Glioma Stem-Like Cells Can Be Targeted in Boron Neutron Capture Therapy with Boronophenylalanine
Simple Summary
Abstract
1. Introduction
2. Results
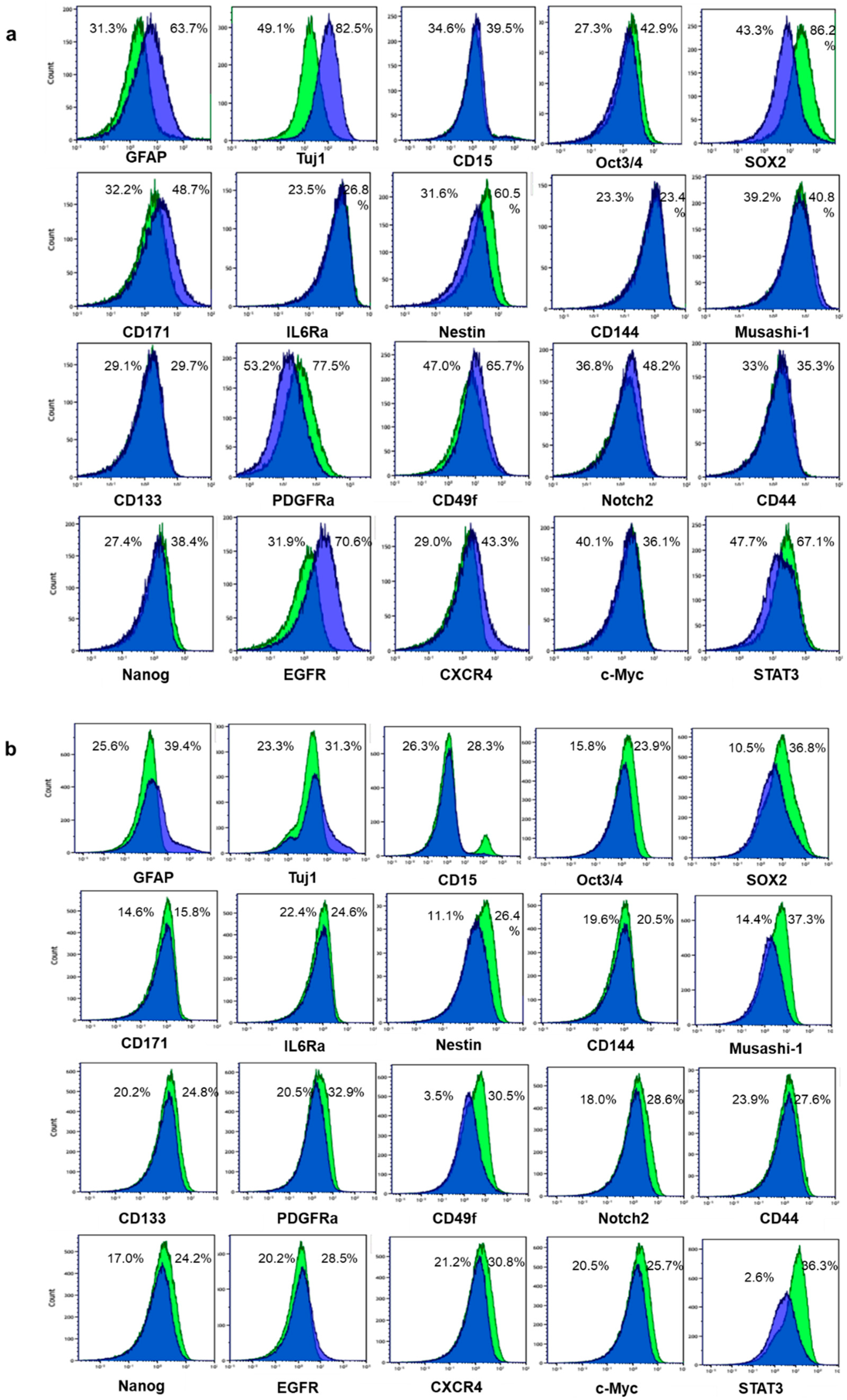
2.1. Differentiation Was Induced to Patient-Derived GSLCs by Fetal Bovine Serum
2.2. Larger Percentages of GSLCs Take up BPA Compared with Differentiated Cells
2.3. Larger Percentages of GSLCs Express CD98 Compared with Differentiated Cells
2.4. Some Stem Markers Have a Stronger Impact on the Uptake of BPA in GSLCs than Proliferation Markers
2.5. GSLCs Take up BPA at Higher Rates than Differentiated Cells in Tumors of Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. BPA Exposure
4.3. Multiparameter Mass Cytometry
4.4. Experimental Orthotopic Tumor Model
4.5. Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide Versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Miyatake, S.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival Benefit of Boron Neutron Capture Therapy for Recurrent Malignant Gliomas. J. Neurooncol. 2009, 91, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron Delivery Agents for Neutron Capture Therapy of Cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Wittig, A.; Sauerwein, W.A.; Coderre, J.A. Mechanisms of Transport of P-borono-phenylalanine through the Cell Membrane in vitro. Radiat. Res. 2000, 153, 173–180. [Google Scholar] [CrossRef]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.A.; Grossman, S.A.; Fisher, J.D.; Shaw, E.G. Prognostic Factors for Survival in Adult Patients with Recurrent Glioma Enrolled Onto the New Approaches to Brain Tumor Therapy CNS Consortium Phase I and II Clinical Trials. J. Clin. Oncol. 2007, 25, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Barth, R.F.; Miyatake, S.I.; Kawabata, S.; Suzuki, M.; Ono, K.; Lehman, N.L. Cerebrospinal Fluid Dissemination of High-Grade Gliomas Following Boron Neutron Capture Therapy Occurs More Frequently in the Small Cell Subtype of IDH1(R132H) Mutation-Negative Glioblastoma. J. Neurooncol. 2017, 133, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Coderre, J.A.; Glass, J.D.; Fairchild, R.D.; Roy, U.; Cohen, S.; Fand, Z. Selective Targeting of Boronophenylalanine to Melanoma in BALB/c Mice for Neutron Capture Therapy. Cancer Res. 1987, 47, 6377–6383. [Google Scholar] [PubMed]
