37, 1244–1245. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, Y.-D.; Hwang, T.-L.; Chuang, L.-F.; Chung, H.-M.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. ChemInform Abstract: Rumphellolide I, a Novel Caryophyllane-Related Tetrahydropyran Norsesquiterpenoid from Gorgonian Coral Rumphella antipathies. Chem. Lett. 2009, 40, 282–283. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Guo, Y.-W. A new norsesquiterpene from Hainan soft coral Sinularia sp. Nat. Prod. Res. 2008, 22, 1359–1364. [Google Scholar] [CrossRef]
- Wang, G.-H.; Ahmed, A.; Sheu, J.-H.; Duh, C.-Y.; Shen, Y.-C.; Wang, L.-T. Suberosols A-D, four new sesquiterpenes with beta-caryophyllene skeletons from a Taiwanese gorgonian coral Subergorgia suberosa. J. Nat. Prod. 2002, 65, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Li, D.-Y.; Li, Y.-C.; Hua, H.-M.; Ma, E.-L.; Li, Z.-L. Caryophyllene Sesquiterpenes from the Marine-Derived FungusAscotrichasp. ZJ-M-5 by the One Strain–Many Compounds Strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.-W.; Cui, C.-B.; Liu, X.-Z.; Che, Y. Cytosporinols A-C, new caryophyllene sesquiterpenoids from Cytospora sp. Nat. Prod. Bioprospect. 2012, 2, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Smetanina, O.F.; Kuznetsova, T.A.; Gerasimenko, A.V.; Kalinovsky, A.I.; Pivkin, M.V.; Dmitrenok, P.C.; Elyakov, G.B. Metabolites of the marine fungus Humicola fuscoatra KMM 4629. Russ. Chem. Bull. 2004, 53, 2643–2646. [Google Scholar] [CrossRef]
- Deyrup, S.T.; Swenson, D.C.; Gloer, J.B.; Wicklow, D.T. Caryophyllene Sesquiterpenoids from a Fungicolous Isolate of Pestalotiopsis disseminata. J. Nat. Prod. 2006, 69, 608–611. [Google Scholar] [CrossRef]
- Pulici, M.; Sugawara, F.; Koshino, H.; Uzawa, A.J.; Yoshida, S.; Lobkovsky, E.; Clardy, J. Pestalotiopsins A and B: New Caryophyllenes from an Endophytic Fungus of Taxus brevifolia. J. Org. Chem. 1996, 61, 2122–2124. [Google Scholar] [CrossRef]
- Xiao, J.; Lin, L.; Hu, J.; Jiao, F.; Duan, D.; Zhang, Q.; Tang, H.-Y.; Gao, J.; Wang, L.; Wang, X. Highly oxygenated caryophyllene-type and drimane-type sesquiterpenes from Pestalotiopsis adusta, an endophytic fungus of Sinopodophyllum hexandrum. RSC Adv. 2017, 7, 29071–29079. [Google Scholar] [CrossRef] [Green Version]
- Magnan, R.F.; Rodrigues-Fo, E.; Daolio, C.; Ferreira, A.G.; De Souza, A.Q.L. Three Highly Oxygenated Caryophyllene Sesquiterpenes from Pestalotiopsis sp., a Fungus Isolated from Bark of Pinus taeda. Z. Nat. C J. Biosci. 2003, 58, 319–324. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Punctaporonins H–M: Caryophyllene-Type Sesquiterpenoids from the Sponge-Associated Fungus Hansfordia sinuosae. Mar. Drugs 2014, 12, 3904–3916. [Google Scholar] [CrossRef] [Green Version]
- Poyser, J.P.; Edwards, R.L.; Anderson, J.R.; Hursthouse, M.B.; Walker, N.P.C.; Sheldrick, G.M.; Whalley, A.J.S. Punctatins A,D,E, and F (antibiotics M95464, M167906, M171950, and M189122), isomeric allylic alcohols from the fungus Poronia punctata: X-ray crystal structures of D and of E acetonide. J. Antibiot. 1986, 39, 167–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-W.; Chan, T.-M.; Terracciano, J.; Boehm, E.; Patel, R.; Chen, G.; Loebenberg, D.; Patel, M.; Gullo, V.; Pramanik, B.; et al. Caryophyllenes from a Fungal Culture of Chrysosporiumpilosum. J. Nat. Prod. 2009, 72, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Kingston, E.; Jeffery, J.C.; Moss, M.O.; Murray, M.; Simpson, T.J.; Sutherland, A. Walleminol and walleminone, novel caryophyllenes from the toxigenic fungus Wallemia sebi. Tetrahedron Lett. 1999, 40, 133–136. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Lindsey, A.S. 663. Sesquiterpenoids. Part I. Evidence for a nine-membered ring in caryophyllene. J. Chem. Soc. 1951, 2988–2991. [Google Scholar] [CrossRef]
- Helmig, D.; Ortega, J.; Duhl, T.; Tanner, D.; Guenther, A.; Harley, P.; Wiedinmyer, C.; Milford, J.; Sakulyanontvittaya, T. Sesquiterpene Emissions from Pine Trees—Identifications, Emission Rates and Flux Estimates for the Contiguous United States. Environ. Sci. Technol. 2007, 41, 1545–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Syamsundar, K.V. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour Fragr. J. 2004, 20, 51–53. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Rodriguez, D.M.; Dao, L.; Patterson, R. Headspace Volatiles Dria baicalensis Georgi Flowers. J. Essent. Oil Bear. Plants 2009, 12, 435–442. [Google Scholar] [CrossRef]
- Gramosa, N.V.; Silveira, E.R. Volatile Constituents of Copaifera langsdorffii from the Brazilian Northeast. J. Essent. Oil Res. 2005, 17, 130–132. [Google Scholar] [CrossRef]
- Van Hac, L.; Luong, N.X.; Dung, N.X.; Klinkby, N.; A Leclercq, P. Volatile Constituents of the Essential Oil of Orthodon dianthera Maxim.(Syn. Mosla dianthera Maxim.) from Vietnam. J. Essent. Oil Res. 2001, 13, 18–20. [Google Scholar] [CrossRef]
- Senatore, F.; Nelly, A.; Piozzi, F. Composition of the Essential Oil of Nepeta curviflora Boiss. (Lamiaceae) from Lebanon. J. Essent. Oil Res. 2005, 17, 268–270. [Google Scholar] [CrossRef]
- Bhatt, R.; Padalia, R.C.; Pande, C. Chemical Composition of the Essential Oil of Colquhounia coccinea Wall. J. Essent. Oil Res. 2009, 21, 74–75. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A. Essential Oil Composition of Four Major Cultivars of Black Pepper (Piper nigrum L.) III. J. Essent. Oil Res. 2003, 15, 155–157. [Google Scholar] [CrossRef]
- Son, L.C.; Dai, D.N.; Thai, T.H.; Huyen, D.D.; Thang, T.D.; Ogunwande, I.A. The leaf essential oils of four Vietnamese species of Cinnamomum (Lauraceae). J. Essent. Oil Res. 2013, 25, 267–271. [Google Scholar] [CrossRef]
- Pino, J.A.; Aguero, J.; Fuentes, V. Essential Oil of Salvia officinalis L. ssp. altissima Grown in Cuba. J. Essent. Oil Res. 2002, 14, 373–374. [Google Scholar] [CrossRef]
- Pino, J.A.; Fernandes, P.; Marbot, R.; Rosado, A.; Fontinha, S.S. Leaf Oils of Helichrysum melaleucum Rchb. ex Holl., Oenanthe divaricata (R. Br.) Mabb. and Persea indica (L.) Spreng. from Madeira. J. Essent. Oil Res. 2004, 16, 487–489. [Google Scholar] [CrossRef]
- Boyom, F.F.; Zollo, P.H.A.; Agnaniet, H.; Menut, C.; Bessière, J.M. Aromatic Plants of Tropical Central Africa. XL. Essential Oils from Uvariodendron calophyllum R.E. Fries Growing in Cameroon. J. Essent. Oil Res. 2005, 17, 128–129. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.; Di Sotto, A.; Leopizzi, M.; Božović, M.; Di Maio, V.; Gullì, M.; Vedova, P.D.; Ammendola, S.; D’Abusco, A.S. Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals. Nutrients 2020, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Bailac, P.N.; Dellacasa, A.D.; Bernasconi, H.O.; Ponzi, M.I.; Firpo, N.H. Essential Oil of Female Plants of Baccharis coridifolia De Candole. J. Essent. Oil Res. 2001, 13, 23–24. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Akalin, E.; Ozhatay, N. Composition of the Essential Oil of Cachrys alpine Bieb. J. Essent. Oil Res. 2004, 16, 167–168. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I.; Craven, L.A.; Lepschi, B.J. The Leaf Essential Oils of the Australian Members of the Genus Callistemon (Myrtaceae). J. Essent. Oil Res. 1998, 10, 595–606. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef] [Green Version]
