Randomized Phase II Study with Cetuximab in Combination with 5-FU and Cisplatin or Carboplatin Vs. Cetuximab in Combination with Paclitaxel and Carboplatin for Treatment of Patients with Relapsed or Metastatic Squamous Cell Carcinoma of the Head and Neck (CETMET Trial)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
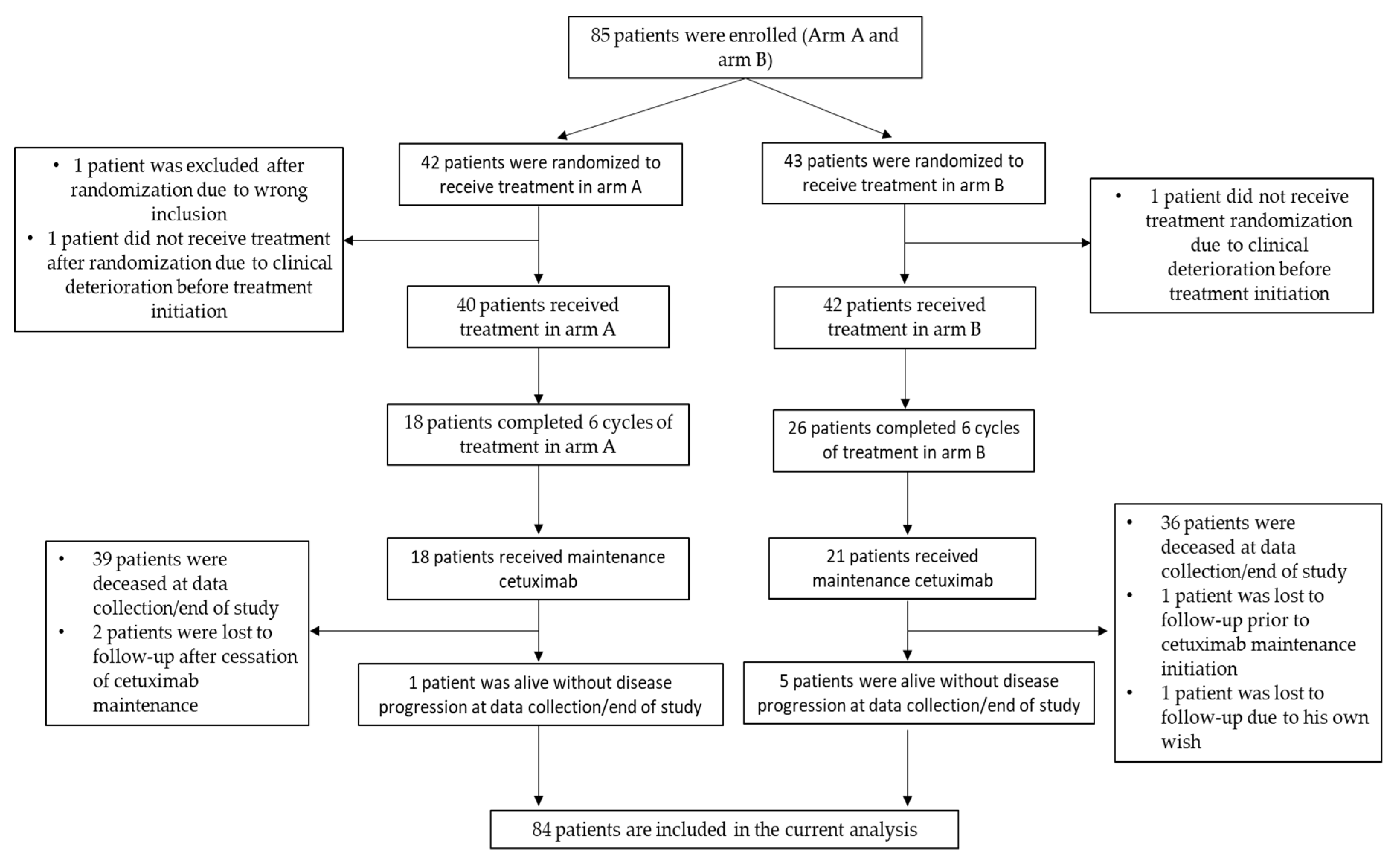
2.1. Patient Characteristics
2.2. Response and Survival Analyses
2.3. Toxicity
3. Discussion
4. Materials and Methods
4.1. Patient Eligibility
4.2. Study Design
4.3. Assessment
4.4. Statistical Analysis
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burtness, B.; Goldwasser, M.A.; Flood, W.; Mattar, B.; Forastiere, A.A.; Eastern Cooperative Oncology. Phase III Randomized Trial of Cisplatin Plus Placebo Compared With Cisplatin Plus Cetuximab in Metastatic/Recurrent Head and Neck Cancer: An Eastern Cooperative Oncology Group Study. J. Clin. Oncol. 2005, 23, 8646–8654. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D. Chemotherapy Options for Patients With Metastatic or Recurrent Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2006, 24, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Lingen, M.W.; Vokes, E.E. The Expanding Role of Systemic Therapy in Head and Neck Cancer. J. Clin. Oncol. 2004, 22, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Stohlmacher-Williams, J.; Davidenko, I.; Licitra, L.; Winquist, E.; Villanueva, C.; Foa, P.; Rottey, S.; Skladowski, K.; Tahara, M.; et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013, 14, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Forastiere, A.A.; Metch, B.; Schuller, D.E.; Ensley, J.F.; Hutchins, L.F.; Triozzi, P.; Kish, J.A.; McClure, S.; Vonfeldt, E.; Williamson, S.K. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: A Southwest Oncology Group study. J. Clin. Oncol. 1992, 10, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Li, Y.; Murphy, B.; Hussain, M.H.; DeConti, R.C.; Ensley, J.; Forastiere, A.A.; Eastern Cooperative Oncology. Randomized Phase III Evaluation of Cisplatin Plus Fluorouracil Versus Cisplatin Plus Paclitaxel in Advanced Head and Neck Cancer (E1395): An Intergroup Trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2005, 23, 3562–3567. [Google Scholar] [CrossRef] [PubMed]
- Lala, M.; Chirovsky, D.; Cheng, J.D.; Mayawala, K. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): A systematic literature review. Oral Oncol. 2018, 84, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Lefebvre, J.L.; Vermorken, J.B. Cetuximab in the management of locoregionally advanced head and neck cancer: Expanding the treatment options? Eur. J. Cancer 2010, 46, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigay, J.; Fayette, J.; Mesia, R.; Lafond, C.; Saada-Bouzid, E.; Geoffrois, L.; Martin, L.; Cupissol, D.; Capitain, O.; Castanie, H.; et al. TPExtreme randomized trial: TPEx versus Extreme regimen in 1st line recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37, 6002. