Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations
Abstract
:Simple Summary
Abstract
1. Introduction
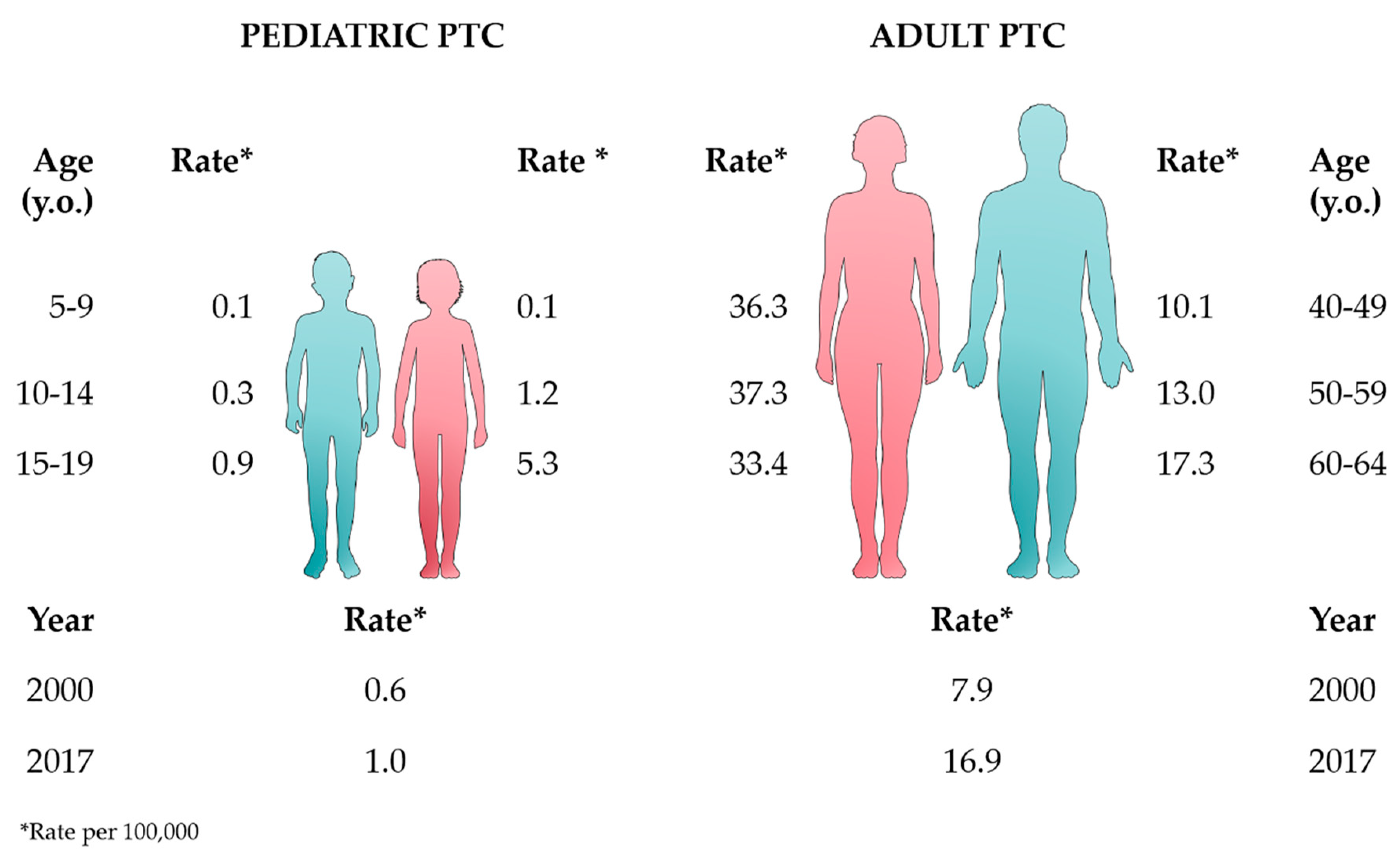
2. Epidemiology and Pathogenesis
3. Clinical Features, Prognosis, and Treatment
4. Molecular Features
4.1. BRAF Alterations
4.2. RET/PTC Rearrangements
4.3. ETV6-NTRK3 Rearrangement
4.4. STRN-ALK Rearrangement
4.5. PAX8-PPARγ Rearrangement
4.6. RAS Mutations
5. Telomere-Related Genomic Instability and Nuclear Architecture
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [Green Version]
- Lise, M.; Franceschi, S.; Buzzoni, C.; Zambon, P.; Falcini, F.; Crocetti, E.; Serraino, D.; Iachetta, F.; Zanetti, R.; Vercelli, M.; et al. Changes in the Incidence of Thyroid Cancer Between 1991 and 2005 in Italy: A Geographical Analysis. Thyroid 2012, 22, 27–34. [Google Scholar] [CrossRef]
- Keinan-Boker, L.; Silverman, B.G. Trends of Thyroid Cancer in Israel: 1980–2012. Rambam Maimonides Med. J. 2016, 7, e0001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubina, A.; Cohen, O.; Barchana, M.; Liphshiz, I.; Vered, I.; Sadetzki, S.; Karasik, A. Time trends of incidence rates of thyroid cancer in Israel: What might explain the sharp increase. Thyroid 2006, 16, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W. Increasing incidence of thyroid cancer in Shanghai, China, 1983–2007. Asia-Pac. J. Public Health 2015, 27, NP223–NP229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.S.; Kim, H.J.; Welch, G. Korea’s Thyroid-Cancer “Epidemic”—Screening and Overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Veiga, L.H.S.; Neta, G.; Aschebrook-Kilfoy, B.; Ron, E.; Devesa, S.S. Thyroid cancer incidence patterns in Sao Paulo, Brazil, and the U.S. SEER program, 1997–2008. Thyroid 2013, 23, 748–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, M.S.; Soerjomataram, I.; Forman, D. Thyroid cancer burden in Central and South America. Cancer Epidemiol. 2016, 44, S150–S157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Kent, W.D.T.; Hall, S.F.; Isotalo, P.A.; Houlden, R.L.; George, R.L.; Groome, P.A. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 2007, 177, 1357–1361. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Semenciw, R.; Ugnat, A.M.; Mao, Y. Increasing thyroid cancer incidence in Canada, 1970–1996: Time trends and age-period-cohort effects. Br. J. Cancer 2001, 85, 1335–1339. [Google Scholar] [CrossRef]
- Uhry, Z.; Colonna, M.; Remontet, L.; Grosclaude, P.; Carré, N.; Couris, C.M.; Velten, M. Estimating infra-national and national thyroid cancer incidence in France from cancer registries data and national hospital discharge database. Eur. J. Epidemiol. 2007, 22, 607–614. [Google Scholar] [CrossRef]
- Colonna, M.; Uhry, Z.; Guizard, A.V.; Delafosse, P.; Schvartz, C.; Belot, A.; Grosclaude, P. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015, 39, 511–518. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Weir, J.; Stockton, D.L.; Brewster, D.H.; Sandeep, T.C.; Strachan, M.W.J. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin. Endocrinol. 2005, 62, 156–162. [Google Scholar] [CrossRef]
- Smailyte, G.; Miseikyte-Kaubriene, E.; Kurtinaitis, J. Increasing thyroid cancer incidence in Lithuania in 1978–2003. BMC Cancer 2006, 6. [Google Scholar] [CrossRef] [Green Version]
- Pandeya, N.; McLeod, D.S.; Balasubramaniam, K.; Baade, P.D.; Youl, P.H.; Bain, C.J.; Allison, R.; Jordan, S.J. Increasing thyroid cancer incidence in Queensland, Australia 1982–2008—True increase or overdiagnosis. Clin. Endocrinol. 2016, 84, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. Cancer Statistics Review, 1975–2017—SEER Statistics. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 13 July 2020).
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. SEER Cancer Statistics Review, 1975–2016. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 3 April 2020).