- Pino, J.A.; Bello, A.; Urquiola, A.; Aguero, J. Leaf Oil of Eugenia rocana Britt. et Wils. from Cuba. J. Essent. Oil Res. 2002, 14, 412–413. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Chinou, I.B.; Harvala, C. Composition and Antibacterial Activity of the Essential Oils of Two Helichrysum stoechas Varieties Growing in the Island of Crete. J. Essent. Oil Res. 2002, 14, 459–461. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Tajbakhsh, M.; Gholami, F.A.; Hosaseinzadeh, M.; Dastoorani, P.; Norouzi, M.; Dabiri, H.A. Composition of the Essential oils of Hippomarathrum microcarpum (M. Bieb.) B. Fedtsch. and Physospermum cornubiense (L.) DC. Iran. J. Essent. Oil Res. 2007, 19, 567–568. [Google Scholar] [CrossRef]
- Katsiotis, S.T.; Langezaal, C.R.; Scheffer, J.J.C. Composition of the essential oils from leaves of various Humulus lupulus L. cultivars. Flavour Fragr. J. 1990, 5, 97–100. [Google Scholar] [CrossRef]
- Cakir, A.; Kordali, S.; Zengin, H.; Izumi, S.; Hirata, T. Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour Fragr. J. 2004, 19, 62–68. [Google Scholar] [CrossRef]
- Rout, P.K.; Naik, S.; Rao, Y.R. Composition of Absolutes of Jasminum sambac L. Flowers Fractionated with Liquid CO2 and Methanol and Comparison with Liquid CO2 Extract. J. Essent. Oil Res. 2010, 22, 398–406. [Google Scholar] [CrossRef]
- Hernandez, T.; Canales, M.; Avila, J.; Bocanegra-García, V.; Martinez, A.; Caballero, J.; De Vivar, A.R.; Lira, R. Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf. (Verbenaceae). J. Ethnopharmacol. 2005, 96, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Evandri, M.; Battinelli, L.; Daniele, C.; Mastrangelo, S.; Bolle, P.; Mazzanti, G. The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem. Toxicol. 2005, 43, 1381–1387. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, T.A.; Horhat, F.G. Chemical Composition and Antimicrobial Activity of Essential Oils of Lavender (Lavandula angustifolia) and Lavandin (Lavandula x intermedia) Grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Naz, T.; Packer, J.; Yin, P.; Brophy, J.J.; Wohlmuth, H.; Renshaw, D.E.; Smith, J.; Yaegl Community Elders; Vemulpad, S.R.; Jamie, J.F. Bioactivity and chemical characterisation of Lophostemon suaveolens—An endemic Australian Aboriginal traditional medicinal plant. Nat. Prod. Res. 2015, 30, 693–696. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. Leaf Essential Oils of Lycopus australis (Lamiaceae), the Australian Gipsywort. J. Essent. Oil Res. 2005, 17, 133–134. [Google Scholar] [CrossRef]
- Limberger, R.P.; Simões-Pires, C.A.; Sobral, M.; Henriques, A.T. Essential Oils ofMarliereaSpecies. J. Essent. Oil Res. 2004, 16, 479–482. [Google Scholar] [CrossRef]
- Nik, Z.B.; Mirza, M. Composition of the Essential Oil of Marrubium astracanicum Jacq. J. Essent. Oil Res. 2003, 15, 342–343. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ranjbaran, J. Chemical composition and antimicrobial activity of Micromeria hedgei Rech. f. oil from Iran. Nat. Prod. Res. 2016, 31, 210–213. [Google Scholar] [CrossRef]
- Sharma, P.; Shah, G.; Sharma, R.; Dhyani, P. Chemical composition and antibacterial activity of essential oil of Nepeta graciliflora Benth. (Lamiaceae). Nat. Prod. Res. 2015, 30, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Kumar, A.; Mishra, P.; Kuppusamy, B.; Padalia, R.C.; Sundaresan, V. Essential oil composition of four Ocimum spp. from the Peninsular India. J. Essent. Oil Res. 2015, 28, 35–41. [Google Scholar] [CrossRef]
- Mockute, D.; Bernotiene, G.; Judzentiene, A. The essential oil of Origanum vulgare L. ssp. vulgare growing wild in Vilnius district (Lithuania). Phytochemistry 2001, 57, 65–69. [Google Scholar] [CrossRef]
- Kula, J.; Majda, T.; Stoyanova, A.; Georgiev, E. Chemical Composition ofOriganum vulgareL. essential Oil from Bulgaria. J. Essent. Oil Bear. Plants 2007, 10, 215–220. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Ozek, T.; Kirimer, N.; Baser, K.H.C.; Bedir, E.; Khan, I.A.; Wedge, D.E. Gas chromatographic–mass spectrometric analysis of essential oils from Pimpinella species gathered from Central and Northern Turkey. J. Chromatogr. A 2006, 1117, 194–205. [Google Scholar] [CrossRef]
- Pino, J.A.; Bello, A.; Urquiola, A.; Aguero, J. Chemical Composition of the Leaf Oil of Plinia dermatodes Urb. from Cuba. J. Essent. Oil Res. 2003, 15, 23–24. [Google Scholar] [CrossRef]
- Pino, J.A.; Bello, A.; Urquiola, A.; Aguero, J. Leaf Oil of Psidium salutare (HBK) Berg. from Cuba. J. Essent. Oil Res. 2003, 15, 19–20. [Google Scholar] [CrossRef]
- Velickovic, D.; Ristić, M.; Velickovic, A. Chemical Composition of the Essential Oils Obtained from the Flower, Leaf and Stem of Salvia aethiopis L. and Salvia glutinosa L. Originating from the Southeast Region of Serbia. J. Essent. Oil Res. 2003, 15, 346–349. [Google Scholar] [CrossRef]
- Mirza, M.; Baher, Z.F. Essential Oil of Stachys lanata Jacq from Iran. J. Essent. Oil Res. 2003, 15, 46–47. [Google Scholar] [CrossRef]
- Raj, G.; George, V.; Pradeep, N.S.; Sethuraman, M.G. Chemical Composition and Antimicrobial Activity of the Leaf Oil from Syzygium gardneri Thw. J. Essent. Oil Res. 2008, 20, 72–74. [Google Scholar] [CrossRef]
- Stojanova, A.; Primova, T.; Anastassov, C. Effect of Mineral Fertilization on the Essential Oil Composition of Tagetes patula L. from Bulgaria. J. Essent. Oil Res. 2000, 12, 609–612. [Google Scholar] [CrossRef]
- Chitsazan, M.H.; Bina, E.; Asgarpanah, J. Essential oil composition of the endemic species Tephrosia persica Boiss. J. Essent. Oil Res. 2013, 26, 141–145. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R. Composition of the Essential Oil of Teucrium orientate L. ssp. orientate from Iran. J. Essent. Oil Res. 2003, 15, 118–119. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Ahmad, V.U. 13C-NMR of Natural Products; Springer: Berlin/Heidelberg, Germany, 1992; pp. 583–584. [Google Scholar]
- Collado, I.G.; Hanson, J.R.; Macías-Sánchez, A.J. Recent advances in the chemistry of caryophyllene. Nat. Prod. Rep. 1998, 15, 187. [Google Scholar] [CrossRef]
- Rogers, D.; Mazhar-ul-Haque. The Molecular and Crystal Structure of Caryophyllene Chlorohydrin. Proc. Chem. Soc. 1963, 371–372. [Google Scholar] [CrossRef]
- Shirahama, H.; Osawa, E.; Matsumoto, T. Conformational Studies on Humulene by Means of Empirical Force Field Calculations. Role of Stable Conformers of Humulene in Biosynthetic and Chemical Reactions. J. Am. Chem. Soc. 1980, 102, 3208–3213. [Google Scholar] [CrossRef]
- Kollner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant. Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talapatra, S.K.; Talapatra, B. Chemistry of Plant. Natural Products; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Sköld, M.; Karlberg, A.-T.; Matura, M.; Börje, A. The fragrance chemical β-caryophyllene—Air oxidation and skin sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ishida, T.; Toyota, M.; Takemoto, T. Terpenoid biotransformation in mammals IV Biotransformation of (+)-longifolene, (-)-caryophyllene, (-)-caryophyllene oxide, (-)-cyclocolorenone, (+)-nootkatone, (-)-elemol, (-)-abietic acid and (+)-dehydroabietic acid in rabbits. Xenobiotica 1986, 16, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.; Fernandes, E.S.; Da Cunha, F.M.; Ferreira, J.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J. Ethnopharmacol. 2007, 110, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Lee, D.-U.; Kim, Y.S.; Kim, H.P. Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch. Pharmacal. Res. 2011, 34, 223–228. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; El-Alim, A.E.-A.F.; Galal, A.A.; El-Sayed, R.G.; El-Naseery, N.I. Anti-arthritic effect of β-caryophyllene and its ameliorative role on methotrexate and/or leflunomide-induced side effects in arthritic rats. Life Sci. 2019, 233, 116750. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomology 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Pieri, F.A.; Souza, M.C.D.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; Da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Veter Res. 2016, 12, 216. [Google Scholar] [CrossRef] [Green Version]