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; De Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Lynggaard, C.D.; Therkildsen, M.H.; Kristensen, C.A.; Specht, L. The EXTREME regimen for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): Treatment outcome in a single institution cohort. Acta Oncol. 2015, 54, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulieres, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Carson, W.E., 3rd; Shapiro, C.L.; Crespin, T.R.; Thornton, L.M.; Andersen, B.L. Cellular Immunity in Breast Cancer Patients Completing Taxane Treatment. Clin. Cancer Res. 2004, 10, 3401–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]

| Arm A (N = 42) | Arm B (N = 43) | p Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | |||||
| Male | 33 | 78.6 | 26 | 60.5 | |
| Female | 9 | 21.4 | 17 | 39.5 | 0.07 |
| Localization | |||||
| Hypopharynx | 4 | 9.5 | 4 | 9.3 | |
| Larynx | 2 | 4.8 | 5 | 11.6 | |
| Oral cavity | 30 | 71.4 | 29 | 67.4 | |
| Oropharynx | 5 | 11.9 | 5 | 11.6 | |
| Missing | 1 | 2.4 | 0 | 0 | 0.734 |
| T stage | |||||
| Τ0 | 2 | 4.8 | 0 | 0 | |
| T1 | 3 | 7.1 | 4 | 9.3 | |
| T2 | 17 | 40.5 | 17 | 39.5 | |
| T3 | 12 | 28.6 | 10 | 23.3 | |
| T4 | 7 | 16.7 | 12 | 27.9 | 0.463 |
| N stage | |||||
| N0 | 9 | 21.4 | 17 | 39.5 | |
| N1 | 6 | 14.3 | 6 | 14 | |
| N2 | 23 | 54.8 | 18 | 41.9 | |
| N3 | 1 | 2.4 | 0 | 0 | |
| Nx | 2 | 4.8 | 2 | 4.7 | 0.409 |
| M stage | |||||
| M0 | 41 | 97.6 | 43 | 100 | |
| M1 | 1 | 2.4 | 0 | 0 | 0.35 |
| HPV status | |||||
| Positive | 11 | 26.2 | 15 | 34.9 | |
| Negative | 24 | 57.1 | 27 | 62.8 | |
| Missing | 7 | 16.7 | 1 | 2.3 | 0.071 |
| Smoking status | |||||
| Former smoker | 28 | 66.7 | 27 | 62.8 | |
| Non-smoker | 9 | 21.4 | 6 | 14 | |
| Smoker | 5 | 11.9 | 9 | 20.9 | |
| Not known | 0 | 0 | 1 | 2.3 | 0.432 |
| Age | |||||
| Mean, stdv | 59.13 | 10.12 | 59.10 | 7.27 | 0.989 |
| Recurrent/metastatic disease | |||||
| Local recurrence | 12 | 36.4 | 21 | 63.6 | |
| Metastatic | 14 | 56 | 11 | 44 | |
| Recurrent and metastatic | 16 | 59.3 | 11 | 40.7 | 0.155 |
| WHO performance Status | |||||
| 0 | 14 | 34.1 | 15 | 34.9 | |
| 1 | 27 | 65.9 | 27 | 62.8 | |
| 2 | 0 | 0 | 1 | 2.3 | 0.61 |
| Prior treatment | |||||
| Neoadjuvant chemotherapy | 5 | 12 | 5 | 11.6 | |
| Radiotherapy | 39 | 93 | 41 | 95 | |
| Chemoradiotherapy | 26 | 62 | 18 | 42 | |
| Surgery | 20 | 48 | 23 | 53.5 | * |
| OS | PFS | TTF | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Gender | ||||||
