Epstein–Barr Virus Promotes B Cell Lymphomas by Manipulating the Host Epigenetic Machinery
Simple Summary
Abstract
1. Introduction
2. Host–Pathogen Interaction during EBV Pathogenesis
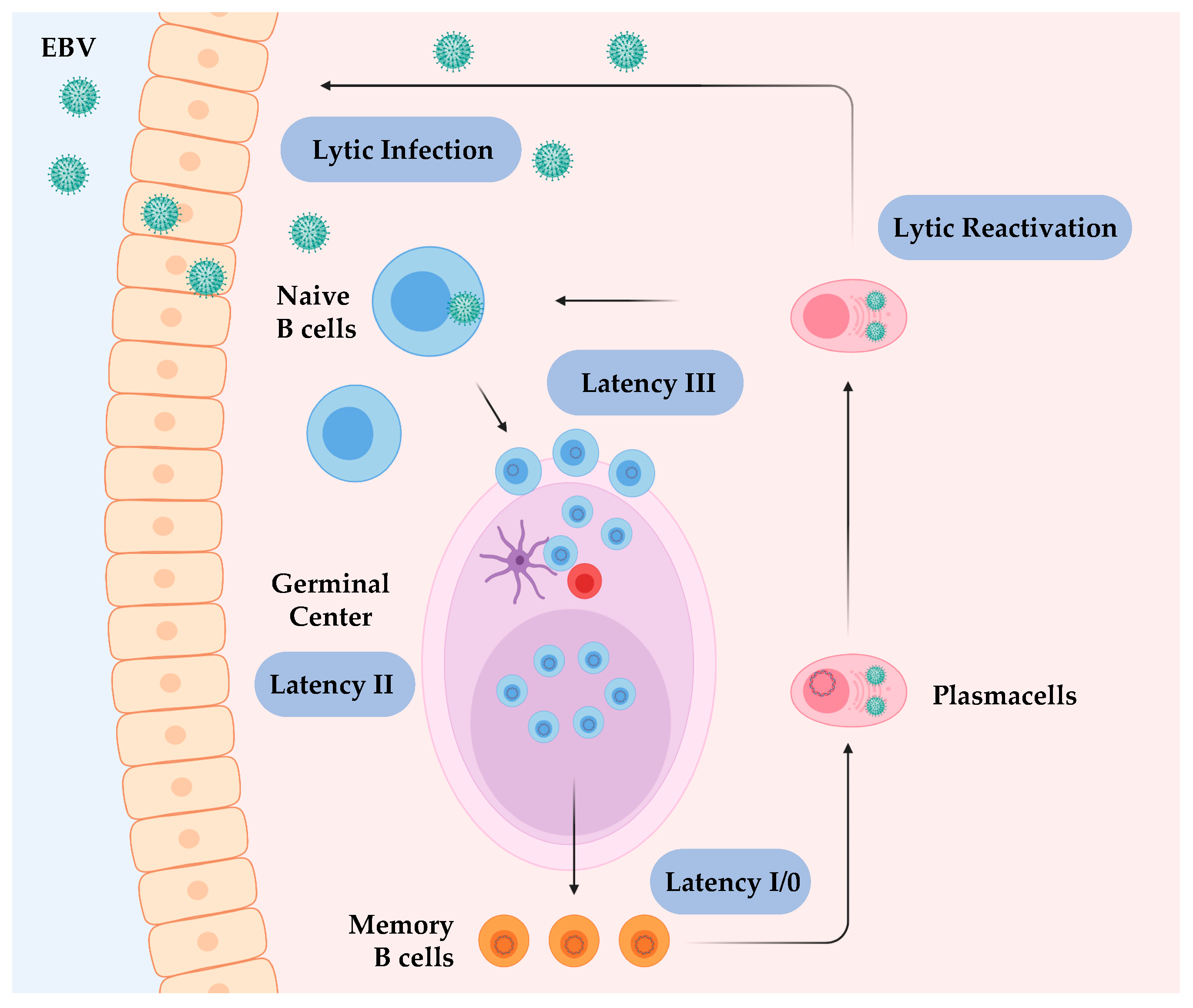
2.1. The Viral Life Cycle: From the Epithelium to B Cells
2.2. EBV Adopts Immune Evasion Strategies to Facilitate Viral Persistence in B Cells
2.3. Targeting B Cells to Establish Viral Persistence
2.4. The Reactivation of EBV Lytic Replication Increases the Pool of Infected B Cells in Immunocompromised Patients
3. Role of Epigenetics in EBV Infection and Cancer Formation
3.1. Latent Proteins Modulate the Host Epigenetic Machinery for Viral Silencing
3.2. Molecular Basis of B Cell Transformation
3.2.1. EBNA2, EBNALP
3.2.2. EBNA3 Family Proteins
3.2.3. Latent Membrane Proteins
4. Inflammation, Epigenetics, and Tumorigenesis
4.1. How Can Chronic Inflammation Drive Cancer Development?
4.2. Cross-Talk between EBV-Mediated Inflammation and Epigenetic Regulation
5. Open Questions
6. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Paksa, A.; Rajagopal, J. The epigenetic basis of cellular plasticity. Curr. Opin. Cell Biol. 2018, 49, 116–122. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Kondilis-Mangum, H.D.; Wade, P.A. Epigenetics and the adaptive immune response. Mol. Asp. Med. 2013, 34, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 51–55. [Google Scholar] [CrossRef]
- Virani, S.; Colacino, J.A.; Kim, J.H.; Rozek, L.S. Cancer Epigenetics: A Brief Review. ILAR J. 2012, 53, 359–369. [Google Scholar] [CrossRef]
- Poreba, E.; Broniarczyk, J.; Goździcka-Józefiak, A. Epigenetic mechanisms in virus-induced tumorigenesis. Clin. Epigenet. 2011, 2, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, A.; Matsusaka, K.; Aburatani, H.; Fukayama, M. Epstein-Barr Virus Infection as an Epigenetic Driver of Tumorigenesis. Cancer Res. 2012, 72, 3445–3450. [Google Scholar] [CrossRef] [PubMed]
- Dandri, M. Epigenetic modulation in chronic hepatitis B virus infection. Semin. Immunopathol. 2020, 42, 173–185. [Google Scholar] [CrossRef]
- Cavallin, L.E.; Goldschmidt-Clermont, P.; Mesri, E.A. Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi’s Sarcoma Associated with HIV/AIDS. PLoS Pathog. 2014, 10, e1004154. [Google Scholar] [CrossRef]
- Soto, D.; Song, C.; McLaughlin-Drubin, M.E. Epigenetic Alterations in Human Papillomavirus-Associated Cancers. Viruses 2017, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H. Primary Epstein-Barr virus infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kempkes, B.; Robertson, E.S. Epstein-Barr virus latency: Current and future perspectives. Curr. Opin. Virol. 2015, 14, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ternák, G. Epstein-Barr virus reactivation. Lancet Infect. Dis. 2003, 3, 271. [Google Scholar] [CrossRef]
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J. Virol. 2019, 93, e00238-19. [Google Scholar] [CrossRef]
- Buschle, A.; Hammerschmidt, W. Epigenetic lifestyle of Epstein-Barr virus. Semin. Immunopathol. 2020, 42, 131–142. [Google Scholar] [CrossRef]
- Kempkes, B.; Spitkovsky, D.; Jansen-Dürr, P.; Ellwart, J.; Kremmer, E.; Delecluse, H.-J.; Rottenberger, C.; Bornkamm, G.; Hammerschmidt, W. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 1995, 14, 88–96. [Google Scholar] [CrossRef]
- Kaiser, C.; Laux, G.; Eick, D.; Jochner, N.; Bornkamm, G.W.; Kempkes, B. The Proto-Oncogene c-myc Is a Direct Target Gene of Epstein-Barr Virus Nuclear Antigen 2. J. Virol. 1999, 73, 4481–4484. [Google Scholar] [CrossRef]