- Ono, K.; Masunaga, S.-I.; Kinashi, Y.; Takagaki, M.; Akaboshi, M.; Kobayashi, T.; Akuta, K. Radiobiological Evidence Suggesting Heterogeneous Microdistribution of Boron compounds in Tumours: Its Relation to Quiescent Cell Population and Tumor Cure in Neutron Capture Therapy. Int. J. Oncol. Biol. Phys. 1996, 34, 1081–1086. [Google Scholar] [CrossRef]
- Chandra, S.; Tjarks, W.; Lorey, D.R., II; Barth, R.F. Quantitative Subcellular Imaging Of Boron Compounds In Individual Mitotic And Interphase Human Glioblastoma Cells With Imaging Secondary Ion Mass Spectrometry. J. Microsc. 2008, 229, 92–103. [Google Scholar] [CrossRef]
- Detta, A.; Cruickshank, G.S. L-Amino Acid Transporter-1 and Boronophenylalanine-Based Boron Neutron Capture Therapy of Human Brain Tumors. Cancer Res. 2009, 69, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell. Stem Cell. 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Gulaia, V.; Kumeiko, V.; Shved, N.; Cicinskas, E.; Rybtsov, S.; Ruzov, A.; Kagansky, A. Molecular Mechanisms Governing the Stem Cell’s Fate in Brain Cancer: Factors of Stemness and Quiescence. Front. Cell Neurosci. 2018, 12, 388. [Google Scholar] [CrossRef]
- Ying, M.; Wang, S.; Sang, Y.; Sun, P.; Lal, B.; Goodwin, C.R.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Laterra, J.; Xia, S. Regulation of Glioblastoma Stem Cells by Retinoic Acid: Role for Notch Pathway Inhibition. Oncogene 2011, 30, 3454–3467. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.L.; Paillaud, E.; Fages, C.; Rochette-Egly, C.; Plassat, J.L.; Jouault, H.; Perzelova, A.; Tardy, M. Effects of a Novel Synthetic Retinoid on Malignant Glioma in Vitro: Inhibition of Cell Proliferation, Induction of Apoptosis and Differentiation. Eur. J. Cancer 2001, 37, 520–530. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, Y.; Xie, X.; Chen, G.; Li, B.; Wei, Y.; Chen, J.; Huang, Q.; Du, Z. Selective Uptake of Boronophenylalanine by Glioma Stem/Progenitor Cells. Appl. Radiat. Isot. 2012, 70, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Z.; Li, B.; Chen, G.; Xie, X.; Wei, Y.; Wu, J.; Zhou, Y.; Du, Z. Boron neutron capture therapy induces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiat. Oncol. 2013, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Oriuchi, N.; Takahashi, T.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Murakami, H.; Shukuya, T.; Kenmotsu, H.; Naito, T.; et al. LAT1 Expression is Closely Associated with Hypoxic Markers and mTOR in Resected Non-Small Cell Lung Cancer. Am. J. Transl. Res. 2011, 3, 468–478. [Google Scholar]