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef] [Green Version]
- Jarząb, B.; Handkiewicz-Junak, D.; Włoch, J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: A qualitative review. Endocr. Relat. Cancer 2005, 12, 773–803. [Google Scholar] [CrossRef]
- Hogan, A.; Zhuge, Y.; Perez, E.; Koniaris, L.; Lew, J.; Sola, J. The incidence of pediatric thyroid cancer is increasing and is higher in girls than in boys and may have an adverse outcome. Clin. Thyroidol. 2009, 21, 10–12. [Google Scholar]
- Vaisman, F.; Corbo, R.; Vaisman, M. Thyroid Carcinoma in Children and Adolescents—Systematic Review of the Literature. J. Thyroid Res. 2011, 2011, 845362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.-C.; Chen, V.W.; Steele, B.; Roffers, S.; Klotz, J.B.; Correa, C.N.; Carozza, S.E. Cancer incidence in adolescents and young adults in the United States, 1992–1997. J. Adolesc. Health 2003, 32, 405–415. [Google Scholar] [CrossRef]
- Cordioli, M.I.; Moraes, L.; Cury, A.N.; Cerutti, J.M. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr. Relat. Cancer 2015, 22, R311–R324. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, R.M.; Ball, D.W.; Byrd, D.; Dilawari, R.A.; Gerard, M.; Duh, Q.; Ehya, H.; Farrar, W.B.; Haddad, R.I.; Kandeel, F.; et al. Thyroid Carcinoma. J. Natl. Compr. Cancer Netw. 2010, 8, 1228–1274. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.M.; Young, J.; Prager, J.; Travers, S. Pediatric Thyroid Cancer. Adv. Pediatr. 2017, 64, 171–190. [Google Scholar] [CrossRef]
- Zimmerman, D.; Hay, I.D.; Gough, I.R.; Goellner, J.R.; Ryan, J.J.; Grant, C.S.; McConahey, W.M. Papillary thyroid carcinoma in children and adults: Long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 1988, 104, 1157–1166. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Karapanou, O.; Tzanela, M.; Vlassopoulou, B.; Kanaka-Gantenbein, C. Differentiated thyroid cancer in childhood: A literature update. Hormones 2017, 16, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Creo, A.; Alahdab, F.; Al Nofal, A.; Thomas, K.; Kolbe, A.; Pittock, S.T. Ultrasonography and the American Thyroid Association Ultrasound-Based Risk Stratification Tool: Utility in Pediatric and Adolescent Thyroid Nodules. Horm. Res. Paediatr. 2018, 90, 93–101. [Google Scholar] [CrossRef]
- Hay, I.D.; Gonzalez-Losada, T.; Reinalda, M.S.; Honetschlager, J.A.; Richards, M.L.; Thompson, G.B. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J. Surg. 2010, 34, 1192–1202. [Google Scholar] [CrossRef]
- Zaydfudim, V.; Feurer, I.D.; Griffin, M.R.; Phay, J.E. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008, 144, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.H.; Kim, J.R.; Jeong, H.C.; Lee, J.S.; Chang, E.S.; Kim, Y.H. Predictive factors of central lymph node metastasis in papillary thyroid carcinoma. Ann. Surg. Treat. Res. 2015, 88, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelczak, M.; David, R.; Franklin, B.; Kessler, M.; Lam, L.; Shah, B. Outcomes of children and adolescents with well-differentiated thyroid carcinoma and pulmonary metastases following 131I treatment: A systematic review. Thyroid 2010, 20, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Handkiewicz-Junak, D.; Wloch, J.; Roskosz, J.; Krajewska, J.; Kropinska, A.; Pomorski, L.; Kukulska, A.; Prokurat, A.; Wygoda, Z.; Jarzab, B. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J. Nucl. Med. 2007, 48, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.P.; Davies, L. Is there really an increased incidence of thyroid cancer? Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Vaccarella, S.; La Vecchia, C.; Bosetti, C.; Malvezzi, M.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer: An epidemic of disease or an epidemic of diagnosis? Int. J. Cancer 2015, 136, 2738–2739. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.G.; Tuttle, R.M.; Davies, L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol. Head Neck Surg. 2016. [Google Scholar] [CrossRef]
- Sholl, L.M.; Barletta, J.A.; Hornick, J.L. Radiation-associated neoplasia: Clinical, pathological and genomic correlates. Histopathology 2017, 70, 70–80. [Google Scholar] [CrossRef]
- Cléro, É.; Doyon, F.; Chungue, V.; Rachédi, F.; Boissin, J.-L.; Sebbag, J.; Shan, L.; Bost-Bezeaud, F.; Petitdidier, P.; Dewailly, É.; et al. Dietary Iodine and Thyroid Cancer Risk in French Polynesia: A Case—Control Study. Thyroid 2012, 22, 422–429. [Google Scholar] [CrossRef]
- Engeland, A.; Tretli, S.; Akslen, L.A.; Bjørge, T. Body size and thyroid cancer in two million Norwegian men and women. Br. J. Cancer 2006, 95, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2016. [Google Scholar] [CrossRef]
- Ron, E.; Lubin, J.H.; Shore, R.E.; Mabuchi, K.; Modan, B.; Pottern, L.M.; Schneider, A.B.; Tucker, M.A.; Boice, J.D. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat. Res. 1995, 141, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Sadetzki, S.; Chetrit, A.; Lubina, A.; Stovall, M.; Novikov, I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J. Clin. Endocrinol. Metab. 2006, 91, 4798–4804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldschmidt, H. Dermatologic Radiotherapy and Thyroid Cancer. Arch. Dermatol. 1977, 113, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Wijnen, M.; van den Heuvel-Eibrink, M.M.; Medici, M.; Peeters, R.P.; van der Lely, A.J.; Neggers, S.J. Risk factors for subsequent endocrine-related cancer in childhood cancer survivors. Endocr. Relat. Cancer 2016, 23, R299–R321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, T. Radiation Doses and Associated Risk from the Fukushima Nuclear Accident. Asia Pac. J. Public Health 2017, 29, 18S–28S. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Hayashi, K.; Scherb, H.; Efird, J.T. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine 2019, 98. [Google Scholar] [CrossRef] [Green Version]
- Toki, H.; Wada, T.; Manabe, Y.; Hirota, S.; Higuchi, T.; Tanihata, I.; Satoh, K.; Bando, M. Relationship between environmental radiation and radioactivity and childhood thyroid cancer found in Fukushima health management survey. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mitsutake, N.; Fukushima, T.; Matsuse, M.; Rogounovitch, T.; Saenko, V.; Uchino, S.; Ito, M.; Suzuki, K.; Suzuki, S.; Yamashita, S. BRAFV600E mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: A different oncogenic profile from Chernobyl. Sci. Rep. 2015, 5, 16976. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Suzuki, S.; Suzuki, S.; Shimura, H.; Saenko, V. Lessons from Fukushima: Latest Findings of Thyroid Cancer after the Fukushima Nuclear Power Plant Accident. Thyroid 2018, 28, 11–22. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Alkhafaji, D.; Tuli, M.; Al-Hindi, H.; Sadiq, B. Bin Comparison of differentiated thyroid cancer in children and adolescents (≤20 years) with young adults. Clin. Endocrinol. 2015, 84, 571–577. [Google Scholar] [CrossRef]