- Vrzalikova, K.; Vockerodt, M.; Leonard, S.; Bell, A.; Wei, W.; Schrader, A.; Wright, K.L.; Kube, D.; Rowe, M.; Woodman, C.B.; et al. Down-regulation of BLIMP1α by the EBV oncogene, LMP-1, disrupts the plasma cell differentiation program and prevents viral replication in B cells: Implications for the pathogenesis of EBV-associated B-cell lymphomas. Blood 2011, 117, 5907–5917. [Google Scholar] [CrossRef]
- Yang, P.; Markowitz, G.J.; Wang, X. The hepatitis B virus-associated tumor microenvironment in hepatocellular carcinoma. Natl. Sci. Rev. 2014, 1, 396–412. [Google Scholar] [CrossRef]
- Martin, M.; Herceg, Z. From hepatitis to hepatocellular carcinoma: A proposed model for cross-talk between inflammation and epigenetic mechanisms. Genome Med. 2012, 4, 8. [Google Scholar] [CrossRef]
- Longnecker, R.M.; Kieff, E.; Cohen, J.I. Epstein-barr Virus. In Fields Virology, 6th ed.; Lippincott Williams and Wilkins (Wolters Kluwer), Two Commence Square: Philadelphia, PA, USA, 2013; Volume 1. [Google Scholar]
- Luzuriaga, K.; Sullivan, J.L. Infectious Mononucleosis. N. Engl. J. Med. 2010, 362, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Houghten, R.A.; Moore, M.D.; Cooper, N.R. Identification of an epitope in the major envelope protein of Epstein-Barr virus that mediates viral binding to the B lymphocyte EBV receptor (CR2). Cell 1989, 56, 369–377. [Google Scholar] [CrossRef]
- Klarenbeek, P.L.; Remmerswaal, E.B.M.; Berge, I.J.M.T.; Doorenspleet, M.E.; Van Schaik, B.D.C.; Esveldt, R.E.E.; Koch, S.D.; Brinke, A.T.; Van Kampen, A.H.C.; Bemelman, F.J.; et al. Deep Sequencing of Antiviral T-Cell Responses to HCMV and EBV in Humans Reveals a Stable Repertoire That Is Maintained for Many Years. PLoS Pathog. 2012, 8, e1002889. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Hayes, G.M.; Liu, H.; Gemmell, L.; Schmeling, D.O.; Radecki, P.; Aguilar, F.; Burbelo, P.D.; Woo, J.; Balfour, H.H.; et al. Kinetics of Epstein-Barr Virus (EBV) Neutralizing and Virus-Specific Antibodies after Primary Infection with EBV. Clin. Vaccine Immunol. 2016, 23, 363–369. [Google Scholar] [CrossRef]
- Lane, H.C.; Masur, H.; Edgar, L.C.; Whalen, G.; Rook, A.H.; Fauci, A.S. Abnormalities of B-Cell Activation and Immunoregulation in Patients with the Acquired Immunodeficiency Syndrome. N. Engl. J. Med. 1983, 309, 453–458. [Google Scholar] [CrossRef]
- Pensieroso, S.; Galli, L.; Nozza, S.; Ruffin, N.; Castagna, A.; Tambussi, G.; Hejdeman, B.; Misciagna, D.; Riva, A.; Malnati, M.; et al. B-cell subset alterations and correlated factors in HIV-1 infection. AIDS 2013, 27, 1209–1217. [Google Scholar] [CrossRef]
- Weiss, G.E.; Traore, B.; Kayentao, K.; Ongoiba, A.; Doumbo, S.; Doumtabe, D.; Kone, Y.; Dia, S.; Guindo, A.; Traore, A.; et al. The Plasmodium falciparum-Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections. PLoS Pathog. 2010, 6, e1000912. [Google Scholar] [CrossRef]
- Hahn, A.M.; Huye, L.E.; Ning, S.; Webster-Cyriaque, J.; Pagano, J.S. Interferon Regulatory Factor 7 Is Negatively Regulated by the Epstein-Barr Virus Immediate-Early Gene, BZLF-1. J. Virol. 2005, 79, 10040–10052. [Google Scholar] [CrossRef]
- Keating, S.; Prince, S.; Jones, M.; Rowe, M. The Lytic Cycle of Epstein-Barr Virus Is Associated with Decreased Expression of Cell Surface Major Histocompatibility Complex Class I and Class II Molecules. J. Virol. 2002, 76, 8179–8188. [Google Scholar] [CrossRef]