- Kwan, K.Y.; Shen, J.; Corey, D.P. C-MYC Transcriptionally Amplifies SOX2 Target Genes to Regulate Self-Renewal in Multipotent Otic Progenitor Cells. Stem Cell Rep. 2015, 4, 47–60. [Google Scholar] [CrossRef]
- Hayashi, K.; Jutabha, P.; Endou, H.; Anzai, N. C-Myc is Crucial for the Expression of LAT1 in MIA Paca-2 Human Pancreatic Cancer Cells. Oncol. Rep. 2012, 28, 862–866. [Google Scholar] [CrossRef]
- Yuan, H.; Corbi, N.; Basilico, C.; Dailey, L. Developmental-Specific Activity of the FGF-4 Enhancer Requires the Synergistic Action of SOX2 and Oct-3. Genes Dev. 1995, 9, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, M.; Fukushima, A.; Okuda, A.; Muramatsu, M. The Gene for the Embryonic Stem Cell Coactivator UTF1 Carries a Regulatory Element which Selectively Interacts with a Complex Composed of Oct-3/4 and SOX2. Mol. Cell. Biol. 1999, 19, 5453–5465. [Google Scholar] [CrossRef]
- Tokuzawa, Y.; Kaiho, E.; Maruyama, M.; Takahashi, K.; Mitsui, K.; Maeda, M.; Niwa, H.; Yamanaka, S. Fbx15 is a Novel Target of Oct3/4 but is Dispensable for Embryonic Stem Cell Self-Renewal and Mouse Development. Mol. Cell. Biol. 2003, 23, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Martin-Santos, A.; Loubiere, L.S.; Gonzalez, A.M.; Stieger, B.; Logan, A.; McCabe, C.J.; Franklyn, J.A.; Kilby, M.D. The Expression of Thyroid Hormone Transporters in the Human Fetal Cerebral Cortex during Early Development and in N-Tera-2 Neurodifferentiation. J. Physiol. 2011, 589, 2827–2845. [Google Scholar] [CrossRef]
- Grzes, K.M.; Swamy, M.; Hukelmann, J.L.; Emslie, E.; Sinclair, L.V.; Cantrell, D.A. Control of Amino Acid Transport Coordinates Metabolic Reprogramming in T-Cell Malignancy. Leukemia 2017, 31, 2771–2779. [Google Scholar] [CrossRef]
- Yin, Y.; Qiu, S.; Peng, Y. Functional Roles of Enhancer of Zeste Homolog 2 in Gliomas. Gene 2016, 576, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.G.; Ryskin, M.; Barsotti, A.M.; Golas, J.; Shi, C.; Miranda, M.; Hosselet, C.; Lemon, L.; Lucas, J.; Karnoub, M.; et al. Reciprocal Regulation of Amino Acid Import and Epigenetic State through Lat1 and EZH2. EMBO J. 2015, 34, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.; Konuma, T.; Lytle, N.K.; Kwon, Y.H.; Ablack, J.N.; Cantor, J.M.; Rizzieri, D.; Chuah, C.; Oehler, V.G.; Broome, E.H.; et al. CD98-Mediated Adhesive Signaling Enables the Establishment and Propagation of Acute Myelogenous Leukemia. Cancer Cell 2016, 30, 792–805. [Google Scholar] [CrossRef]