- Ames-Sibin, A.P.; Barizão, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Natali, M.R.M.; Bracht, A.; Comar, J.F. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Da Fonsêca, D.V.; Filho, C.D.S.M.B.; Lima, T.C.; De Almeida, R.N.; De Sousa, É.B.V. Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy. Biomology 2019, 9, 835. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; Da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric Cytoprotection of the Non-Steroidal Anti-Inflammatory Sesquiterpene, β-Caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Chang, H.J.; Lee, S.K.; Kim, H.J.; Hwang, J.K.; Chun, H.S. Amelioration of dextran sulphate sodium-induced colitis in mice by oral administration of b-caryophyllene, a sesquiterpene. Life Sci. 2007, 80, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a Potential Protective Agent against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24, 1929. [Google Scholar] [CrossRef] [Green Version]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Varga, Z.V.; Matyas, C.; Erdelyi, K.; Cinar, R.; Nieri, D.; Chicca, A.; Nemeth, B.T.; Paloczi, J.; Lajtos, T.; Corey, L.; et al. β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2017, 175, 320–334. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Segat, G.C.; Manjavachi, M.N.; Matias, D.O.; Passos, G.F.; Freitas, C.S.; Da Costa, R.; Calixto, J.B. Antiallodynic effect of β-caryophyllene on paclitaxel-induced peripheral neuropathy in mice. Neuropharmacology 2017, 125, 207–219. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; González-Trujano, M.E.; Narváez-González, F.; Pérez-Ortega, G.; Rivero-Cruz, F.; Aguilar, M.I. Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil. Molecules 2020, 25, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-Caryophyllene Suppresses Hypoxia-Induced Neuroinflammatory Responses by Inhibiting NF-κB Activation in Microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, Z.; Wang, B.; Guo, S. Trans-caryophyllene inhibits amyloid β (Aβ) oligomer-induced neuroinflammation in BV-2 microglial cells. Int. Immunopharmacol. 2017, 51, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. β-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free. Radic. Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Taee, H.; Azimullah, S.; Meeran, M.N.; Almheiri, M.K.A.; Al Jasmi, R.A.; Tariq, S.; Ab Khan, M.; Adeghate, E.; Ojha, S. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Chen, Y.; Yang, M. β-Caryophyllene inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells. Int. Immunopharmacol. 2020, 84, 106556. [Google Scholar] [CrossRef]
- Sain, S.; Naoghare, P.K.; Sivanesan, S.; Daiwile, A.; Krishnamurthi, K.; Arrigo, P.; Chakrabarti, T. Beta Caryophyllene and Caryophyllene Oxide, Isolated from Aegle Marmelos, as the Potent Anti-inflammatory Agents against Lymphoma and Neuroblastoma Cells. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Chaudhuri, S.; Kubo, Y.; Sanchez, Y.; Ogura, T.; Saito, T.; Ishikawa, H.; Haraguchi, H. Cytotoxic and Antioxidative Sesquiterpenoids from Heterotheca inuloides. Planta Med. 1996, 62, 427–430. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2012, 109, 394–401. [Google Scholar] [CrossRef]
- Amiel, E.; Ofir, R.; Dudai, N.; Soloway, E.; Rabinsky, T.; Rachmilevitch, S. β-Caryophyllene, a Compound Isolated from the Biblical Balm of Gilead (Commiphora gileadensis), Is a Selective Apoptosis Inducer for Tumor Cell Lines. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.-S.; Hong, J.Y.; Lee, J.-H.; Lee, H.-J.; Park, J.Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [Green Version]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandhiran, D.; Sankaranarayanan, C.; Murali, R.; Babukumar, S.; Vinothkumar, V. β-Caryophyllene promotes oxidative stress and apoptosis in KB cells through activation of mitochondrial-mediated pathway -An in-vitro and in-silico study. Arch. Physiol. Biochem. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, V.; Kotakonda, M.; Periyannan, V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020, 34, e22514. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.-S.; Chun, H.S.; Kwon, S.-H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet–induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [Green Version]
- Dahham, S.S.; Shah, A.M.; Majid, A. β- caryophyllene, a natural sesquiterpene isolated from agar wood inhibits growth and metastasis of human colorectal cancer by modulation of multiple targets in vitro and in vivo. J. Cancer Sci. Ther. 2014, 7, 10. [Google Scholar]
- Campos, M.I.D.C.; Vieira, W.D.A.; Campos, C.N.; Aarestrup, F.M.; Aarestrup, B.J.V. Atorvastatin and trans-caryophyllene for the prevention of leukopenia in an experimental chemotherapy model in Wistar rats. Mol. Clin. Oncol. 2015, 3, 825–828. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing Properties of β-Caryophyllene and β-Caryophyllene Oxide in Combination with Doxorubicin in Human Cancer Cells. Anticancer. Res. 2017, 37, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, S.; Abete, L.; Cocchiola, R.; Mazzanti, G.; Eufemi, M.; Di Sotto, A. Caryophyllane sesquiterpenes inhibit DNA-damage by tobacco smoke in bacterial and mammalian cells. Food Chem. Toxicol. 2018, 111, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in HepG2 Cells: An in Vitro and in Silico Study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef] [Green Version]
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gullì, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; De Paula, J.R.; De Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Kim, H.-B.; Lee, S.; Kim, M.-J.; Kim, K.-J.; Han, G.; Han, S.-Y.; Lee, E.-A.; Yoon, J.-H.; Kim, D.-O.; et al. Antidepressant-like effects of β-caryophyllene on restraint plus stress-induced depression. Behav. Brain Res. 2020, 380, 112439. [Google Scholar] [CrossRef]
- Leonhardt, V.; Leal-Cardoso, J.H.; Lahlou, S.; Albuquerque, A.A.; Porto, R.S.; Celedônio, N.R.; De Oliveira, A.C.; Farias-Pereira, R.; Silva, L.P.; Garcia-Teófilo, T.M.; et al. Antispasmodic effects of essential oil of Pterodon polygalaeflorus and its main constituent β-caryophyllene on rat isolated ileum. Fundam. Clin. Pharmacol. 2009, 24, 749–758. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Ambrož, M.; Boušová, I.; Skarka, A.; Skarkova, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. The Influence of Sesquiterpenes from Myrica rubra on the Antiproliferative and Pro-Oxidative Effects of Doxorubicin and Its Accumulation in Cancer Cells. Molecules 2015, 20, 15343–15358. [Google Scholar] [CrossRef] [Green Version]
- Pavithra, P.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef]