- Morrison, T.E.; Mauser, A.; Klingelhutz, A.J.; Kenney, S.C. Epstein-Barr Virus Immediate-Early Protein BZLF1 Inhibits Tumor Necrosis Factor Alpha-Induced Signaling and Apoptosis by Downregulating Tumor Necrosis Factor Receptor 1. J. Virol. 2004, 78, 544–549. [Google Scholar] [CrossRef]
- Morrison, T.E.; Mauser, A.; Wong, A.; Ting, J.P.-Y.; Kenney, S.C. Inhibition of IFN-γ Signaling by an Epstein-Barr Virus Immediate-Early Protein. Immunity 2001, 15, 787–799. [Google Scholar] [CrossRef]
- Horst, D.; Burmeister, W.P.; Boer, I.G.J.; Van Leeuwen, D.; Buisson, M.; Gorbalenya, A.E.; Wiertz, E.J.H.J.; Ressing, M.E. The “Bridge” in the Epstein-Barr Virus Alkaline Exonuclease Protein BGLF5 Contributes to Shutoff Activity during Productive Infection. J. Virol. 2012, 86, 9175–9187. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Thomas, W.; Van Leeuwen, D.; Middeldorp, J.M.; Wiertz, E.J.H.J.; Ressing, M.E.; Rowe, M. The DNase of Gammaherpesviruses Impairs Recognition by Virus-Specific CD8+ T Cells through an Additional Host Shutoff Function. J. Virol. 2007, 82, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Thorley-Lawson, D.A. EBV Persistence—Introducing the Virus. Curr. Top. Microbiol. Immunol. 2015, 390, 151–209. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Dawson, C.W.; Eliopoulos, A.G. The expression and function of Epstein-Barr virus encoded latent genes. Mol. Pathol. 2000, 53, 238–247. [Google Scholar] [CrossRef]
- Kis, L.L.; Salamon, D.; Persson, E.K.; Nagy, N.; Scheeren, F.A.; Spits, H.; Klein, G.; Klein, E. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C- and activation of LMP-1-promoter. Proc. Natl. Acad. Sci. USA 2009, 107, 872–877. [Google Scholar] [CrossRef]
- Woellmer, A.; Hammerschmidt, W. Epstein–Barr virus and host cell methylation: Regulation of latency, replication and virus reactivation. Curr. Opin. Virol. 2013, 3, 260–265. [Google Scholar] [CrossRef]
- Mansouri, S.; Pan, Q.; Blencowe, B.J.; Claycomb, J.M.; Frappier, L. Epstein-Barr Virus EBNA1 Protein Regulates Viral Latency through Effects on let-7 MicroRNA and Dicer. J. Virol. 2014, 88, 11166–11177. [Google Scholar] [CrossRef]
- Babcock, G.J.; Decker, L.L.; Freeman, R.B.; Thorley-Lawson, D.A. Epstein-Barr Virus–Infected Resting Memory B Cells, Not Proliferating Lymphoblasts, Accumulate in the Peripheral Blood of Immunosuppressed Patients. J. Exp. Med. 1999, 190, 567–576. [Google Scholar] [CrossRef]
- Simone, O.; Bejarano, M.T.; Pierce, S.K.; Antonaci, S.; Wahlgren, M.; Troye-Blomberg, M.; Donati, D. TLRs innate immunereceptors and Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) CIDR1α-driven human polyclonal B-cell activation. Acta Trop. 2011, 119, 144–150. [Google Scholar] [CrossRef]
- Chêne, A.; Donati, D.; Guerreiro-Cacais, A.O.; Levitsky, V.; Chen, Q.I.; Falk, K.; Orem, J.; Kironde, F.; Wahlgren, M.; Bejarano, M.T. A Molecular Link between Malaria and Epstein–Barr Virus Reactivation. PLoS Pathog. 2007, 3, e80. [Google Scholar] [CrossRef]
- Moormann, A.M.; Chelimo, K.; Sumba, O.P.; Lutzke, M.L.; Ploutz-Snyder, R.; Newton, D.; Kazura, J.; Rochford, R. Exposure to Holoendemic Malaria Results in Elevated Epstein-Barr Virus Loads in Children. J. Infect. Dis. 2005, 191, 1233–1238. [Google Scholar] [CrossRef]
- Angeletti, P.C.; Zhang, L.; Wood, C. The Viral Etiology of AIDS-Associated Malignancies. Adv. Pharmacol. 2008, 56, 509–557. [Google Scholar] [CrossRef]