- Aderetti, D.A.; Hira, V.V.V.; Molenaar, R.J.; van Noorden, C.J.F. The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 346–354. [Google Scholar] [CrossRef]
- Niles, R.M. Signaling Pathways in Retinoid Chemoprevention and Treatment of Cancer. Mutat. Res. 2004, 555, 97–105. [Google Scholar] [CrossRef]
- Soprano, D.R.; Teets, B.W.; Soprano, K.J. Role of Retinoic Acid in the Differentiation of Embryonal Carcinoma and Embryonic Stem Cells. Vitam. Horm. 2007, 75, 69–95. [Google Scholar] [CrossRef]
- de la Iglesia, N.; Puram, S.V.; Bonni, A. STAT3 Regulation of Glioblastoma Pathogenesis. Curr. Mol. Med. 2009, 9, 580–590. [Google Scholar] [CrossRef]
- Kondo, N.; Sakurai, Y.; Hirota, Y.; Tanaka, H.; Watanabe, T.; Nakagawa, Y.; Narabayashi, M.; Kinashi, Y.; Miyatake, S.; Hasegawa, M.; et al. DNA Damage Induced by Boron Neutron Capture Therapy is Partially Repaired by DNA Ligase IV. Radiat. Environ. Biophys. 2016, 55, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Fujimoto, T.; Sudo, T.; Fujita, I.; Imabori, M.; Moritake, H.; Sugimoto, T.; Sakuma, Y.; Takeuchi, T.; Kawabata, S.; et al. Boron neutron capture therapy for clear cell sarcoma (CCS): Biodistribution study of p-borono-l-phenylalanine in CCS-bearing animal models. Appl. Radiat. Isot. 2011, 69, 1721–1724. [Google Scholar] [CrossRef]
- Futamura, G.; Kawabata, S.; Nonoguchi, N.; Hiramatsu, R.; Toho, T.; Tanaka, H.; Masunaga, S.I.; Hattori, Y.; Kirihata, M.; Ono, K.; et al. Evaluation of a novel sodium borocaptate-containing unnatural amino acid as a boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat. Oncol. 2017, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.; Synowitz, M. CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol. 2014, 128, 347–362. [Google Scholar] [CrossRef] [PubMed]


| BPA+ | No. 1 Stem | No. 1 DC | No. 2 Stem | No. 2 DC |
|---|---|---|---|---|
| Total | 56.0 | 25.7 | 35.8 | 21.5 |
| Differentiation + | ||||
| GFAP | 20.5 | 18.4 | 10.5 | 8.6 |
| Tuj1 | 21.2 | 24.9 | 11.1 | 9.4 |
| Stem + | ||||
| Oct3/4 | 29.4 | 9.3 | 11.4 | 4.9 |
| CD15 | 19.2 | 10.6 | 11.0 | 6.1 |
| CD171 | 21.3 | 13.9 | 8.0 | 4.2 |
| IL6Ra | 15.1 | 6.4 | 8.1 | 4.1 |