- Di Sotto, A.; Evandri, M.G.; Mazzanti, G. Antimutagenic and mutagenic activities of some terpenes in the bacterial reverse mutation assay. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 653, 130–133. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Mazzanti, G.; Di Sotto, A. Mutagenicity of cigarette butt waste in the bacterial reverse mutation assay: The protective effects of β-caryophyllene and β-caryophyllene oxide. Environ. Toxicol. 2015, 31, 1319–1328. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Inhibition by beta-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat. Res. 2010, 699, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jasso, D.; Álvarez-González, I.; Madrigal-Bujaidar, E. Clastogenicity of beta-caryophyllene in mouse. Biol. Pharm. Bull. 2009, 32, 520–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-González, I.; Madrigal-Bujaidar, E.; Castro-García, S. Antigenotoxic capacity of beta-caryophyllene in mouse, and evaluation of its antioxidant and GST induction activities. J. Toxicol. Sci. 2014, 39, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Hurtado, P.; González-Castañeda, R.E.; Beas-Zarate, C.; Flores-Soto, M.E.; Viveros-Paredes, J.M. β-Caryophyllene Reduces DNA Oxidation and the Overexpression of Glial Fibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus of d-Galactose-Induced Aged BALB/c Mice. J. Med. Food 2020, 23, 515–522. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef]
- Suijun, W.; Zhen, Y.; Ying, G.; Yanfang, W. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, V.S.; Kaur, G. Insulinotropic and antidiabetic effects of β-caryophyllene with l -arginine in type 2 diabetic rats. J. Food Biochem. 2020, 44, e13156. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Doleski, P.H.; Boligon, A.A.; Stefani, L.M.; Monteiro, S.G. Hypolipidemic effect of β-caryophyllene to treat hyperlipidemic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 390, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kamikubo, R.; Kai, K.; Tsuji-Naito, K.; Akagawa, M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. [Google Scholar] [CrossRef]
- Cho, H.-I.; Hong, J.-M.; Choi, J.-W.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef]
- Tan, J.W.; Israf, D.A.; Tham, C.L. Major Bioactive Compounds in Essential Oils Extracted from the Rhizomes of Zingiber zerumbet (L) Smith: A Mini-Review on the Anti-allergic and Immunomodulatory Properties. Front. Pharmacol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghelardini, C.; Galeotti, N.; Di Cesare, M.L.; Mazzanti, G.; Bartolini, A. Local anaesthetic activity of b-caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Di Sotto, A.; Accolla, M.L.; Castelli, F. Differential Scanning Calorimetry Study on the Interaction of β-Caryophyllene and β-Caryophyllene Oxide with Phospholipid Bilayers. Thermochim. Acta 2015, 600, 28–34. [Google Scholar] [CrossRef]
- Lucca, L.G.; De Matos, S.P.; Borille, B.T.; Dias, D.D.O.; Teixeira, H.F.; Veiga, V.F.; Limberger, R.P.; Koester, L.S. Determination of β-caryophyllene skin permeation/retention from crude copaiba oil (Copaifera multijuga Hayne) and respective oil-based nanoemulsion using a novel HS-GC/MS method. J. Pharm. Biomed. Anal. 2015, 104, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Kim, H.J.; Chun, H.S. Quantitative structure−activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci. 2007, 80, 835–841. [Google Scholar] [CrossRef]
- Chang, H.-J.; Kim, J.; Lee, J.-C.; Kim, W.-K.; Chun, H.S. Protective Effect of β-Caryophyllene, a Natural Bicyclic Sesquiterpene, Against Cerebral Ischemic Injury. J. Med. Food 2013, 16, 471–480. [Google Scholar] [CrossRef]
- Assis, L.; Straliotto, M.; Engel, D.; Hort, M.; Dutra, R.C.; De Bem, A. β-Caryophyllene protects the C6 glioma cells against glutamate-induced excitotoxicity through the Nrf2 pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef]
- Viveros-Paredes, J.M.; González-Castañeda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; López-Roa, R.I.; Vázquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of β-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s Disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Fontes, L.B.; Dias, D.D.S.; Aarestrup, B.J.; Aarestrup, F.M.; Filho, A.A.D.S.; Corrêa, J.O.A. β-Caryophyllene ameliorates the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Biomed. Pharmacother. 2017, 91, 257–264. [Google Scholar] [CrossRef]
- Zhang, Q.; An, R.; Tian, X.; Yang, M.; Li, M.; Lou, J.; Xu, L.; Dong, Z. β-Caryophyllene Pretreatment Alleviates Focal Cerebral Ischemia-Reperfusion Injury by Activating PI3K/Akt Signaling Pathway. Neurochem. Res. 2017, 42, 1459–1469. [Google Scholar] [CrossRef]
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.; Wakte, P.; Shinde, D. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Souza, A.B.; Martins, C.H.G.; Souza, M.G.M.; Furtado, N.A.J.C.; Heleno, V.C.G.; De Sousa, J.P.B.; Rocha, E.M.P.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.S.; et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytotherapy Res. 2011, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Michel, L.; Chaumont, J.-P.; Millet-Clerc, J. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathology 1999, 148, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, S.K.; Kim, K.-D.; Nam, D.; Chung, W.-S.; Jang, H.-J.; Lee, S.-G.; Shim, B.S.; Sethi, G.; Ahn, K. β-Caryophyllene oxide potentiates TNFα-induced apoptosis and inhibits invasion through down-modulation of NF-κB-regulated gene products. Apoptosis 2013, 19, 708–718. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.-H.; Sethi, G.; Ahn, K.S. β-caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2013, 53, 793–806. [Google Scholar] [CrossRef]
- Jun, N.J.; Mosaddik, A.; Moon, J.Y.; Jang, K.C.; Lee, D.S.; Ahn, K.S.; Cho, S.K. Cytotoxic activity of β-caryophyllene oxide isolated from Jeju guava (Psidium cattleianum Sabine) leaf. Rec. Nat. Prod. 2011, 5, 242–246. [Google Scholar]
- Ryu, N.H.; Park, K.-R.; Kim, S.-H.; Yun, H.-M.; Nam, D.; Lee, S.-G.; Jang, H.-J.; Ahn, K.S.; Shim, B.S.; Choi, S.-H.; et al. A Hexane Fraction of Guava Leaves (Psidium guajava L.) Induces Anticancer Activity by Suppressing AKT/Mammalian Target of Rapamycin/Ribosomal p70 S6 Kinase in Human Prostate Cancer Cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.-I.; Rhee, K.-J.; Eom, Y.-B. Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can. J. Microbiol. 2020, 66, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Wanas, A.S.; Radwan, M.M.; Mehmedic, Z.; Jacob, M.; Khan, I.A.; Elsohly, M.A. Antifungal activity of the volatiles of high potency Cannabis sativa L. against Cryptococcus neoformans. Rec. Nat. Prod. 2016, 10, 214–220. [Google Scholar]
- Govindarajan, M.; Benelli, G. α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene α-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [Green Version]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.-C. Antitumor Activity of Balsam Fir Oil: Production of Reactive Oxygen Species Induced by α-Humulene as Possible Mechanism of Action. Planta Med. 2003, 69, 402–407. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008, 41, 1002–1012. [Google Scholar] [CrossRef]
- Legault, J.; Côté, P.A.; Ouellet, S.; Simard, S.; Pichette, A. Iso-caryophyllene cytotoxicity induced by lipid peroxidation and membrane permeabilization in L-929 cells. J. App. Pharm. Sci. 2013, 3, 25–31. [Google Scholar]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Chicca, A.; Caprioglio, D.; Minassi, A.; Petrucci, V.; Appendino, G.; Taglialatela-Scafati, O.; Gertsch, J. Functionalization of β-Caryophyllene Generates Novel Polypharmacology in the Endocannabinoid System. ACS Chem. Biol. 2014, 9, 1499–1507. [Google Scholar] [CrossRef]