- Lee, Y.A.; Jung, H.W.; Kim, H.Y.; Choi, H.; Kim, H.-Y.; Hah, J.H.; Park, D.J.; Chung, J.-K.; Yang, S.W.; Shin, C.H.; et al. Pediatric Patients with Multifocal Papillary Thyroid Cancer Have Higher Recurrence Rates than Adult Patients: A Retrospective Analysis of a Large Pediatric Thyroid Cancer Cohort over 33 Years. J. Clin. Endocrinol. Metab. 2015, 100, 1619–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, I.D.; Johnson, T.R.; Kaggal, S.; Reinalda, M.S.; Iniguez-Ariza, N.M.; Grant, C.S.; Pittock, S.T.; Thompson, G.B. Papillary Thyroid Carcinoma (PTC) in Children and Adults: Comparison of Initial Presentation and Long-Term Postoperative Outcome in 4432 Patients Consecutively Treated at the Mayo Clinic during Eight Decades (1936–2015). World J. Surg. 2018, 42, 329–342. [Google Scholar] [CrossRef]
- Hogan, A.R.; Zhuge, Y.; Perez, E.A.; Koniaris, L.G.; Lew, J.I.; Sola, J.E. Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. J. Surg. Res. 2009, 156, 167–172. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.C.; Gaze, M.N.; Szychot, E.; Rozalén García, V.; Brain, C.; Dattani, M.; Spoudeas, H.; Hindmarsh, P.; Abdel-Aziz, T.E.; Bomanji, J.; et al. Treating papillary and follicular thyroid cancer in children and young people: Single UK-center experience between 2003 and 2018. J. Pediatr. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rah, C.S.; Kim, W.W.; Lee, Y.M.; Kim, W.G.; Song, D.E.; Chung, K.W.; Kim, S.C.; Hong, S.J.; Sung, T.Y. Recent Trends in the Clinicopathological Features of Thyroid Nodules in Pediatric Patients: A Single Tertiary Center Experience over 25 Years. Int. J. Endocrinol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr. Relat. Cancer 2006, 13, 427–453. [Google Scholar] [CrossRef]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The diagnosis and management of thyroid nodules a review. JAMA J. Am. Med. Assoc. 2018, 319, 919–924. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Lerner, J.; Goldfarb, M. Pediatric Thyroid Microcarcinoma. Ann. Surg. Oncol. 2015, 22, 4187–4192. [Google Scholar] [CrossRef]
- Bernier, M.O.; Withrow, D.R.; Berrington de Gonzalez, A.; Lam, C.J.K.; Linet, M.S.; Kitahara, C.M.; Shiels, M.S. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 2019, 125, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, S.; Perez, E.A.; Tashiro, J.; Lew, J.I.; Sola, J.E.; Hogan, A.R. Pediatric papillary thyroid carcinoma: Outcomes and survival predictors in 2504 surgical patients. Pediatr. Surg. Int. 2016, 32, 201–208. [Google Scholar] [CrossRef]
- Chow, S.M.; Law, S.C.; Mendenhall, W.M.; Au, S.K.; Yau, S.; Mang, O.; Lau, W.H. Differentiated thyroid carcinoma in childhood and adolescence-clinical course and role of radioiodine. Pediatr. Blood Cancer 2004, 42, 176–183. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, D.; Huang, Y.; Chen, S.; Zeng, W.; Zhou, L.; Zhou, W.; Wang, M.; Feng, H.; Wei, W.; et al. Factors associated with distant metastasis in pediatric thyroid cancer: Evaluation of the seer database. Endocr. Connect. 2019, 8, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dottorini, M.E.; Vignati, A.; Mazzucchelli, L.; Lomuscio, G.; Colombo, L. Differentiated thyroid carcinoma in children and adolescents: A 37-year experience in 85 patients. J. Nucl. Med. 1997, 38, 669–675. [Google Scholar] [PubMed]
- Kuo, S.-F.; Chao, T.-C.; Hsueh, C.; Chuang, W.-Y.; Yang, C.-H.; Lin, J.-D. Prognosis and Risk Stratification in Young Papillary Thyroid Carcinoma Patients. Endocr. J. 2008, 55, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaisman, F.; Bulzico, D.A.; Pessoa, C.H.; Bordallo, M.A.; de Mendonça, U.B.; Dias, F.L.; Coeli, C.M.; Corbo, R.; Vaisman, M. Prognostic factors of a good response to initial therapy in children and adolescents with differentiated thyroid cancer. Clinics 2011, 66, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, M.V.; Savva, N.N.; Krasko, O.V.; Zborovskaya, A.A.; Mankovskaya, S.V.; Schmid, K.W.; Demidchik, Y.E. Clinical and Pathologic Features of “Sporadic” Papillary Thyroid Carcinoma Registered in the Years 2005 to 2008 in Children and Adolescents of Belarus. Thyroid 2012, 22, 1016–1024. [Google Scholar] [CrossRef]
- Pires, B.P.; Alves, P.A.G.; Bordallo, M.A.; Bulzico, D.A.; Lopes, F.P.; Farias, T.; Dias, F.; Lima, R.A.; Santos Gisler, I.C.; Coeli, C.M.; et al. Prognostic Factors for Early and Long-Term Remission in Pediatric Differentiated Thyroid Carcinoma: The Role of Sex, Age, Clinical Presentation, and the Newly Proposed American Thyroid Association Risk Stratification System. Thyroid 2016, 26, 1480–1487. [Google Scholar] [CrossRef]
- Cordioli, M.I.; Moraes, L.; Alves, M.T.; Delcelo, R.; Monte, O.; Longui, C.A.; Cury, A.N.; Cerutti, J.M. Thyroid-specific genes expression uncovered age-related differences in pediatric thyroid carcinomas. Int. J. Endocrinol. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyrazoğlu, Ş.; Bundak, R.; Baş, F.; Yeğen, G.; Şanlı, Y.; Darendeliler, F. Clinicopathological characteristics of papillary thyroid cancer in children with emphasis on pubertal status and association with BRAFV600E mutation. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Hampson, S.; Stephens, D.; Wasserman, J.D. Young age is associated with increased rates of residual and recurrent paediatric differentiated thyroid carcinoma. Clin. Endocrinol. 2018, 89, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Galuppini, F.; Vianello, F.; Censi, S.; Barollo, S.; Bertazza, L.; Carducci, S.; Colato, C.; Manso, J.; Rugge, M.; Iacobone, M.; et al. Differentiated Thyroid Carcinoma in Pediatric Age: Genetic and Clinical Scenario. Front. Endocrinol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferri, E.L.; Massoll, N. Management of papillary and follicular (differentiated) thyroid cancer: New paradigms using recombinant human thyrotropin. Endocr. Relat. Cancer 2002, 9, 227–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzaferri, E.L.; Kloos, R.T. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 2001, 86, 1447–1463. [Google Scholar] [CrossRef]
- Demidchik, Y.E.; Demidchik, E.P.; Reiners, C.; Biko, J.; Mine, M.; Saenko, V.A.; Yamashita, S. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann. Surg. 2006, 243, 525–532. [Google Scholar] [CrossRef]
- Lazar, L.; Lebenthal, Y.; Steinmetz, A.; Yackobovitch-Gavan, M.; Phillip, M. Differentiated Thyroid Carcinoma in Pediatric Patients: Comparison of Presentation and Course between Pre-Pubertal Children and Adolescents. J. Pediatr. 2009, 154, 708–714. [Google Scholar] [CrossRef]
- Van Santen, H.M.; Aronson, D.C.; Vulsma, T.; Tummers, R.F.; Geenen, M.M.; De Vijlder, J.J.; Van Den Bos, C. Frequent adverse events after treatment for childhood-onset differentiated thyroid carcinoma: A single institute experience. Eur. J. Cancer 2004, 40, 1743–1751. [Google Scholar] [CrossRef]
- Schneider, R.; Reiners, C. The Effect of Levothyroxine Therapy on Bone Mineral Density: A Systematic Review of the Literature. Exp. Clin. Endocrinol. Diabetes 2003, 111, 455–470. [Google Scholar] [CrossRef]
- Fridman, M.; Krasko, O.; Branovan, D.I.; Dabryian, S.; Pisarenko, A.; Lo, C.Y.; Lam, A.K. yin Factors affecting the approaches and complications of surgery in childhood papillary thyroid carcinomas. Eur. J. Surg. Oncol. 2019, 45, 2078–2085. [Google Scholar] [CrossRef] [Green Version]