- Lindahl, T.; Adams, A.; Bjursell, G.; Bornkamm, G.W.; Kaschka-Dierich, C.; Jehn, U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J. Mol. Biol. 1976, 102, 511–530. [Google Scholar] [CrossRef]
- Comet, I.; Riising, E.M.; Leblanc, B.; Helin, K. Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat. Rev. Cancer 2016, 16, 803–810. [Google Scholar] [CrossRef]
- Murata, T.; Kondo, Y.; Sugimoto, A.; Kawashima, D.; Saito, S.; Isomura, H.; Kanda, T.; Tsurumi, T. Epigenetic Histone Modification of Epstein-Barr Virus BZLF1 Promoter during Latency and Reactivation in Raji Cells. J. Virol. 2012, 86, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, X.; Choi, G.C.G.; Cao, Y.; Ambinder, R.F.; Tao, Q. Methylation profiling of Epstein-Barr virus immediate-early gene promoters, BZLF1 and BRLF1in tumors of epithelial, NK- and B-cell origins. BMC Cancer 2012, 12, 125. [Google Scholar] [CrossRef]
- Hughes, D.J.; Marendy, E.M.; Dickerson, C.A.; Yetming, K.D.; Sample, C.E.; Sample, J.T. Contributions of CTCF and DNA Methyltransferases DNMT1 and DNMT3B to Epstein-Barr Virus Restricted Latency. J. Virol. 2011, 86, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, F.; Sanchez-Alcaraz, T.; Wang, S.; Holowaty, M.N.; Sheng, Y.; Frappier, L. EBNA1-Mediated Recruitment of a Histone H2B Deubiquitylating Complex to the Epstein-Barr Virus Latent Origin of DNA Replication. PLoS Pathog. 2009, 5, e1000624. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Li, H.-P.; Lu, Y.-J.; Hsueh, C.; Liang, Y.; Chen, C.-L.; Tsao, S.W.; Tse, K.-P.; Yu, J.-S.; Chang, Y.-S. Activation of DNA Methyltransferase 1 by EBV LMP1 Involves c-Jun NH2-Terminal Kinase Signaling. Cancer Res. 2006, 66, 11668–11676. [Google Scholar] [CrossRef]
- Peng, H.; Chen, Y.; Gong, P.; Cai, L.; Lyu, X.; Jiang, Q.; Wang, J.; Lu, J.; Yao, K.; Liu, K.; et al. Higher methylation intensity induced by EBV LMP1 via NF-κB/DNMT3b signaling contributes to silencing of PTEN gene. Oncotarget 2016, 7, 40025–40037. [Google Scholar] [CrossRef]
- Leonard, S.; Wei, W.; Anderton, J.; Vockerodt, M.; Rowe, M.; Murray, P.G.; Woodman, C.B. Epigenetic and Transcriptional Changes Which Follow Epstein-Barr Virus Infection of Germinal Center B Cells and Their Relevance to the Pathogenesis of Hodgkin’s Lymphoma. J. Virol. 2011, 85, 9568–9577. [Google Scholar] [CrossRef]
- Anderton, J.A.; Bose, S.; Vockerodt, M.; Vrzalikova, K.; Wei, W.; Kuo, M.; Helin, K.; Christensen, J.; Rowe, M.; Murray, P.G.; et al. The H3K27me3 demethylase, KDM6B, is induced by Epstein–Barr virus and over-expressed in Hodgkin’s Lymphoma. Oncogene 2011, 30, 2037–2043. [Google Scholar] [CrossRef]
- Roy, S.G.; Robertson, E.S.; Saha, A. Epigenetic Impact on EBV Associated B-Cell Lymphomagenesis. Biomolecules 2016, 6, 46. [Google Scholar] [CrossRef]
- Kang, M.-S.; Kieff, E. Epstein–Barr virus latent genes. Exp. Mol. Med. 2015, 47, e131. [Google Scholar] [CrossRef]
- Wu, D.Y.; Krumm, A.; Schubach, W.H. Promoter-Specific Targeting of Human SWI-SNF Complex by Epstein-Barr Virus Nuclear Protein 2. J. Virol. 2000, 74, 8893–8903. [Google Scholar] [CrossRef]
- Lu, F.; Wiedmer, A.; Martin, K.A.; Wickramasinghe, P.J.M.S.; Kossenkov, A.V.; Lieberman, P.M. Coordinate Regulation of TET2 and EBNA2 Controls the DNA Methylation State of Latent Epstein-Barr Virus. J. Virol. 2017, 91, e00804-17. [Google Scholar] [CrossRef]