- Rauter, A.P.; Branco, I.; Bermejo, J.; González, A.G.; García-Grávalos, M.D.; Feliciano, A.S. Bioactive humulene derivatives from Asteriscus vogelii. Phytochemistry 2001, 56, 167–171. [Google Scholar] [CrossRef]
- Haque, A.; Jantan, I.; Arshad, L.; Bukhari, S.N.A. Exploring the immunomodulatory and anticancer properties of zerumbone. Food Funct. 2017, 8, 3410–3431. [Google Scholar] [CrossRef]
- European Food Safety Autority (EFSA). Flavouring group evaluation 78 (FGE.78)—Consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromatic hydrocarbons evaluated by EFSA in FGE.25-scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food (AFC). EFSA J. 2009, 931, 1–59. [Google Scholar]
- European Food Safety Autority (EFSA). Scientific Opinion on Flavouring Group Evaluation 82, Revision 1 (FGE.82Rev1): Consideration of Epoxides evaluated by the JECFA (65th meeting). EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA J. 2014, 12, 3708. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Autority (EFSA). Scientific Opinion on Flavouring Group Evaluation 78, Revision 2 (FGE.78Rev2): Consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic hydrocarbons evaluated by EFSA in FGE.25Rev3. EFSA J. 2015, 13, 4067. [Google Scholar]
- European Food Safety Autority (EFSA). Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of eight compounds belonging to chemical group 31 (aliphatic and aromatic hydrocarbons) when used as flavourings for all animal species and categories. EFSA J. 2016, 14, 4339. [Google Scholar]
- Opdyke, D.L. Monographs on fragrance raw materials: Caryophyllene. Food Cosmet. Toxicol. 1973, 11, 1059–1060. [Google Scholar] [CrossRef]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological Evaluation of β-Caryophyllene Oil. Int. J. Toxicol. 2016, 35, 558–567. [Google Scholar] [CrossRef]
- Oliveira, G.L.D.S.; Machado, K.C.; Machado, K.C.; Silva, A.P.D.S.C.D.; Feitosa, C.M.; Almeida, F.R.D.C. Non-clinical toxicity of β-caryophyllene, a dietary cannabinoid: Absence of adverse effects in female Swiss mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.; Diop, M.M.; Harman, C.L.; Hayashi, S.-M.; Krammer, G.; et al. Dietary administration of β-caryophyllene and its epoxide to Sprague-Dawley rats for 90 days. Food Chem. Toxicol. 2020, 135, 110876. [Google Scholar] [CrossRef]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Castelli, F.; Sarpietro, M.G.; Mazzanti, G. Genotoxicity assessment of β-caryophyllene oxide. Regul. Toxicol. Pharmacol. 2013, 66, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Hemann, M.T. DNA Damage-Mediated Induction of a Chemoresistant Niche. Cell 2010, 143, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Barry, S.P.; Townsend, P.A.; Knight, R.A.; Scarabelli, T.M.; Latchman, D.S.; Stephanou, A. STAT3 modulates the DNA damage response pathway. Int. J. Exp. Pathol. 2010, 91, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Zhuang, G.; Cao, Y.; Du, P.; Kim, H.-J.; Settleman, J. Drug Resistance via Feedback Activation of Stat3 in Oncogene-Addicted Cancer Cells. Cancer Cell 2014, 26, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant natural modulators in breast cancer prevention: Status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef]
- De Flora, S. Mechanisms of inhibitors of mutagenesis and carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 151–158. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Myslivečková, Z.; Szotáková, B.; Špičáková, A.; Lněničková, K.; Ambrož, M.; Kubicek, V.; Krasulová, K.; Anzenbacher, P.; Skálová, L. The inhibitory effects of β-caryophyllene, β-caryophyllene oxide and α-humulene on the activities of the main drug-metabolizing enzymes in rat and human liver in vitro. Chem. Interact. 2017, 278, 123–128. [Google Scholar] [CrossRef]
- Chatelut, É.; Delord, J.-P.; Canal, P. Toxicity patterns of cytotoxic drugs. Investig. New Drugs 2003, 21, 141–148. [Google Scholar] [CrossRef]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Griggs, J.J. Reducing the toxicity of anticancer therapy: New strategies. Leuk. Res. 1998, 22, S27–S33. [Google Scholar] [CrossRef]
- Steele, V.E. Current mechanistic approaches to the chemoprevention of cancer. J. Biochem. Mol. Biol. 2003, 36, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Hari, S.; Vasudevan, V.; Kasibhotla, S.; Reddy, D.; Venkatappa, M.; Devaiah, D. Anti-inflammatory Dietary Supplements in the Chemoprevention of Oral Cancer. Cancer Res. Front. 2016, 2, 380–395. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, Y.-X. Cytokines that target immune killer cells against tumors. Cell. Mol. Immunol. 2020, 17, 1–6. [Google Scholar] [CrossRef]
- Chen, H.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yuan, J.; Yang, X. Data of cytotoxicity, p53 and Akt downstream proteins and physiological indexes in hepatocellular carcinoma cells or HepG2-bearing nude mouse model administered by α-Humulene. Data Brief. 2020, 29, 105325. [Google Scholar] [CrossRef]
- Stark, G. Functional Consequences of Oxidative Membrane Damage. J. Membr. Biol. 2005, 205, 1–16. [Google Scholar] [CrossRef]
- Fariss, M.W. Role of Mitochondria in Toxic Oxidative Stress. Mol. Interv. 2005, 5, 94–111. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Božović, M.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Shahwar, D.; Ullah, S.; Khan, M.A.; Ahmad, N.; Saeed, A.; Ullah, S. Anticancer activity of Cinnamon tamala leaf constituents towards human ovarian cancer cells. Pak. J. Pharm. Sci. 2015, 28, 969–972. [Google Scholar]
- Nony, P.A.; Kennett, S.B.; Glasgow, W.C.; Olden, K.; Roberts, J.D.; Stebbins, J.L.; Jung, D.; Leone, M.; Zhang, X.-K.; Pellecchia, M. 15(S)-Lipoxygenase-2 Mediates Arachidonic Acid-stimulated Adhesion of Human Breast Carcinoma Cells through the Activation of TAK1, MKK6, and p38 MAPK. J. Biol. Chem. 2005, 280, 31413–31419. [Google Scholar] [CrossRef] [Green Version]
- Shureiqi, I.; Lippman, S.M. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001, 61, 6307–6312. [Google Scholar]
- Liu, H.; Yang, G.; Tang, Y.; Cao, D.; Qi, T.; Qi, Y.; Fan, G. Physicochemical characterization and pharmacokinetics evaluation of β-caryophyllene/β-cyclodextrin inclusion complex. Int. J. Pharm. 2013, 450, 304–310. [Google Scholar] [CrossRef]
- Lou, J.; Teng, Z.; Zhang, L.; Yang, J.; Ma, L.; Wang, F.; Tian, X.; An, R.; Yang, M.; Zhang, Q.; et al. β-Caryophyllene/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Improves Cognitive Deficits in Rats with Vascular Dementia through the Cannabinoid Receptor Type 2 -Mediated Pathway. Front. Pharmacol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Quintans, J.D.S.S.; Araújo, A.A.; Brito, R.G.; Santos, P.L.; Quintans, J.D.S.S.; Menezes, P.P.; Serafini, M.R.; Silva, G.F.; Carvalho, F.M.; Brogden, N.K.; et al. β-caryophyllene, a dietary cannabinoid, complexed with β-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 2016, 149, 34–41. [Google Scholar] [CrossRef]
- Neves, J.K.D.O.; Apolinário, A.C.; Saraiva, K.L.A.; Da Silva, D.T.C.; Reis, M.Y.D.F.A.; Damasceno, B.P.G.L.; Adalberto, P., Jr.; Galvão, M.A.M.; Soares, L.A.L.; Júnior, V.F.D.V.; et al. Microemulsions containing Copaifera multijuga Hayne oil-resin: Challenges to achieve an efficient system for β-caryophyllene delivery. Ind. Crop. Prod. 2018, 111, 185–192. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. 2011, 3, 279–296. [Google Scholar] [CrossRef]