- Maxon, H.R. Quantitative radioiodine therapy in the treatment of differentiated thyroid cancer. Q. J. Nucl. Med. 1999, 43, 313–323. [Google Scholar] [PubMed]
- Zidan, J.; Hefer, E.; Iosilevski, G.; Drumea, K.; Stein, M.E.; Kuten, A.; Israel, O. Efficacy of i131 ablation therapy using different doses as determined by postoperative thyroid scan uptake in patients with differentiated thyroid cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Nasr, C.; Bischoff, L.; Busaidy, N.L.; Byrd, D.; Callender, G.; Dickson, P.; Duh, Q.Y.; Ehya, H.; Goldner, W.; et al. Thyroid carcinoma, version 2.2018 featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2018, 16, 1429–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid cancer in the pediatric population. Genes 2019, 10, 723. [Google Scholar] [CrossRef] [Green Version]
- Van Wyngaarden, M.; McDougall, I.R. What is the role of 1100 MBq (<30 mCi) radioiodine 131I in the treatment of patients with differentiated thyroid cancer? Nucl. Med. Commun. 1996, 17, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W.; Moreno-Reyes, R.; Van den Bruel, A.; Zira, C.; Feldt-Rasmussen, U.; et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Schlumberger, M.; Pacini, F.; Wiersinga, W.M.; Toft, A.; Smit, J.W.A.; Franco, F.S.; Lind, P.; Limbert, E.; Jarzab, B.; Jamar, F.; et al. Follow-up and management of differentiated thyroid carcinoma: A European perspective in clinical practice. Eur. J. Endocrinol. 2004, 151, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Biondi, B.; Fazio, S.; Carella, C.; Amato, G.; Cittadini, A.; Lupoli, G.; Saccà, L.; Bellastella, A.; Lombardi, G. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. J. Clin. Endocrinol. Metab. 1993, 77, 334–338. [Google Scholar] [CrossRef]
- Matuszewska, G.; Roskosz, J.; Wloch, J.; Jurecka-Tuleja, B.; Hasse-Lazar, K.; Kowalczyk, B.; Jarzab, B. Evaluation of effects of L-thyroxine therapy in differentiated thyroid carcinoma on the cardiovascular system—Prospective study. Wiad. Lek. 2001, 54, 373–377. [Google Scholar] [PubMed]
- Chow, S.M.; Yau, S.; Lee, S.H.; Leung, W.M.; Law, S.C.K. Pregnancy outcome after diagnosis of differentiated thyroid carcinoma: No deleterious effect after radioactive iodine treatment. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Pontikides, N. Gonadal effect of radiation from 131I in male patients with thyroid carcinoma. Arch. Androl. 2005, 51, 171–175. [Google Scholar] [CrossRef]
- Wichers, M.; Benz, E.; Palmedo, H.; Biersack, H.J.; Grünwald, F.; Klingmüller, D. Testicular function after radioiodine therapy for thyroid carcinoma. Eur. J. Nucl. Med. 2000, 27, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y. Molecular diagnosis of thyroid tumors. Arch. Pathol. Lab. Med. 2011, 135, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Haugen, B.R.; Schlumberger, M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013, 381, 1058–1069. [Google Scholar] [CrossRef] [Green Version]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, N.; Cao, J.; Sofiadis, A.; Dinets, A.; Zedenius, J.; Larsson, C.; Xu, D. The age-and shorter telomere-dependent tert promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 2014, 33, 4978–4984. [Google Scholar] [CrossRef] [Green Version]
- Kimura, E.T.; Nikiforova, M.N.; Zhu, Z.; Knauf, J.A.; Nikiforov, Y.E.; Fagin, J.A. High Prevalence of BRAF Mutations in Thyroid Cancer: Genetic Evidence for Constitutive Activation of the RET/PTC-RAS-BRAF Signaling Pathway in Papillary Thyroid Carcinoma. Cancer Res. 2003, 63, 1454–1457. [Google Scholar]
- Cohen, Y.; Xing, M.; Mambo, E.; Gou, Z.; Wu, G.; Trink, B.; Beller, U.; Westra, W.H.; Ladenson, P.W.; Sidransky, D.; et al. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. 2003, 95, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.U.; Oler, G.; Nozima, B.H.N.; Moysés, R.A.; Cerutti, J.M. BRAF V600E and decreased NIS and TPO expression are associated with aggressiveness of a subgroup of papillary thyroid microcarcinoma. Eur. J. Endocrinol. 2015, 173, 525–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oler, G.; Cerutti, J.M. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas. Cancer 2009, 115, 972–980. [Google Scholar] [CrossRef]
- Al-Salam, S.; Sharma, C.; Afandi, B.; Al Dahmani, K.; Al-Zahrani, A.S.; Al Shamsi, A.; Al Kaabi, J. BRAF and KRAS mutations in papillary thyroid carcinoma in the United Arab Emirates. PLoS ONE 2020, 15, e0231341. [Google Scholar] [CrossRef] [Green Version]
- Rusinek, D.; Pfeifer, A.; Cieslicka, M.; Kowalska, M.; Pawlaczek, A.; Krajewska, J.; Szpak-Ulczok, S.; Tyszkiewicz, T.; Halczok, M.; Czarniecka, A.; et al. TERT Promoter Mutations and Their Impact on Gene Expression Profile in Papillary Thyroid Carcinoma. Cancers 2020, 12, 1597. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Trovisco, V.; Soares, P.; Máximo, V.; Magalhães, J.; Salvatore, G.; Santoro, M.; Bogdanova, T.; Tronko, M.; Abrosimov, A.; et al. BRAF mutations are not a major event in post-chernobyl childhood thyroid carcinomas. J. Clin. Endocrinol. Metab. 2004, 89, 4267–4271. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, A.; Namba, H.; Saenko, V.A.; Ashizawa, K.; Ohtsuru, A.; Ito, M.; Ishikawa, N.; Sugino, K.; Ito, K.; Jeremiah, S.; et al. Low frequency of BRAFT1796A mutations in childhood thyroid carcinomas. J. Clin. Endocrinol. Metab. 2004, 89, 4280–4284. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, A.S.; Murugan, A.K.; Qasem, E.; Alswailem, M.; Al-Hindi, H.; Shi, Y. Single Point Mutations in Pediatric Differentiated Thyroid Cancer. Thyroid 2017, 27, 189–196. [Google Scholar] [CrossRef]
- Geng, J.; Wang, H.; Liu, Y.; Tai, J.; Jin, Y.; Zhang, J.; He, L.; Fu, L.; Qin, H.; Song, Y.; et al. Correlation between BRAFV600E mutation and clinicopathological features in pediatric papillary thyroid carcinoma. Sci. China Life Sci. 2017, 60, 729–738. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Labourier, E.; Sullivan, L.; LiVolsi, V.; Li, Y.; Xiao, R.; Beaudenon-Huibregtse, S.; Kazahaya, K.; Adzick, N.S.; Baloch, Z.; et al. Molecular Testing for Oncogenic Gene Alterations in Pediatric Thyroid Lesions. Thyroid 2018, 28, 60–67. [Google Scholar] [CrossRef]
- Henke, L.E.; Perkins, S.M.; Pfeifer, J.D.; Ma, C.; Chen, Y.; Dewees, T.; Grigsby, P.W. BRAF V600E mutational status in pediatric thyroid cancer. Pediatr. Blood Cancer 2014, 61, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Alswailem, M.; Alswailem, A.A.; Al-Hindi, H.; Goljan, E.; Alsudairy, N.; Abouelhoda, M. Genetic Alterations in Pediatric Thyroid Cancer Using a Comprehensive Childhood Cancer Gene Panel. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, E.; Hosler, G.; Zahurak, M.; Cohen, Y.; Sidransky, D.; Westra, W.H. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod. Pathol. 2005, 18, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Ricarte-Filho, J.C.; Li, S.; Garcia-Rendueles, M.E.R.; Montero-Conde, C.; Voza, F.; Knauf, J.A.; Heguy, A.; Viale, A.; Bogdanova, T.; Thomas, G.A.; et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J. Clin. Investig. 2013, 123, 4935–4944. [Google Scholar] [CrossRef] [Green Version]