- Lu, F.; Chen, H.-S.; Kossenkov, A.V.; Dewispeleare, K.; Won, K.J.; Lieberman, P.M. EBNA2 Drives Formation of New Chromosome Binding Sites and Target Genes for B-Cell Master Regulatory Transcription Factors RBP-jκ and EBF1. PLoS Pathog. 2016, 12, e1005339. [Google Scholar] [CrossRef]
- Portal, D.; Zhou, H.; Zhao, B.; Kharchenko, P.V.; Lowry, E.; Wong, L.; Quackenbush, J.; Holloway, D.; Jiang, S.; Lu, Y.; et al. Epstein-Barr virus nuclear antigen leader protein localizes to promoters and enhancers with cell transcription factors and EBNA2. Proc. Natl. Acad. Sci. USA 2013, 110, 18537–18542. [Google Scholar] [CrossRef]
- Chen, A.; DiVisconte, M.; Jiang, X.; Quink, C.; Wang, F. Epstein-Barr Virus with the Latent Infection Nuclear Antigen 3B Completely Deleted Is Still Competent for B-Cell Growth Transformation In Vitro. J. Virol. 2005, 79, 4506–4509. [Google Scholar] [CrossRef]
- Jiang, S.; Willox, B.; Zhou, H.; Holthaus, A.M.; Wang, A.; Shi, T.T.; Maruo, S.; Kharchenko, P.V.; Johannsen, E.C.; Kieff, E.; et al. Epstein-Barr Virus Nuclear Antigen 3C binds to BATF/IRF4 or SPI1/IRF4 composite sites and recruits Sin3A to repress CDKN2A. Proc. Natl. Acad. Sci. USA 2013, 111, 421–426. [Google Scholar] [CrossRef]
- Paschos, K.; Parker, G.A.; Watanatanasup, E.; White, R.E.; Allday, M.J. BIM promoter directly targeted by EBNA3C in polycomb-mediated repression by EBV. Nucleic Acids Res. 2012, 40, 7233–7246. [Google Scholar] [CrossRef]
- Touitou, R.; Hickabottom, M.; Parker, G.; Crook, T.; Allday, M.J. Physical and Functional Interactions between the Corepressor CtBP and the Epstein-Barr Virus Nuclear Antigen EBNA3C. J. Virol. 2001, 75, 7749–7755. [Google Scholar] [CrossRef]
- Maruo, S.; Zhao, B.; Johannsen, E.; Kieff, E.; Zou, J.; Takada, K. Epstein-Barr virus nuclear antigens 3C and 3A maintain lymphoblastoid cell growth by repressing p16INK4Aand p14ARFexpression. Proc. Natl. Acad. Sci. USA 2011, 108, 1919–1924. [Google Scholar] [CrossRef]
- Zhang, B.; Kracker, S.; Yasuda, T.; Casola, S.; Vanneman, M.; Hömig-Hölzel, C.; Wang, Z.; Derudder, E.; Li, S.; Chakraborty, T.; et al. Immune Surveillance and Therapy of Lymphomas Driven by Epstein-Barr Virus Protein LMP1 in a Mouse Model. Cell 2012, 148, 739–751. [Google Scholar] [CrossRef]
- Rowe, M.; Peng-Pilon, M.; Huen, D.S.; Hardy, R.; Croom-Carter, D.; Lundgren, E.; Rickinson, A.B. Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: A B-cell-specific response that is delayed relative to NF-kappa B activation and to induction of cell surface markers. J. Virol. 1994, 68, 5602–5612. [Google Scholar] [CrossRef]
- Kieser, A.; Kilger, E.; Gires, O.; Ueffing, M.; Kolch, W.; Hammerschmidt, W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997, 16, 6478–6485. [Google Scholar] [CrossRef]
- Incrocci, R.; Barse, L.; Stone, A.; Vagvala, S.; Montesano, M.; Subramaniam, V.; Swanson-Mungerson, M. Epstein-Barr Virus Latent Membrane Protein 2A (LMP2A) enhances IL-10 production through the activation of Bruton’s tyrosine kinase and STAT3. Virology 2017, 500, 96–102. [Google Scholar] [CrossRef]
- Yuan, K.; Lei, Y.; Chen, H.-N.; Chen, Y.; Zhang, T.; Li, K.; Xie, N.; Wang, K.; Feng, X.; Pu, Q.; et al. HBV-induced ROS accumulation promotes hepatocarcinogenesis through Snail-mediated epigenetic silencing of SOCS3. Cell Death Differ. 2016, 23, 616–627. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shinjo, K.; Shimizu, Y.; Sano, T.; Yamao, K.; Gao, W.; Fujii, M.; Osada, H.; Sekido, Y.; Murakami, S.; et al. Hepatitis Virus Infection Affects DNA Methylation in Mice With Humanized Livers. Gastroenterology 2014, 146, 562–572. [Google Scholar] [CrossRef]