- Khan, K.H.; Blanco-Codesido, M.; Molife, L.R. Cancer therapeutics: Targeting the apoptotic pathway. Crit. Rev. Oncol. 2014, 90, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Bioenergy 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Shore, G.C.; Papa, F.R.; Oakes, S.A. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 2011, 23, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Ilagan, E.; Manning, B.D. Emerging Role of mTOR in the Response to Cancer Therapeutics. Trends Cancer 2016, 2, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Janku, F.; Wheler, J.J.; Westin, S.N.; Moulder, S.L.; Naing, A.; Tsimberidou, A.M.; Fu, S.; Falchook, G.S.; Hong, D.S.; Garrido-Laguna, I.; et al. PI3K/AKT/mTOR Inhibitors in Patients With Breast and Gynecologic Malignancies Harboring PIK3CA Mutations. J. Clin. Oncol. 2012, 30, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-kB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Dejardin, E. The alternative NF-κB pathway from biochemistry to biology: Pitfalls and promises for future drug development. Biochem. Pharmacol. 2006, 72, 1161–1179. [Google Scholar] [CrossRef]
- Han, L.; Yang, L.; Liu, B.; Cheng, X. Trans-caryophyllene suppresses tumor necrosis factor (TNFα)-induced inflammation in human chondrocytes. Eur. Food Res. Technol. 2014, 239, 1061–1066. [Google Scholar] [CrossRef]
- Johnston, P.A.; Grandis, J.R. STAT3 SIGNALING: Anticancer Strategies and Challenges. Mol. Interv. 2011, 11, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Mohammad, I.S.; Liu, Z. Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors (Review). Oncol. Lett. 2020, 19, 2585–2594. [Google Scholar] [CrossRef]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial–Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, K.; Fry, E.A. Aberrant Expression of Cyclin D1 in Cancer. Signal. Transduct. Insights 2015, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Agami, R.; Bernards, R. Distinct Initiation and Maintenance Mechanisms Cooperate to Induce G1 Cell Cycle Arrest in Response to DNA Damage. Cell 2000, 102, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Jirawatnotai, S.; Sittithumcharee, G. Paradoxical roles of cyclin D1 in DNA stability. DNA Repair 2016, 42, 56–62. [Google Scholar] [CrossRef]
- André, N.; Tsai, K.; Carre, M.; Pasquier, E. Metronomic Chemotherapy: Direct Targeting of Cancer Cells after all? Trends Cancer 2017, 3, 319–325. [Google Scholar] [CrossRef]
- Ambrož, M.; Matoušková, P.; Skarka, A.; Zajdlová, M.; Žáková, K.; Skálová, L. The Effects of Selected Sesquiterpenes from Myrica rubra Essential Oil on the Efficacy of Doxorubicin in Sensitive and Resistant Cancer Cell Lines. Molecules 2017, 22, 1021. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, U.; Kroemer, H.K. The ABC Transporters MDR1 and MRP2: Multiple Functions in Disposition of Xenobiotics and Drug Resistance. Drug Metab. Rev. 2004, 36, 669–701. [Google Scholar] [CrossRef]
- Ohnuma, S.; Ambudkar, S.V. Discovering Natural Product Modulators to Overcome Multidrug Resistance in Cancer Chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 609–620. [Google Scholar] [CrossRef]
- Zhou, S.-F. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 2008, 38, 802–832. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahad, A.; Khar, R.K.; Jaggi, M.; Aqil, M.; Iqbal, Z.; Ahmad, F.J.; Talegaonkar, S. The emerging role of P-glycoprotein inhibitors in drug delivery: A patent review. Expert Opin. Ther. Patents 2011, 21, 561–576. [Google Scholar] [CrossRef]
- Wink, M. Evolutionary Advantage and Molecular Modes of Action of Multi-Component Mixtures Used in Phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Kioka, N.; Kato, H.; Matsuo, M.; Ueda, K. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem. J. 2007, 401, 597–605. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, W.; Wang, L.; Tian, Z.; Zhang, J. Deactivation of Signal Transducer and Activator of Transcription 3 Reverses Chemotherapeutics Resistance of Leukemia Cells via Down-Regulating P-gp. PLoS ONE 2011, 6, e20965. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, P.; Zhang, B.; Wang, A.; Yang, M. Role of STAT3 decoy oligodeoxynucleotides on cell invasion and chemosensitivity in human epithelial ovarian cancer cells. Cancer Genet. Cytogenet. 2010, 197, 46–53. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Zalba, S.; Hagen, T.L.T. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef] [Green Version]
- Bassiony, M.; Aluko, A.V.; Radosevich, J.A. Immunotherapy and Cancer. In Precision Medicine in Oncology; Wiley: Hoboken, NJ, USA, 2020; pp. 133–156. [Google Scholar]
- Carmona, F.; Pereira, A.M.S. Herbal medicines: Old and new concepts, truths and misunderstandings. Rev. Bras. Farm. 2013, 23, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Borgonetti, V.; Governa, P.; Montopoli, M.; Biagi, M. Cannabis sativa L. Constituents and Their Role in Neuroinflammation. Curr. Bioact. Compd. 2019, 15, 147–158. [Google Scholar] [CrossRef]
- Santos, P.S.; Oliveira, T.C.; Júnior, L.M.R.; Figueiras, A.; Nunes, L.C. β-caryophyllene Delivery Systems: Enhancing the Oral Pharmacokinetic and Stability. Curr. Pharm. Des. 2018, 24, 3440–3453. [Google Scholar] [CrossRef]
- Heinrich, M.; Appendino, G.; Efferth, T.; Fürst, R.; Izzo, A.A.; Kayser, O.; Pezzuto, J.M.; Viljoen, A. Best practice in research—Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 2020, 246, 112230. [Google Scholar] [CrossRef]
- Izzo, A.A.; Teixeira, M.; Alexander, S.P.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Ji, Y.; Kendall, D.A.; Panattieri, R.A.; et al. A practical guide for transparent reporting of research on natural products in the British Journal of Pharmacology: Reproducibility of natural product research. Br. J. Pharmacol. 2020, 177, 2169–2178. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]











| Compound (PubChem Compound ID) | Cancer Site | Combined Treatment/Subjects | Comments | References |
|---|---|---|---|---|
| Drugs | ||||
| Tamoxifen (2733526), raloxifene (5035), lasofoxifene (216416), arzoxifene (179337) | Breast | None/healthy and high-risk women | Significant decrease in cancer risk and recurrence; higher tolerability of raloxifene, lasofoxifene and arzoxifene than tamoxifene | [16,17,18] |
| Finasteride (57363), dutasteride (6918296) | Prostate | None/low and high-risk men | Significant decrease in prostate cancer risk; controversial increased risk of high-grade disease | [19,20] |
| Metformin (4091) | Breast | Anthracyclines, platinum, taxanes, capecitabine, cyclophosphamide, doxorubicin/breast cancer patients and high-risk women | Significant reduction in the breast cancer risk and increase in progression-free survival | [21] |
| Colorectal | None/high-risk subjects | Lacking effects | [22] | |
| Endometrial | Medroxyprogesterone acetate/patients with atypical endometrial hyperplasia | Inhibition of disease relapse; further studies required | [23] | |
| Lung | Chemotherapy/lung cancer patients with diabetes | Favorable survival outcome; further studies required | [24] | |
| Prostate | None/prostate cancer patients with or without diabetes | Some evidence of reduced cancer risk; further studies required | [25] | |
| Celecoxib (2662) | Gastric | First-line chemotherapy, radiotherapy/Patients with metastatic or postoperative recurrent advanced gastric cancer | Clinical benefits and safety; further studies required | [26] |
| Colorectal | None/high-risk patients | Significant reduction in colorectal adenomas; further studies required | [27] | |