- Givens, D.J.; Buchmann, L.O.; Agarwal, A.M.; Grimmer, J.F.; Hunt, J.P. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma. Laryngoscope 2014, 124, E389–E393. [Google Scholar] [CrossRef]
- Prasad, M.L.; Vyas, M.; Horne, M.J.; Virk, R.K.; Morotti, R.; Liu, Z.; Tallini, G.; Nikiforova, M.N.; Christison-Lagay, E.R.; Udelsman, R.; et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 2016, 122, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Cordioli, M.I.; Moraes, L.; Bastos, A.U.; Besson, P.; Alves, M.T.; Delcelo, R.; Monte, O.; Longui, C.; Cury, A.N.; Cerutti, J.M. Fusion Oncogenes Are the Main Genetic Events Found in Sporadic Papillary Thyroid Carcinomas from Children. Thyroid 2017, 27, 182–188. [Google Scholar] [CrossRef]
- Sisdelli, L.; Cordioli, M.I.; Vaisman, F.; Moraes, L.; Colozza-Gama, G.A.; Alves, P.A.G.; Araújo, M.L.; Alves, M.T.S.; Monte, O.; Longui, C.A.; et al. AGK-BRAF is associated with distant metastasis and younger age in pediatric papillary thyroid carcinoma. Pediatr. Blood Cancer 2019, 66, 1–7. [Google Scholar] [CrossRef]
- Nikita, M.E.; Jiang, W.; Cheng, S.-M.; Hantash, F.M.; McPhaul, M.J.; Newbury, R.O.; Phillips, S.A.; Reitz, R.E.; Waldman, F.M.; Newfield, R.S. Mutational Analysis in Pediatric Thyroid Cancer and Correlations with Age, Ethnicity, and Clinical Presentation. Thyroid 2016, 26, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Gertz, R.J.; Nikiforov, Y.; Rehrauer, W.; McDaniel, L.; Lloyd, R.V. Mutation in BRAF and Other Members of the MAPK Pathway in Papillary Thyroid Carcinoma in the Pediatric Population. Arch. Pathol. Lab. Med. 2016, 140, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciampi, R.; Knauf, J.A.; Kerler, R.; Gandhi, M.; Zhu, Z.; Nikiforova, M.N.; Rabes, H.M.; Fagin, J.A.; Nikiforov, Y.E. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Investig. 2005, 115, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efanov, A.A.; Brenner, A.V.; Bogdanova, T.I.; Kelly, L.M.; Liu, P.; Little, M.P.; Wald, A.I.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. Investigation of the relationship between radiation dose and gene mutations and fusions in post-chernobyl thyroid cancer. J. Natl. Cancer Inst. 2018, 110, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, M.; Mitsutake, N.; Matsuse, M.; Fukushima, T.; Suzuki, S.; Matsumoto, Y.; Ookouchi, C.; Mizunuma, H.; Nakamura, I.; Nakano, K.; et al. The clinicopathological results of thyroid cancer with BRAFV600E mutation in the young population of Fukushima. J. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef]
- Bastos, A.U.; de Jesus, A.C.; Cerutti, J.M. ETV6-NTRK3 and STRN-ALK kinase fusions are recurrent events in papillary thyroid cancer of adult population. Eur. J. Endocrinol. 2018, 178, 85–93. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Chmielecki, J.; Gay, L.; Johnson, A.; Chudnovsky, J.; Yelensky, R.; Lipson, D.; Ali, S.M.; Elvin, J.A.; et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int. J. Cancer 2015, 138, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Picarsic, J.L.; Buryk, M.A.; Ozolek, J.; Ranganathan, S.; Monaco, S.E.; Simons, J.P.; Witchel, S.F.; Gurtunca, N.; Joyce, J.; Zhong, S.; et al. Molecular Characterization of Sporadic Pediatric Thyroid Carcinoma with the DNA/RNA ThyroSeq v2 Next-Generation Sequencing Assay. Pediatr. Dev. Pathol. 2016, 19, 115–122. [Google Scholar] [CrossRef]
- Vanden Borre, P.; Schrock, A.B.; Anderson, P.M.; Morris, J.C.; Heilmann, A.M.; Holmes, O.; Wang, K.; Johnson, A.; Waguespack, S.G.; Ou, S.I.; et al. Pediatric, Adolescent, and Young Adult Thyroid Carcinoma Harbors Frequent and Diverse Targetable Genomic Alterations, Including Kinase Fusions. Oncologist 2017, 22, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Pekova, B.; Sykorova, V.; Dvorakova, S.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Astl, J.; Vlcek, P.; Kodetova, D.; Vcelak, J.; et al. RET, NTRK, ALK, BRAF and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid 2020. [Google Scholar] [CrossRef]
- Santoro, M.; Carlomagno, F. Central Role of RET in Thyroid Cancer. Cold Spring Harb. Perspect. Biol. 2013, 5, a009233. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Moccia, M.; Federico, G.; Carlomagno, F. Ret gene fusions in malignancies of the thyroid and other tissues. Genes 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekova, B.; Dvorakova, S.; Sykorova, V.; Vacinova, G.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Vlcek, P.; Sykorova, P.; Kodetova, D.; et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr. Connect. 2019, 8, 796–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisarchik, A.; Ermak, G.; Demidchik, E.; Mikhalevich, L.; Kartel, N.; Figge, J. Low prevalence of the ret/PTC3r1 rearrangement in a series of papillary thyroid carcinomas presenting in Belarus ten years post-Chernobyl. Thyroid 1998, 8, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Unger, K.; Zitzelsberger, H.; Salvatore, G.; Santoro, M.; Bogdanova, T.; Braselmann, H.; Kastner, P.; Zurnadzhy, L.; Tronko, N.; Hutzler, P.; et al. Heterogeneity in the distribution of RET/PTC rearrangements within individual post-chernobyl papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2004, 89, 4272–4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforov, Y.E.; Rowland, J.M.; Bove, K.E.; Monforte-Munoz, H.; Fagin, J.A. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997, 57, 1690–1694. [Google Scholar]
- Kjellman, P.; Learoyd, D.L.; Messina, M.; Weber, G.; Höög, A.; Wallin, G.; Larsson, C.; Robinson, B.G.; Zedenius, J. Expression of the RET proto-oncogene in papillary thyroid carcinoma and its correlation with clinical outcome. Br. J. Surg. 2001, 88, 557–563. [Google Scholar] [CrossRef]
- Iyama, K.; Matsuse, M.; Mitsutake, N.; Rogounovitch, T.; Saenko, V.A.; Suzuki, K.; Ashizawa, M.; Ookouchi, C.; Suzuki, S.S.S.; Mizunuma, H.; et al. Identification of Three Novel Fusion Oncogenes, SQSTM1/NTRK3, AFAP1L2/RET, and PPFIBP2/RET in Thyroid Cancers of Young Patients in Fukushima. Thyroid 2017, 27, 811–818. [Google Scholar] [CrossRef]
- Hamatani, K.; Eguchi, H.; Koyama, K.; Mukai, M.; Nakachi, K.; Kusunoki, Y. A novel RET rearrangement (ACBD5/RET) by pericentric inversion, inv(10)(p12.1;q11.2), in papillary thyroid cancer from an atomic bomb survivor exposed to high-dose radiation. Oncol. Rep. 2014, 32, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Klugbauer, S.; Demidchik, E.P.; Lengfelder, E.; Rabes, H.M. Detection of a novel type of RET rearrangement (PTC5) in thyroid carcinomas after chernobyl and analysis of the involved RET-fused gene RFG5. Cancer Res. 1998, 58, 198–203. [Google Scholar]
- Klugbauer, S.; Jauch, A.; Lengfelder, E.; Demidchik, E.; Rabes, H.M. A novel type of RET rearrangement (PTC8) in childhood papillary thyroid carcinomas and characterization of the involved gene (RFG8). Cancer Res. 2000, 60, 7028–7032. [Google Scholar]