- Niwa, T.; Tsukamoto, T.; Toyoda, T.; Mori, A.; Tanaka, H.; Maekita, T.; Ichinose, M.; Tatematsu, M.; Ushijima, T. Inflammatory Processes Triggered by Helicobacter pylori Infection Cause Aberrant DNA Methylation in Gastric Epithelial Cells. Cancer Res. 2010, 70, 1430–1440. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nat. Cell Biol. 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef]
- Robinson, K.; White, J.R.; Winter, J.A. Differential inflammatory response to Helicobacter pylori infection: Etiology and clinical outcomes. J. Inflamm. Res. 2015, 8, 137–147. [Google Scholar] [CrossRef]
- Compare, D.; Rocco, A.; Liguori, E.; Armiento, F.P.D.; Persico, G.; Masone, S.; Coppola-Bottazzi, E.; Suriani, R.; Romano, M.; Nardone, G. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J. Clin. Pathol. 2011, 64, 677–682. [Google Scholar] [CrossRef]
- Ding, S.-Z.; Fischer, W.; Kaparakis-Liaskos, M.; Liechti, G.W.; Merrell, D.S.; Grant, P.A.; Ferrero, R.L.; Crowe, S.E.; Haas, R.; Hatakeyama, M.; et al. Helicobacter pylori-Induced Histone Modification, Associated Gene Expression in Gastric Epithelial Cells, and Its Implication in Pathogenesis. PLoS ONE 2010, 5, e9875. [Google Scholar] [CrossRef]
- Gong, G.; Waris, G.; Tanveer, R.; Siddiqui, A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF- B. Proc. Natl. Acad. Sci. USA 2001, 98, 9599–9604. [Google Scholar] [CrossRef]
- Kaneto, H. Detection of hypermethylation of the p16INK4A gene promoter in chronic hepatitis and cirrhosis associated with hepatitis B or C virus. Gut 2001, 48, 372–377. [Google Scholar] [CrossRef]
- Arzumanyan, A.; Friedman, T.; Kotei, E.; Ng, I.O.-L.; Lian, Z.; Feitelson, M.A. Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene 2011, 31, 563–572. [Google Scholar] [CrossRef]
- Nielsen, K.O.; Mirza, A.H.; Kaur, S.; Jacobsen, K.S.; Winther, T.N.; Glebe, D.; Pociot, F.; Høgh, B.; Størling, J. Hepatitis B virus suppresses the secretion of insulin-like growth factor binding protein 1 to facilitate anti-apoptotic IGF-1 effects in HepG2 cells. Exp. Cell Res. 2018, 370, 399–408. [Google Scholar] [CrossRef]
- Yang, L.F.; He, J.; Chen, L.-B.; Wang, G. Hepatitis B virus X protein upregulates expression of SMYD3 and C-MYC in HepG2 cells. Med. Oncol. 2008, 26, 445–451. [Google Scholar] [CrossRef]
- Torgbor, C.; Awuah, P.; Deitsch, K.; Kalantari, P.; Duca, K.A.; Thorley-Lawson, D.A. A Multifactorial Role for P. falciparum Malaria in Endemic Burkitt’s Lymphoma Pathogenesis. PLoS Pathog. 2014, 10, e1004170. [Google Scholar] [CrossRef]
- Kalchschmidt, J.S.; Bashford-Rogers, R.; Paschos, K.; Gillman, A.C.; Styles, C.T.; Kellam, P.; Allday, M.J. Epstein-Barr virus nuclear protein EBNA3C directly induces expression of AID and somatic mutations in B cells. J. Exp. Med. 2016, 213, 921–928. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Qiao, X.; Cerutti, A. CpG DNA Induces IgG Class Switch DNA Recombination by Activating Human B Cells through an Innate Pathway That Requires TLR9 and Cooperates with IL-10. J. Immunol. 2004, 173, 4479–4491. [Google Scholar] [CrossRef]
- Van Gent, M.; Griffin, B.D.; Berkhoff, E.G.; Van Leeuwen, D.; Boer, I.G.J.; Buisson, M.; Hartgers, F.C.; Burmeister, W.P.; Wiertz, E.J.; Ressing, M.E. EBV Lytic-Phase Protein BGLF5 Contributes to TLR9 Downregulation during Productive Infection. J. Immunol. 2010, 186, 1694–1702. [Google Scholar] [CrossRef]