| Prostate | Radiotherapy/patients with prostate cancer | Significant improvement in radiotherapy efficacy and lowering in the relapse rates; further studies required | [28] | |
| Aspirin (2244) | Colorectal | None/patients with first-time colorectal cancer | Preliminary evidence for reduced colorectal cancer risk; further studies required | [29] |
| Glioma | None/glioma patients | Slight reduction in glioma risk; further studies required | [30] | |
| Lovastatin (53232), atorvastatin (60823), pravastatin (54687), simvastatin (54454), fluvastatin (446155) | Breast, prostate, lung, skin, colorectal, liver | None/healthy, high-risk and cancer patients | Controversial evidence of reduced cancer risk; further studies required | [31,32,33] |
| Natural Substances | ||||
| Curcumin (969516) | Colorectal | None or in combination with avastin-FOLFIRI, irinotecan, FOLFOX, 5-fluorouracil/cancer patients | Preliminary evidence of synergistic effects and chemoresistance reduction; further studies required | [34] |
| Resveratrol (445154) | Colorectal | None/cancer patients | Preliminary evidence of cancer reduction; further studies required | [35] |
| Sulforaphane (5350) | Breast | None/high-risk subjects | Preliminary evidence of cancer risk reduction; further studies required | [36] |
| Prostate | None/high-risk subjects | Preliminary evidence of cancer risk reduction; further studies required | [37] | |
| β-Carotene (5280489) | Breast | None/healthy or high-risk subjects | Preliminary evidence of cancer risk reduction; further studies required | [38] |
| Lycopene (446925) | Prostate | None/healthy or high-risk subjects | Preliminary evidence of cancer risk reduction; further studies required | [39] |
| Plant Species | Plant Part | Composition | References |
|---|---|---|---|
| Baccharis coridifolia D.C. | Aerial parts | β-Caryophyllene 10.8%, β-caryophyllene oxide 9.8%, α-humulene 0.4%, isocaryophyllene 34.3% | [83] |
| Cachrys alpina Bieb. | Aerial parts | β-Caryophyllene 2.5%, α-humulene 33.2%, α-humulene epoxide II 2.2% | [84] |
| Callistemon polandii (Bonpl.) DC. | Leaves | β-Caryophyllene 28.2%, β-caryophyllene oxide 13.5%, α-humulene 21.7% | [85] |
| Cannabis sativa L. | Inflorescences | β-Caryophyllene 7.6–29.8%, β-caryophyllene oxide 0.8–9.5%, α-humulene 2.2–10.1%, isocaryophyllene < 0.05–0.4% | [86] |
| Cinnamomum iners Reinw. ex Blume | Leaves | β-Caryophyllene 35.9% | [76] |
| Colquhounia coccinea Wall | Leaves and Flowers | β-Caryophyllene 44.1% in leaves and 53.2% in flowers | [74] |
| Copaifera langsdorffii Desf. | Balsam oil from bark Leaves | β-Caryophyllene 53.3%, α-humulene 6.1% β-Caryophyllene 16.6%, β-caryophyllene oxide 1.3%, α-humulene 2.9% | [71] |
| Eugenia caryophyllata (syn. Syzygium aromaticum (L.) Merr.) | Floral buds and leaves | β-Caryophyllene 17.4%, β-caryophyllene oxide 0.4%, α-humulene 2.1%, isocaryophyllene 0.5% | [68,69] |
| Eugeniarocana Britt. et Wils. | Leaves | β-Caryophyllene0.1%, β-caryophyllene oxide 57.7%, α-humulene epoxide II 9.9%, 14-hydroxy-9-epi-P-caryophyllene 10.3% | [87] |
| Helichrysum melaleucum Rchb. ex Holl. | Aerial parts | β-Caryophyllene 35.4% | [78] |
| Helichrysum stoechas ssp. barrelieri var. spathulatum | Aerial parts | β-Caryophyllene 27.9–33.6%, β-caryophyllene oxide 1.6–6.5%, α-humulene 13.4–21.1% | [88] |
| Hippomarathrum microcarpum (M. Bieb.) B. Fedtsch. | Aerial parts | β-Caryophyllene 15.8%, β-caryophyllene oxide 2.7%, α-humulene 3.2% | [89] |
| Humulus lupulus L. | Inflorescences | β-Caryophyllene 4.8–28.8%, β-caryophyllene oxide 2.3–8.6%, α-humulene 2.6–23.0% | [90] |
| Hypericum heterophyllum Vent. | Aerial parts | β-Caryophyllene 4.5%, α-humulene 2.4%, isocaryophyllene 17.1% | [91] |
| Jasminum sambac (L.) Aiton | Flowers | β-Caryophyllene 0.3%, α-humulene 0.2%, isocaryophyllene 13.7% | [92] |
| Lantana achyranthifolia Desf. | Aerial parts | α-Humulene 10.7%, isocaryophyllene 16.7% | [93] |
| Lantana camara L. | Leaves | α-Humulene 3.8%, isocaryophyllene 10.7% | [93] |
| Lavandula angustifolia M. | Essential oil from flowers | β-Caryophyllene 4.9%, β-caryophyllene oxide 0.5%, α-humulene 0.4% | [94,95] |
| Lophostemon suaveolens | Fresh leaves | β-Caryophyllene 2.5%, α-humulene 1.5% | [96] |
| Lycopus australis R.Br. | Leaves | β-Caryophyllene 10.2%, β-caryophyllene oxide 1.8%, α-humulene 19.5% | [97] |
| Marliereaobscura O. Berg. | Leaves | β-Caryophyllene oxide 37.20% | [98] |
| Marrubiumastracanicum Jacq | Leaves | β-Caryophyllene 13.1%, β-caryophyllene oxide 35.8, α-humulene 0.9% | [99] |
| Micromeria hedgei L. | Aerial parts | β-Caryophyllene 6.5%, β-caryophyllene oxide 4.7%, α-humulene 3.3% | [100] |
| Nepeta curviflora Boiss. | Aerial parts | β-Caryophyllene 50.2% | [73] |
| Nepeta graciliflora B. | Aerial parts | β-Caryophyllene 5.3, β-caryophyllene oxide 12.2% | [101] |
| Ocimum basilicum L. | Aerial and wooden parts | β-Caryophyllene 1.9%, β-caryophyllene oxide 0.7%, α-humulene 0.4% | [102,103] |
| Origanum vulgare L. | Leaves and stems | β-Caryophyllene 1.1–1.5%, β-caryophyllene oxide 0.1–2.5% | [104,105] |
| Orthodon dianthera Maxim. | Aerial parts | β-Caryophyllene 52.9% | [72] |
| Physospermum cornubiense (L.) DC. | Aerial parts | β-Caryophyllene 15.4% and β-caryophyllene oxide 24.5% | [89] |
| Pimpinella spp. | Aerial parts | β-Caryophyllene 0.1–3.6%, β-caryophyllene oxide 2.5%, α-humulene 1–1.6% | [106] |
| Piper nigrum L. | Berries | β-Caryophyllene 47.5%, β-caryophyllene oxide 4.0%, α-humulene 0.4% | [75] |
| Pliniadermatodes Urb. | Leaves | β-Caryophyllene 0.9%, β-caryophyllene oxide 62.1%, α-humulene 0.1% | [107] |
| Psidiumsalutare (HBK) Berg. | Leaves | β-Caryophyllene 4.8%, β-caryophyllene oxide 39.8% | [108] |
| Salvia glutinosa L. | Leaves | β-Caryophyllene 5–9%, β-caryophyllene oxide 24.3–28.9%, α-humulene 5.9% | [109] |
| Salvia officinalis L. ssp. altissima | Aerial part | β-Caryophyllene 31.8%, β-caryophyllene oxide 23.2%, α-humulene 1.0%, 1 4-hydroxy-g-epi-(E)-caryophyllene 0.6%, humulene epoxide II 0.2% | [77] |
| Scutellaria californica A. Gray | Flowers | β-Caryophyllene 56.2% | [70] |
| Stachys lanata K. Koch | Aerial parts | β-Caryophyllene 12.6%, α-humulene 24.9%, β-caryophyllene oxide 0.3% | [110] |
| Syzygiumgardneri Thw. | Leaves | β-Caryophyllene 5.3%, β-caryophyllene oxide 49.6%, α-humulene 1.7% | [111] |
| Tagetes patula L. | Flower | β-Caryophyllene 0.3%, β-caryophyllene oxide 48.4% | [112] |
| Tephrosiacinerea Pers. | Aerial parts | β-Caryophyllene oxide 63.9% | [113] |
| Tephrosiadensiflora (Hook. f.) | Aerial parts | β-Caryophyllene 45.0%, β-caryophyllene oxide 5.2%, | [113] |
| Tephrosiapersica Boiss. | Aerial parts | β-Caryophyllene 6.8%, β-caryophyllene oxide 7.0%, | [113] |
| Teucriumorientale L. | Aerial parts | β-Caryophyllene 9.3%, β-caryophyllene oxide 33.5%, α-humulene 1.7%, isocaryophyllene 0.7% | [114] |
| Uvariodendron calophyllum RE Fries | Stem bark | β-Caryophyllene 32.5% | [79] |
| Zingiber nimmonii (J. Graham) Dalzell | Rhyzomes | β-Caryophyllene 42.2%, α-humulene 27.7% | [80] |
| Compound | General Pharmacological Properties | Type of Pharmacological Evidence | References |
|---|---|---|---|
| β-Caryophyllene (syn. trans-caryophyllene, E-caryophyllene) | Analgesic | in vitro and in vivo | [123,124] |
| Antiallergic | in vitro and in vivo | [125,126] | |
| Antiarthritic | in vitro and in vivo | [127,128,129] | |
| Antibacterial | in vitro | [130,131,132] | |
| Anticonvulsant | in vivo | [133] | |