- Salassidis, K.; Bruch, J.; Zitzelsberger, H.; Lengfelder, E.; Kellerer, A.M.; Bauchinger, M. Translocation t(10;14)(q11.2;q22.1) fusing the kinectin to the RET gene creates a novel rearranged form (PTC8) of the RET proto-oncogene in radiation-induced childhood papillary thyroid carcinoma. Cancer Res. 2000, 60, 2786–2789. [Google Scholar]
- Fugazzola, L.; Pierotti, M.; Vigano, E.; Pacini, F.; Vorontsova, T.; Bongarzone, I. Molecular and biochemical analysis of RET/PTC4, a novel oncogenic rearrangement between RET and ELE1 genes, in a post-Chernobyl papillary thyroid cancer. Oncogene 1996, 13, 1093–1097. [Google Scholar]
- Yoo, S.-K.; Lee, S.; Kim, S.; Jee, H.-G.; Kim, B.-A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.-K.; Shin, J.-Y.; et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Kelly, L.M.; Liu, P.; Brenner, A.V.; Little, M.P.; Bogdanova, T.I.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2013, 120, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, M.; Carlomagno, F. Oncogenic rearrangements driving ionizing radiation-associated human cancer. J. Clin. Investig. 2013, 123, 4566–4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.M.; Barila, G.; Liu, P.; Evdokimova, V.N.; Trivedi, S.; Panebianco, F.; Gandhi, M.; Carty, S.E.; Hodak, S.P.; Luo, J.; et al. Identification of the transforming STRN-ALK fusion asa potential therapeutic target in the aggressive forms of thyroid cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 4233–4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, P.; Rebocho, A.P.; Soares, R.J.; Magalhães, J.; Roque, L.; Trovisco, V.; Vieira De Castro, I.; Cardoso-De-Oliveira, M.; Fonseca, E.; Soares, P.; et al. PAX8-PPARγ rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforova, M.N.; Biddinger, P.W.; Caudill, C.M.; Kroll, T.G.; Nikiforov, Y.E. PAX8-PPARγ rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am. J. Surg. Pathol. 2002, 26, 1016–1023. [Google Scholar] [CrossRef]
- French, C.A.; Alexander, E.K.; Cibas, E.S.; Nose, V.; Laguette, J.; Faquin, W.; Garber, J.; Moore, F.; Fletcher, J.A.; Larsen, P.R.; et al. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am. J. Pathol. 2003, 162, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- de Jesus Paniza, A.C.; Mendes, T.B.; Viana, M.D.; Thomaz, D.M.; Chiappini, P.; Colozza-Gama, G.A.; Lindsey, S.C.; de Carvalho, M.B.; Alves, V.A.; Curioni, O.; et al. Revised criteria for diagnosis of NIFTP reveals a better correlation with tumor biological behavior. Endocr. Connect. 2019, 8, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; Gaspar da Rocha, A.; Batista, R.; Vinagre, J.; Martins, M.J.; Costa, G.; Ribeiro, C.; Carrilho, F.; Leite, V.; Lobo, C.; et al. TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J. Clin. Endocrinol. Metab. 2017, 102, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panebianco, F.; Nikitski, A.V.; Nikiforova, M.N.; Nikiforov, Y.E. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med. 2019, 8, 5831–5839. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2020, 67, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Onder, S.; Ozturk Sari, S.; Yegen, G.; Sormaz, I.C.; Yilmaz, I.; Poyrazoglu, S.; Sanlı, Y.; Giles Senyurek, Y.; Kapran, Y.; Mete, O. Classic Architecture with Multicentricity and Local Recurrence, and Absence of TERT Promoter Mutations are Correlates of BRAF V600E Harboring Pediatric Papillary Thyroid Carcinomas. Endocr. Pathol. 2016, 27, 153–161. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Qasem, E.; Murugan, A.K.; Al-Hindi, H.N.; AlKhafaji, D.; Almohanna, M.; Xing, M.; Alhomaidah, D.; AlSwailem, M. Uncommon TERT Promoter Mutations in Pediatric Thyroid Cancer. Thyroid 2016, 26, 235–241. [Google Scholar] [CrossRef]
- Oishi, N.; Kondo, T.; Nakazawa, T.; Mochizuki, K.; Inoue, T.; Kasai, K.; Tahara, I.; Yabuta, T.; Hirokawa, M.; Miyauchi, A.; et al. Frequent BRAF V600E and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan. Endocr. Pathol. 2017, 28, 103–111. [Google Scholar] [CrossRef]
- Cordioli, M.I.; Moraes, L.; Carvalheira, G.; Sisdelli, L.; Alves, M.T.; Delcelo, R.; Monte, O.; Longui, C.A.; Cury, A.N.; Cerutti, J.M. AGK-BRAF gene fusion is a recurrent event in sporadic pediatric thyroid carcinoma. Cancer Med. 2016, 5, 1535–1541. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Ghuzlan, A.A.; Kakudo, K.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.H.; Sadow, P.M. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Diagnostic updates and molecular advances. Semin. Diagn. Pathol. 2020, 37, 213–218. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Project, C.G.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Paton, E.L.; Turner, J.A.; Schlaepfer, I.R. Overcoming Resistance to Therapies Targeting the MAPK Pathway in BRAF-Mutated Tumours. J. Oncol. 2020, 2020, 1079827. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, K.E.; Myong, J.P.; Park, J.H.; Jeon, Y.K.; Min, H.S.; Park, S.Y.; Jung, K.C.; Koo, D.H.; Youn, Y.K. BRAFV600Emutation is associated with tumor aggressiveness in papillary thyroid cancer. World J. Surg. 2012, 36, 310–317. [Google Scholar] [CrossRef]
- Choi, E.K.; Chong, A.; Ha, J.-M.; Jung, C.K.; O, J.H.; Kim, S.H. Clinicopathological characteristics including BRAF V600E mutation status and PET/CT findings in papillary thyroid carcinoma. Clin. Endocrinol. 2017, 87, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaka, Y.; Itoh, F.; Tahira, T.; Ikeda, I.; Sugimura, T.; Tucker, J.; Fertitta, A.; Carrano, A.V.; Nagao, M. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989, 4, 1519–1521. [Google Scholar]
- Takaya, K.; Yoshimasa, T.; Arai, H.; Tamura, N.; Miyamoto, Y.; Itoh, H.; Nakao, K. Expression of the RET proto-oncogene in normal human tissues, pheochromocytomas, and other tumors of neural crest origin. J. Mol. Med. 1996, 74, 617–621. [Google Scholar] [CrossRef]
- Grieco, M.; Santoro, M.; Berlingieri, M.T.M.; Melillo, R.M.R.; Donghi, R.; Bongarzone, I.; Pierotti, M.A.; Della Porta, G.; Fusco, A.; Vecchio, G.; et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990, 60, 557–563. [Google Scholar] [CrossRef]
- Staubitz, J.I.; Schad, A.; Springer, E.; Rajalingam, K.; Lang, H.; Roth, W.; Hartmann, N.; Musholt, T.J. Novel rearrangements involving the RET gene in papillary thyroid carcinoma. Cancer Genet. 2019, 230, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Rabes, H.M.; Demidchik, E.P.; Sidorow, J.D.; Implications, C.; Lengfelder, E.; Beimfohr, C.; Hoelzel, D. Pattern of Radiation-induced RET and NTRK1 Rearrangements in 191 Post-Chernobyl Papillary Thyroid Carcinomas: Biological, Phenotypic, and Clinical Implications. Clin. Res. Cancer 2000, 6, 1093–1103. [Google Scholar]