| SOX2 | 51.9 | 15.2 | 15.5 | 2.9 |
| Nestin | 35.0 | 8.3 | 12.8 | 3.6 |
| CD144 | 11.6 | 5.3 | 6.8 | 3.9 |
| Musashi-1 | 28.1 | 14.5 | 19.0 | 8.2 |
| CD133 | 18.8 | 9.0 | 11.6 | 5.8 |
| PDGFRα | 44.9 | 15.6 | 12.0 | 4.6 |
| Notch2 | 18.9 | 12.1 | 11.8 | 4.6 |
| CD44 | 19.1 | 9.9 | 11.6 | 5.9 |
| Nanog | 20.6 | 6.9 | 11.5 | 5.0 |
| STAT3 | 44.9 | 17.2 | 16.2 | 1.1 |
| CXCR4 | 18.7 | 12.2 | 13.5 | 5.7 |
| c-Myc | 27.1 | 11.6 | 13.2 | 5.7 |
| CD49f | 29.8 | 18.7 | 15.0 | 2.0 |
| CD98+ | No. 1 Stem | No. 1 DC | No. 2 Stem | No. 2 DC |
|---|---|---|---|---|
| Stem+ | ||||
| Oct3/4 | 29.2 | 13.3 | 15.6 | 3.6 |
| CD15 | 19.3 | 14.8 | 16.1 | 4.9 |
| CD171 | 23.0 | 22.0 | 11.1 | 3.1 |
| IL6Ra | 15.1 | 9.1 | 11.8 | 3.5 |
| SOX2 | 51.1 | 20.4 | 20.9 | 2.2 |
| Nestin | 34.8 | 11.8 | 18.3 | 3.0 |
| CD144 | 11.7 | 7.8 | 9.3 | 3.0 |
| Musashi-1 | 27.5 | 20.0 | 21.4 | 2.9 |
| CD133 | 19.2 | 12.9 | 16.5 | 4.7 |
| PDGFRa | 47.7 | 23.2 | 18.2 | 3.9 |
| Notch2 | 18.3 | 16.6 | 17.2 | 3.5 |
| Nanog | 20.3 | 9.5 | 15.9 | 3.8 |
| STAT3 | 43.5 | 23.7 | 23.6 | 0.8 |
| CXCR4 | 21.1 | 22.8 | 19.7 | 4.4 |
| c-Myc | 27.7 | 17.5 | 18.4 | 4.6 |
| CD49f | 29.4 | 26.5 | 22.8 | 1.6 |
| Stem or Differentiated Marker | Co-Expression of BPA (%) |
|---|---|
| SOX2 | 100 |
| Nestin | 100 |
| GFAP | 36.9 (±18.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, N.; Hikida, M.; Nakada, M.; Sakurai, Y.; Hirata, E.; Takeno, S.; Suzuki, M. Glioma Stem-Like Cells Can Be Targeted in Boron Neutron Capture Therapy with Boronophenylalanine. Cancers 2020, 12, 3040. https://doi.org/10.3390/cancers12103040
Kondo N, Hikida M, Nakada M, Sakurai Y, Hirata E, Takeno S, Suzuki M. Glioma Stem-Like Cells Can Be Targeted in Boron Neutron Capture Therapy with Boronophenylalanine. Cancers. 2020; 12(10):3040. https://doi.org/10.3390/cancers12103040
Chicago/Turabian StyleKondo, Natsuko, Masaki Hikida, Mitsutoshi Nakada, Yoshinori Sakurai, Eishu Hirata, Satoshi Takeno, and Minoru Suzuki. 2020. "Glioma Stem-Like Cells Can Be Targeted in Boron Neutron Capture Therapy with Boronophenylalanine" Cancers 12, no. 10: 3040. https://doi.org/10.3390/cancers12103040
APA StyleKondo, N., Hikida, M., Nakada, M., Sakurai, Y., Hirata, E., Takeno, S., & Suzuki, M. (2020). Glioma Stem-Like Cells Can Be Targeted in Boron Neutron Capture Therapy with Boronophenylalanine. Cancers, 12(10), 3040. https://doi.org/10.3390/cancers12103040