- Fathallah, I.; Parroche, P.; Gruffat, H.; Zannetti, C.; Johansson, H.; Yue, J.; Manet, E.; Tommasino, M.; Sylla, B.S.; Hasan, U.A. EBV Latent Membrane Protein 1 Is a Negative Regulator of TLR9. J. Immunol. 2010, 185, 6439–6447. [Google Scholar] [CrossRef]
- Miller, W.E.; Cheshire, J.L.; Raab-Traub, N. Interaction of Tumor Necrosis Factor Receptor-Associated Factor Signaling Proteins with the Latent Membrane Protein 1 PXQXT Motif Is Essential for Induction of Epidermal Growth Factor Receptor Expression. Mol. Cell. Biol. 1998, 18, 2835–2844. [Google Scholar] [CrossRef]
- Iannetti, A.; LeDoux, A.C.; Tudhope, S.J.; Sellier, H.; Zhao, B.; Mowla, S.; Moore, A.; Hummerich, H.; Gewurz, B.E.; Cockell, S.J.; et al. Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence. PLoS Genet. 2014, 10, e1004642. [Google Scholar] [CrossRef]
- Geiger, T.R.; Martin, J.M. The Epstein-Barr Virus-Encoded LMP-1 Oncoprotein Negatively Affects Tyk2 Phosphorylation and Interferon Signaling in Human B Cells. J. Virol. 2006, 80, 11638–11650. [Google Scholar] [CrossRef]
- Chen, H.; Hutt-Fletcher, L.; Cao, L.; Hayward, S.D. A Positive Autoregulatory Loop of LMP1 Expression and STAT Activation in Epithelial Cells Latently Infected with Epstein-Barr Virus. J. Virol. 2003, 77, 4139–4148. [Google Scholar] [CrossRef]
- Pan, Y.-M.; Wang, C.-G.; Zhu, M.; Xing, R.; Cui, J.-T.; Li, W.-M.; Yu, D.-D.; Wang, S.-B.; Zhu, W.; Ye, Y.-J.; et al. STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer. Mol. Cancer 2016, 15, 79. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.Y.; Marzec, M.T.; Raghunath, P.N.; Nagasawa, T.; Wasik, M.A. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 6948–6953. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, P.; Herrmann, A.; Yang, C.; Xin, H.; Wang, Z.; Hoon, D.S.B.; Forman, S.J.; Jove, R.; Riggs, A.D.; et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc. Natl. Acad. Sci. USA 2012, 109, 7765–7769. [Google Scholar] [CrossRef]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Keasler, V.V.; Hodgson, A.J.; Madden, C.R.; Slagle, B.L. Enhancement of Hepatitis B Virus Replication by the Regulatory X Protein In Vitro and In Vivo. J. Virol. 2006, 81, 2656–2662. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, H.; Lee, S.Y.; Kim, S.S.; Jang, K.L. Hepatitis B Virus X Protein Stimulates Virus Replication Via DNA Methylation of the C-1619 in Covalently Closed Circular DNA. Mol. Cells 2018, 42, 67–78. [Google Scholar]
- Gailhouste, L.; Liew, L.C.; Yasukawa, K.; Hatada, I.; Tanaka, Y.; Nakagama, H.; Ochiya, T. Differentiation Therapy by Epigenetic Reconditioning Exerts Antitumor Effects on Liver Cancer Cells. Mol. Ther. 2018, 26, 1840–1854. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, E.; Ma, Z.; Pei, R.; Jiang, M.; Schlaak, J.F.; Roggendorf, M.; Lu, M. Modulation of hepatitis B virus replication and hepatocyte differentiation by MicroRNA-1. Hepatology 2011, 53, 1476–1485. [Google Scholar] [CrossRef]
- Hui, K.; Chiang, A.K.S. Suberoylanilide hydroxamic acid induces viral lytic cycle in Epstein-Barr virus-positive epithelial malignancies and mediates enhanced cell death. Int. J. Cancer 2010, 126, 2479–2489. [Google Scholar] [CrossRef]