- Zhu, Z.; Ciampi, R.; Nikiforova, M.N.; Gandhi, M.; Nikiforov, Y.E. Prevalence of RET/PTC Rearrangements in Thyroid Papillary Carcinomas: Effects of the Detection Methods and Genetic Heterogeneity. J. Clin. Endocrinol. Metab. 2006, 91, 3603–3610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Qadri, Q.; Makhdoomi, M.J.; Wani, M.A.; Malik, A.A.; Niyaz, M.; Masoodi, S.R.; Andrabi, K.I.; Ahmad, R.; Mudassar, S. RET/PTC Gene Rearrangements in Thyroid Carcinogenesis: Assessment and Clinico-Pathological Correlations. Pathol. Oncol. Res. 2020, 26, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bounacer, A.; Wicker, R.; Caillou, B.; Cailleux, A.F.; Sarasin, A.; Schlumberger, M.; Suárez, H.G. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene 1997, 15, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeniran, A.J.; Zhu, Z.; Gandhi, M.; Steward, D.L.; Fidler, J.P.; Giordano, T.J.; Biddinger, P.W.; Nikiforov, Y.E. Correlation Between Genetic Alterations and Microscopic Features, Clinical Manifestations, and Prognostic Characteristics of Thyroid Papillary Carcinomas. Am. J. Surg. Pathol. 2006, 30, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.S.; Hong, S.; Park, C.S. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid 2009, 19, 1225–1231. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Regalbuto, C.; Pellegriti, G.; Moleti, M.; Caff, A.; Squatrito, S.; Vigneri, R. Outcome of the Diffuse Sclerosing Variant of Papillary Thyroid Cancer: A Meta-Analysis. Thyroid 2016, 26, 1285–1292. [Google Scholar] [CrossRef]
- Elisei, R.; Romei, C.; Vorontsova, T.; Cosci, B.; Veremeychik, V.; Kuchinskaya, E.; Basolo, F.; Demidchik, E.P.; Miccoli, P.; Pinchera, A.; et al. RET/PTC rearrangements in thyroid nodules: Studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J. Clin. Endocrinol. Metab. 2001, 86, 3211–3216. [Google Scholar] [CrossRef]
- Prescott, J.D.; Zeiger, M.A. The RET oncogene in papillary thyroid carcinoma. Cancer 2015. [Google Scholar] [CrossRef]
- Kroll, T.G.; Sarraf, P.; Pecciarini, L.; Chen, C.J.; Mueller, E.; Spiegelman, B.M.; Fletcher, J.A. PAX8-PPARγ1 fusion in oncogene human thyroid carcinoma. Science 2000, 289, 1357–1360. [Google Scholar] [CrossRef]
- Ballester, L.Y.; Sarabia, S.F.; Sayeed, H.; Patel, N.; Baalwa, J.; Athanassaki, I.; Hernandez, J.A.; Fang, E.; Quintanilla, N.M.; Roy, A.; et al. Integrating Molecular Testing in the Diagnosis and Management of Children with Thyroid Lesions. Pediatr. Dev. Pathol. 2016, 19, 94–100. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Lynch, R.A.; Biddinger, P.W.; Alexander, E.K.; Dorn, G.W.; Tallini, G.; Kroll, T.G.; Nikiforov, Y.E. RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: Evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 2318–2326. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.R.; Espadinha, C.; Catarino, A.L.; Moniz, S.; Pereira, T.; Sobrinho, L.G.; Leite, V. Expression of PAX8-PPARγ1 Rearrangements in Both Follicular Thyroid Carcinomas and Adenomas. J. Clin. Endocrinol. Metab. 2002, 87, 3947–3952. [Google Scholar] [CrossRef] [Green Version]
- Dwight, T.; Thoppe, S.R.; Foukakis, T.; Lui, W.O.; Wallin, G.; Höög, A.; Frisk, T.; Larsson, C.; Zedenius, J. Involvement of the PAX8/peroxisome proliferator-activated receptor γ rearrangement in follicular thyroid tumors. J. Clin. Endocrinol. Metab. 2003, 88, 4440–4445. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J.; Vousden, K.; Ozanne, B. The involvement of activated ras genes in determining the transformed phenotype. Proc. R. Soc. Lond. Biol. Sci. 1985, 226, 99–106. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Nikiforova, M.N.; Gnepp, D.R.; Fagin, J.A. Prevalence of mutations of ras and p53 in benign and malignant thyroid tumors from children exposed to radiation after the Chernobyl nuclear accident—PubMed. Oncogene 1996, 13, 687–693. [Google Scholar] [PubMed]
- Jang, E.K.; Song, D.E.; Sim, S.Y.; Kwon, H.; Choi, Y.M.; Jeon, M.J.; Han, J.M.; Kim, W.G.; Kim, T.Y.; Shong, Y.K.; et al. NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid 2014, 24, 1275–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleal, K.; Norris, K.; Baird, D. Telomere length dynamics and the evolution of cancer genome architecture. Int. J. Mol. Sci. 2018, 19, 482. [Google Scholar] [CrossRef] [Green Version]
- Maciejowski, J.; De Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [Green Version]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [Green Version]
- di Fagagna, F.D. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- De Lange, T. How telomeres solve the end-protection problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobinoff, A.P.; Pickett, H.A. Alternative Lengthening of Telomeres: DNA Repair Pathways Converge. Trends Genet. 2017, 33, 921–932. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, M.; Berardinelli, F.; Sgura, A. Telomere length maintenance in cancer: At the crossroad between telomerase and alternative lengthening of telomeres (ALT). Int. J. Mol. Sci. 2018, 19, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturini, L.; Daidone, M.G.; Motta, R.; Collini, P.; Spreafico, F.; Terenziani, M.; Piva, L.; Radice, P.; Perotti, D.; Zaffaroni, N. Telomere maintenance in wilms tumors: First evidence for the presence of alternative lengthening of telomeres mechanism. Genes Chromosom. Cancer 2011, 50, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Hakin-Smith, V.; Jellinek, D.A.; Levy, D.; Carroll, T.; Teo, M.; Timperley, W.R.; McKay, M.J.; Reddel, R.R.; Royds, J.A. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet 2003, 361, 836–838. [Google Scholar] [CrossRef]
- Omori, Y.; Nakayama, F.; Li, D.; Kanemitsu, K.; Semba, S.; Ito, A.; Yokozaki, H. Alternative lengthening of telomeres frequently occurs in mismatch repair system-deficient gastric carcinoma. Cancer Sci. 2009, 100, 413–418. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hoffman, A.R.; Otero, J.; Huang, H.-Y.; Zhao, Z.; Mazumdar, M.; Gorlick, R.; Meyers, P.; Healey, J.H.; Ladanyi, M. Divergent patterns of telomere maintenance mechanisms among human sarcomas: Sharply contrasting prevalence of the alternative lengthening of telomeres mechanism in Ewing’s sarcomas and osteosarcomas. Genes Chromosom. Cancer 2004, 41, 155–162. [Google Scholar] [CrossRef]
- Else, T.; Giordano, T.J.; Hammer, G.D. Evaluation of telomere length maintenance mechanisms in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2008, 93, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Villa, R.; Daidone, M.G.; Motta, R.; Venturini, L.; De Marco, C.; Vannelli, A.; Kusamura, S.; Baratti, D.; Deraco, M.; Costa, A.; et al. Multiple mechanisms of telomere maintenance exist and differentially affect clinical outcome in diffuse malignant peritoneal mesothelioma. Clin. Cancer Res. 2008, 14, 4134–4140. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Peng, M.; Song, Q. The co-expression of telomerase and ALT pathway in human breast cancer tissues. Tumor Biol. 2014, 35, 4087–4093. [Google Scholar] [CrossRef]
- Xue, Y.; Li, L.; Zhang, D.; Wu, K.; Chen, Y.; Zeng, J.; Wang, X.; He, D. Twisted Epithelial-to-Mesenchymal Transition Promotes Progression of Surviving Bladder Cancer T24 Cells with hTERT-Dysfunction. PLoS ONE 2011, 6, e27748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojovic, B.; Booth, R.E.; Jin, Y.; Zhou, X.; Crowe, D.L. Alternative lengthening of telomeres in cancer stem cells in vivo. Oncogene 2015, 34, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, P.; Leão, R.; Lipman, T.; Campbell, B.; Lee, D.; Price, A.; Zhang, C.; Heidari, A.; Stephens, D.; Boerno, S.; et al. A cancer specific hypermethylation signature of the TERT promoter predicts biochemical relapse in prostate cancer: A retrospective cohort study. Oncotarget 2016, 7, 57726–57736. [Google Scholar] [CrossRef] [Green Version]