| Male | 1.00 | ref. | 1.00 | ref. | 1.00 | ref. |
| Female | 1.09 | 0.63–1.89 | 0.99 | 0.58–1.68 | 1.03 | 0.61–1.74 |
| Treatment | ||||||
| Arm A | 1.00 | ref. | 1.00 | ref. | 1.00 | ref. |
| Arm B | 0.70 | 0.42–1.15 | 0.65 | 0.40–1.05 | 0.62 | 0.39–1.01 |
| Adverse Events | Arm A (N = 27) | Arm B (N = 24) | Total |
|---|---|---|---|
| Anemia | 3 | 1 | 4 |
| Anorexia | 3 | 1 | 4 |
| Bleeding from mouth and nose | 1 | 1 | 2 |
| Colon perforation | 1 | 0 | 1 |
| Dysphagia | 2 | 2 | 4 |
| Fatigue | 6 | 4 | 10 |
| Febrile neutropenia | 6 | 3 | 9 |
| Gingivostomatitis | 1 | 0 | 1 |
| Heart failure | 1 | 0 | 1 |
| Hypokalemia | 1 | 2 | 3 |
| Infections | 9 | 3 | 12 |
| Leukopenia | 0 | 4 | 4 |
| Mucositis oral | 3 | 0 | 3 |
| Nausea | 4 | 2 | 6 |
| Neuropathy—sensory | 0 | 2 | 2 |
| Pain | 1 | 0 | 1 |
| Rash/acne | 0 | 1 | 1 |
| Syncope | 1 | 0 | 1 |
| Thromboembolic events | 3 | 0 | 3 |
| Thrombocytopenia | 2 | 2 | 4 |
| Ulcerations on fingers and nails | 1 | 0 | 1 |
| Vomiting | 1 | 1 | 2 |
| Diarrhea | 1 | 0 | 1 |
| Hand and foot syndrome | 0 | 1 | 1 |
| Hypomagnesemia | 0 | 1 | 1 |
| Motoric neuropathy | 0 | 1 | 1 |
| Sepsis | 0 | 1 | 1 |
| Syncope | 1 | 0 | 1 |
| Weight loss | 1 | 0 | 1 |
| Total events | 61 | 41 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakonas, G.; Specht, L.; Kristensen, C.A.; Moreno, M.H.C.; Haugen Cange, H.; Soderstrom, K.; Friesland, S. Randomized Phase II Study with Cetuximab in Combination with 5-FU and Cisplatin or Carboplatin Vs. Cetuximab in Combination with Paclitaxel and Carboplatin for Treatment of Patients with Relapsed or Metastatic Squamous Cell Carcinoma of the Head and Neck (CETMET Trial). Cancers 2020, 12, 3110. https://doi.org/10.3390/cancers12113110
Tsakonas G, Specht L, Kristensen CA, Moreno MHC, Haugen Cange H, Soderstrom K, Friesland S. Randomized Phase II Study with Cetuximab in Combination with 5-FU and Cisplatin or Carboplatin Vs. Cetuximab in Combination with Paclitaxel and Carboplatin for Treatment of Patients with Relapsed or Metastatic Squamous Cell Carcinoma of the Head and Neck (CETMET Trial). Cancers. 2020; 12(11):3110. https://doi.org/10.3390/cancers12113110
Chicago/Turabian StyleTsakonas, Georgios, Lena Specht, Claus Andrup Kristensen, Maria Herlestam Calero Moreno, Hedda Haugen Cange, Karin Soderstrom, and Signe Friesland. 2020. "Randomized Phase II Study with Cetuximab in Combination with 5-FU and Cisplatin or Carboplatin Vs. Cetuximab in Combination with Paclitaxel and Carboplatin for Treatment of Patients with Relapsed or Metastatic Squamous Cell Carcinoma of the Head and Neck (CETMET Trial)" Cancers 12, no. 11: 3110. https://doi.org/10.3390/cancers12113110