| Antifungal | in vitro | [91] | |
| Anti-inflammatory | in vitro and in vivo | [123,124,125,129,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149] | |
| Antioxidant | in vitro and in vivo | [123,132,135,138,147,148,149,150,151] | |
| Antiproliferative | in vitro and in vivo | [45,123,124,152,153,154,155,156,157,158,159,160,161,162,163] | |
| Anxiolytic/antidepressant | in vivo | [164,165,166] | |
| Antispasmodic | in vivo | [167] | |
| Chemosensitizing | in vitro | [160,161,162,163,168,169,170] | |
| Genoprotective | in vitro and in vivo | [163,171,172,173,174,175,176] | |
| Hypoglycemic | in vitro and in vivo | [135,177,178,179] | |
| Hypolipidemic | in vitro and in vivo | [180,181] | |
| Immunomodulatory | in vitro and in vivo | [125,136,145] | |
| Local anesthetic | in vitro and in vivo | [184] | |
| Membrane permeability modulation | in vitro | [123,185,186] | |
| Neuroprotective | in vitro and in vivo | [123,187,188,189,190,191,192,193,194] | |
| β-Caryophyllene oxide | Analgesic | in vivo | [129,195] |
| Antibacterial | in vitro | [196] | |
| Antifungal | in vitro | [197] | |
| Anti-inflammatory | in vitro and in vivo | [149,195,198] | |
| Antiproliferative | in vitro | [124,198,199,200,201,202,203,204] | |
| Chemosensitizing | in vitro and in vivo | [160,162,169,199,200] | |
| Genoprotective | in vitro | [161,174] | |
| α-Humulene | Antibacterial | in vitro | [205] |
| Antifungal/Antiparasitic | in vitro | [206,207] | |
| Anti-inflammatory | in vitro | [134,208] | |
| Antiproliferative | in vitro and in vivo | [45,168,209,210,211] | |
| Chemosensitizing | in vitro | [169,200] | |
| Isocaryophyllene (syn. γ-caryophyllene) | Antiproliferative | in vitro | [168,211,212] |
| Antifungal | in vitro | [206] |
| Compound | IC50 [μM]/Time Exposure | Cancer Cells/Type a | Outcome | Mechanisms | References |
|---|---|---|---|---|---|
| In vitro studies | |||||
| β-Caryophyllene | 18.6–23.5 μM/nr | HeLa, BT-20, B-16, HIB | Cytotoxicity | nr | [151] |
| 0.02 μM/2 h | BS-24-1, MoFir | Cytotoxicity and apoptosis | DNA ladder and ↑ caspase-3 activity | [152] | |
| 137–270 μM/48 h | A549, AsPC-1, HT-29, NCI-H358 | Cytotoxicity | G1 cell cycle arrest, ↓ cyclin D1, cyclin E, cyclin-dependent protein kinase (CDK) -2, -4, and -6, RB phosphorylation, ↑ p21CIP1/WAF1 and p27KIP1 | [153] | |
| ≈122–150 b μM/24 h | U-373 MG, U-87 MG | Cytotoxicity, switch of autophagy to apoptosis | Cell cycle inhibition, ↑ caspases 3 and 9 activity, ↓ Beclin-1, LC3 and p62/SQSTM1, CB2-mediated anti-inflammatory effects (↓ NF-kB, TNF-α and Jun N-Terminal Kinase, ↑ PPARγ) | [154] | |
| ≈196 b μM/24 h | KB | Cytotoxicity and apoptosis | Apoptosis induction, inhibition of metastasization, ↓NF-kB and PI3K/Akt signalings | [155] | |
| ≈20 b μM/24 h | MG-63 | Cytotoxicity, apoptosis and inflammation | Induction via ROS and JAK1/STAT3 signaling pathway | [156] | |
| 19–285 μM/24 h | HCT 116, HT29, PANC-1 | Cytotoxicity, apoptosis, inhibition of clonogenicity, migration and invasion | Nuclear condensation and fragmentation pathways, disruption of mitochondrial membrane potential | [157] | |
| >250 μM/24 h | PC3, MCF-7, ME-180, K562 | Lack of cytotoxicity | [158] | ||
| 5 and 10 µM c/9 days | HCT 116 spheroid | Inhibition of spheroid formation | [132,158] | ||
| 1103.3 μM/24 h | Caco-2 | Cytotoxicity | [160] | ||
| 311.2–368.5 μM/24 h | CCRF/CEM, CEM/ADR5000 | Cytotoxicity | [160] | ||
| 379.5 μM/2 h | HepG2 | Cytotoxicity | [162] | ||
| 251–265 μM/2 h double and triple d | |||||
| 197 μM/24 h | |||||
| 121 μM/48 h | |||||
| 113 μM/72 h | |||||
| 171.5 μM/2 h | Mz-ChA-1 | Cytotoxicity | [163] | ||
| 139.5 μM/2 h double d | and apoptosis | ||||
| 124 μM/24 h | |||||
| 90 μM/72 h | |||||
| >250 μM/nr | MCF-7, PC-3, A-549, DLD-1, M4BEU and CT-26 | Lack of cytotoxicity | [209] | ||
| 93 μM/24 h | MDA-MB468 | Cytotoxicity | [242] | ||
| 220 μM/24 h | HepG2 | Cytotoxicity | [242] | ||
| β-Caryophyllene oxide | 12.3 μM/nr | HeLa | Cytotoxicity | [151] | |
| 235.2–297.8 μM/24 h | CCRF/CEM, CEM/ADR5000 | Cytotoxicity | [162] | ||
| 332.3 μM/24 h | Caco-2 | Cytotoxicity | [160] | ||
| 379.5 μM/2 h | HepG2 | Cytotoxicity | [162] | ||
| 251–265 μM/2 h double and triple d | |||||
| 195 μM/24 h | |||||
| 162 μM/48 h | |||||
| 152.5 μM/72 h | |||||
| up to 100 μM/4 h followed by 72 h restoring | Alexander or PCL/PRF/5 wild-type and MDR phenotype (Alexander/R) | Lack of cytotoxicity | [199] | ||
| 30–50 μM c | PC-3, MCF-7 | Apoptosis | ↓ PI3K/Akt/mTOR/S6K1 pathways and ↑ROS-mediated MAPKs | [201] | |
| 30 μM c | U266, MM1.S, DU145, MDAMB-231 | Apoptosis and inhibition of proliferation and invasiveness | Inhibition of constitutive and inducible STAT3 signaling, induction of SHP-1 Protein Tyrosine Phosphatase | [202] | |
| 3.7–29.4 μM/96 h | HepG2, HeLa, AGS, SNU-1, SNU-16 | Cytotoxicity | [203] | ||
| 50 μM c/6 h | PC-3 | Apoptosis | Inhibition of Akt/mTOR/S6K1 signaling | [204] | |
| >250 μM/nr | MCF-7, PC-3, A-549, DLD-1, M4BEU and CT-26 | Lack of cytotoxicity | [209] | ||
| 41 μM/48 h | A-2780 | Cytotoxicity | [243] | ||
| α-Humulene | 50–73 μM/nr | MCF-7, PC-3, A-549, DLD-1, M4BEU and CT-26 | Cytotoxicity | Pro-oxidant effects | [209] |
| ≈32 b μM/48 h | MCF-7, DLD-1 and L-929 | Cytotoxicity | nr | [168] | |
| ≈53.8–83.1 μM/12 h | Huh7, SMMC-7721, HepG2 and Hep3B | Cytotoxicity | Inhibition of Akt signaling and apoptosis signaling activation | [210] | |
| Isocaryophyllene | 34–87 μM/nr | MCF-7, PC-3, A-549, DLD-1, M4BEU, L-929 and CT-26 | Cytotoxicity | nr | [209] |
| <32 c μM/48 h | MCF-7, DLD-1 and L-929 | Cytotoxicity | nr | [168] | |
| ≈100 b μM/48 h | L-929 | Cytotoxicity | Pro-oxidant effects, membrane permeabilization and cell shrinking | [222] | |
| In vivo studies | |||||
| β-Caryophyllene | High-fat diet (HFD) supplemented with 0.15 and 0.3% of sesquiterpene | B16F10-bearing C57BL/6N mice | Anticancer effects | Inhibition of solid tumor growth, metastasis, angiogenesis and lymphangiogenesis, apoptosis induction, activation of Bax and caspase-3, ↓ mRNA expressions of HIF-1α, VEGF-A, CD31 and VE-cadherin induced by HFD | [157] |
| 50, 100, and 200 mg/kg/day/nr | Orthotopically xenograft model of colon cancer | Anticancer effects | Reduction in tumor growth and vascularization | [158] | |
| α-Humulene | 10–20 mg/kg i.p. f/every 2 days for 4 weeks | HepG2-bearing nude mouse | Anticancer effects | Inhibition of Akt signaling and apoptosis signaling activation; evidence of side effects in mice | [210,239] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sotto, A.; Mancinelli, R.; Gullì, M.; Eufemi, M.; Mammola, C.L.; Mazzanti, G.; Di Giacomo, S. Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence. Cancers 2020, 12, 3034. https://doi.org/10.3390/cancers12103034
Di Sotto A, Mancinelli R, Gullì M, Eufemi M, Mammola CL, Mazzanti G, Di Giacomo S. Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence. Cancers. 2020; 12(10):3034. https://doi.org/10.3390/cancers12103034
Chicago/Turabian StyleDi Sotto, Antonella, Romina Mancinelli, Marco Gullì, Margherita Eufemi, Caterina Loredana Mammola, Gabriela Mazzanti, and Silvia Di Giacomo. 2020. "Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence" Cancers 12, no. 10: 3034. https://doi.org/10.3390/cancers12103034
APA StyleDi Sotto, A., Mancinelli, R., Gullì, M., Eufemi, M., Mammola, C. L., Mazzanti, G., & Di Giacomo, S. (2020). Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence. Cancers, 12(10), 3034. https://doi.org/10.3390/cancers12103034