- Hui, K.; Ho, D.N.; Tsang, C.; Middeldorp, J.M.; Tsao, G.S.; Chiang, A.K.S. Activation of lytic cycle of Epstein-Barr virus by suberoylanilide hydroxamic acid leads to apoptosis and tumor growth suppression of nasopharyngeal carcinoma. Int. J. Cancer 2012, 131, 1930–1940. [Google Scholar] [CrossRef]
- Hui, K.F.; Leung, Y.Y.; Yeung, P.L.; Middeldorp, J.M.; Chiang, A.K.S. Combination of SAHA and bortezomib up-regulates CDKN2A and CDKN1A and induces apoptosis of Epstein-Barr virus-positive Wp-restricted Burkitt lymphoma and lymphoblastoid cell lines. Br. J. Haematol. 2014, 167, 639–650. [Google Scholar] [CrossRef]
- Lemoine, M.; Derenzini, E.; Buglio, D.; Medeiros, L.J.; Davis, R.E.; Zhang, J.; Ji, Y.; Younes, A. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood 2012, 119, 4017–4025. [Google Scholar] [CrossRef]
- Maly, J.J.; Christian, B.A.; Zhu, X.; Wei, L.; Sexton, J.L.; Jaglowski, S.M.; Devine, S.M.; Fehniger, T.A.; Wagner-Johnston, N.D.; Phelps, M.A.; et al. A Phase I/II Trial of Panobinostat in Combination with Lenalidomide in Patients with Relapsed or Refractory Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, 347–353. [Google Scholar] [CrossRef]
- Clozel, T.; Yang, S.; Elstrom, R.L.; Tam, W.; Martin, P.; Kormaksson, M.; Banerjee, S.; VasanthaKumar, A.; Čuljković, B.; Scott, D.W.; et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B-cell lymphoma. Cancer Discov. 2013, 3, 1002–1019. [Google Scholar] [CrossRef]

| Latent Transcripts | Latency Program | Functions | B Cell Lymphoma |
|---|---|---|---|
| EBNA2 | III | Major EBV-encoded transcriptional activator, inducing gene transcription, such as cMyc. | Diffuse large B cell lymphomas (DLBCLs), Immunoblastic lymphomas |
| EBNA-LP | III | Acts as a co-transactivator of EBNA-2 expression by deregulating HDAC (HDAC4 and HDAC5) activities. | Diffuse large B cell lymphomas (DLBCLs), Immunoblastic lymphomas |
| EBNA3A/C | III | BCL2L11 promoter repression through CpG-methylation and recruiting PRC2 complex, H3K27me3 heterochromatic mark. Represses CDKN2A through recruiting CtBP, depositing H3K27me3. Transcriptional regulation through interacting with several HATs and HDACs. Inhibits CDKN2B transcriptions through induction of H3K27me3 heterochromatic mark. | Diffuse large B cell lymphomas (DLBCLs), Immunoblastic lymphomas |
| LMP1 | III/II/I/0 | Interacts with the DNA methyltransferase DNMT3B, regulating cellular apoptosis by elevating antiapoptotic Bcl2 expression Induces DNMT1, which is involved in the JNK-AP-1 pathway | Diffuse large B cell lymphomas (DLBCLs), Immunoblastic lymphoma, Hodgkin lymphoma, Burkitt lymphoma |
| LMP2A | III/II/I/0 | Induces DNMT1, which is involved in the JNK-AP-1 pathway. | Diffuse large B cell lymphomas (DLBCLs), Immunoblastic lymphoma, Hodgkin lymphoma, Burkitt lymphoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, A. Epstein–Barr Virus Promotes B Cell Lymphomas by Manipulating the Host Epigenetic Machinery. Cancers 2020, 12, 3037. https://doi.org/10.3390/cancers12103037
Di Pietro A. Epstein–Barr Virus Promotes B Cell Lymphomas by Manipulating the Host Epigenetic Machinery. Cancers. 2020; 12(10):3037. https://doi.org/10.3390/cancers12103037
Chicago/Turabian StyleDi Pietro, Andrea. 2020. "Epstein–Barr Virus Promotes B Cell Lymphomas by Manipulating the Host Epigenetic Machinery" Cancers 12, no. 10: 3037. https://doi.org/10.3390/cancers12103037
APA StyleDi Pietro, A. (2020). Epstein–Barr Virus Promotes B Cell Lymphomas by Manipulating the Host Epigenetic Machinery. Cancers, 12(10), 3037. https://doi.org/10.3390/cancers12103037