- Donati, B.; Ciarrocchi, A. Telomerase and telomeres biology in thyroid cancer. Int. J. Mol. Sci. 2019, 20, 2887. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, Z.; Chen, T.; Zeng, W.; Guo, Y.; Huang, T. TERT promoter Mutation and Its Association with Clinicopathological Features and Prognosis of Papillary Thyroid Cancer: A Meta-analysis. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Geng, J.; Liu, Y.; Guo, Y.; Wang, H.; Tai, J.; Jin, Y.; Zhang, J.; Yu, Y.; Wang, S.; Song, Y.; et al. Correlation between TERT C228T and clinic-pathological features in pediatric papillary thyroid carcinoma. Sci. China Life Sci. 2019, 62, 1563–1571. [Google Scholar] [CrossRef]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef] [Green Version]
- Mai, S.; Garini, Y. The significance of telomeric aggregates in the interphase nuclei of tumor cells. J. Cell. Biochem. 2006, 97, 904–915. [Google Scholar] [CrossRef]
- Gadji, M.; Pozzo, A.R. From cellular morphology to molecular and epigenetic anomalies of myelodysplastic syndromes. Genes Chromosom. Cancer 2019, 58, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Caria, P.; Dettori, T.; Frau, D.V.; Lichtenzstejn, D.; Pani, F.; Vanni, R.; Mai, S. Characterizing the three-dimensional organization of telomeres in papillary thyroid carcinoma cells. J. Cell. Physiol. 2019, 234, 5175–5185. [Google Scholar] [CrossRef]
- Mai, S. The three-dimensional cancer nucleus. Genes Chromosom. Cancer 2019, 58, 462–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmont, A.S.; Zhai, Y.; Thilenius, A. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J. Cell Biol. 1993, 123, 1671–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmont, A.S.; Bignone, F.; Ts’O, P.O.P. The relative intranuclear positions of barr bodies in XXX non-transformed human fibroblasts. Exp. Cell Res. 1986, 165, 165–179. [Google Scholar] [CrossRef]
- Fritz, A.J.; Sehgal, N.; Pliss, A.; Xu, J.; Berezney, R. Chromosome territories and the global regulation of the genome. Genes Chromosom. Cancer 2019, 58, 407–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforova, M.N.; Stringer, J.R.; Blough, R.; Medvedovic, M.; Fagin, J.A.; Nikiforov, Y.E. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 2000, 290, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Medvedovic, M.; Stringer, J.R.; Nikiforov, Y.E. Interphase chromosome folding determines spatial proximity of genes participating in carcinogenic RET/PTC rearrangements. Oncogene 2006, 25, 2360–2366. [Google Scholar] [CrossRef] [Green Version]
- Elder, A.D.; Domin, A.; Kaminski Schierle, G.S.; Lindon, C.; Pines, J.; Esposito, A.; Kaminski, C.F. A quantitative protocol for dynamic measurements of protein interactions by Förster resonance energy transfer-sensitized fluorescence emission. J. Royal Soc. Interface 2009, 6, S59–S81. [Google Scholar] [CrossRef]
- Zink, D.; Fischer, A.H.; Nickerson, J.A. Nuclear structure in cancer cells. Nat. Rev. Cancer 2004, 4, 677–687. [Google Scholar] [CrossRef]
- Wegel, E.; Göhler, A.; Lagerholm, B.C.; Wainman, A.; Uphoff, S.; Kaufmann, R.; Dobbie, I.M. Imaging cellular structures in super-resolution with SIM, STED and Localisation Microscopy: A practical comparison. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Heintzmann, R.; Cremer, C.G. Laterally modulated excitation microscopy: Improvement of resolution by using a diffraction grating. In Optical Biopsies and Microscopic Techniques III; Bigio, I.J., Schneckenburger, H., Slavik, J., Svanberg, K., Viallet, P.M., Eds.; SPIE: Bellingham, WA, USA, 1999; Volume 3568, pp. 185–196. [Google Scholar]
- Gustafsson, M.G.L.; Shao, L.; Carlton, P.M.; Wang, C.J.R.; Golubovskaya, I.N.; Cande, W.Z.; Agard, D.A.; Sedat, J.W. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 2008, 94, 4957–4970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righolt, C.H.; Guffei, A.; Knecht, H.; Young, I.T.; Stallinga, S.; Van Vliet, L.J.; Mai, S. Differences in nuclear DNA organization between lymphocytes, hodgkin and reed-sternberg cells revealed by structured illumination microscopy. J. Cell. Biochem. 2014, 115, 1441–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righolt, C.H.; Knecht, H.; Mai, S. DNA Superresolution Structure of Reed-Sternberg Cells Differs between Long-Lasting Remission versus Relapsing Hodgkin’s Lymphoma Patients. J. Cell. Biochem. 2016, 117, 1633–1637. [Google Scholar] [CrossRef]
- Sathitruangsak, C.; Righolt, C.H.; Klewes, L.; Tammur, P.; Ilus, T.; Tamm, A.; Punab, M.; Olujohungbe, A.; Mai, S. Quantitative superresolution microscopy reveals differences in nuclear dna organization of multiple myeloma and monoclonal gammopathy of undetermined significance. J. Cell. Biochem. 2015, 116, 704–710. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Pozzo, A.; Booth, S.; Yu, P.L.I.; Singh, M.; Selivanova, G.; Mai, S. p53 CRISPR Deletion Affects DNA Structure and Nuclear Architecture. J. Clin. Med. 2020, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Pozzo, A.; Kuzyk, A.; Gartner, J.; Mai, S. MYCN overexpression is linked to significant differences in nuclear DNA organization in neuroblastoma. SPG BioMed 2019. [Google Scholar] [CrossRef]
- Ajaezi, G.C.; Eisele, M.; Contu, F.; Lal, S.; Rangel-Pozzo, A.; Mai, S.; Gough, K.M. Near-field infrared nanospectroscopy and super-resolution fluorescence microscopy enable complementary nanoscale analyses of lymphocyte nuclei. Analyst 2018, 143, 5926–5934. [Google Scholar] [CrossRef]

| Reference | n | Distant Met. (%) | LN Met. (%) | Mean Age (y.o.) | Gender F:M | Mean size (cm) | Mean Follow-up (years) | % NED | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Zimmerman et al. [29] | 58 | 6.9 | 89.7 | < 17 | 2.2: 1 | 3.1 | 26.7 | 52 | 14%* |
| Dottorini et al. [68] | 85 | 18.8 | 60 | 14.7 | 2.86: 1 | X | 9.25 | 63.5 | 0 |
| Kuo et al. [69] | 77 | 18 | 6.4 | 12.9 | 3.3: 1 | 6.93 | 8.2 | 89.6 | 0 |
| Vaisman et al. [70] | 65 | 29.2 | 61.5 | 14 | 3: 1 | 2.99 | 12.6 | 50.8 | 0 |
| Fridman et al. [71] | 94 | 20 | 66 | 15.1 | 3: 1 | 1.2 | 4.2 | 97 | 0 |
| Pires et al. [72] | 118 | 26.9 | 67.3 | 13.3 | 2.6: 1 | 2.5 | 8 | 63.5 | 0 |
| Cordioli et al. [73] | 38 | 26.3 | 73.7 | 11.8 | 3.2: 1 | 2.6 | 7.8 | 54.1 | 0 |
| Poyrazoğlu et al. [74] | 75 | 13.3 | 45.3 | 12.4 | 2.1: 1 | 2.2 | 4.3 | 65.3 | 1 patient |
| Hampson et al. [75] | 62 | 19.3 | 46.7 | 13.8 | 2.5: 1 | 2.3 | 3.6 | 59.6 | Not reported |
| Galuppini et al. [76] | 59 | 20.8 | 51 | 14.4 | 2.7: 1 | 2.0 | 5.9 | 66.7 | Not reported |
| Genetic Alterations | Adult PTC | Pediatric PTC | ||
|---|---|---|---|---|
| Sporadic | Post-Chernobyl | Post-Fukushima | ||
| BRAFV600E | 27–83% [100,102,103,104,105,106,107,108] | 0–63% [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123] | 0–17% [26,117,124,125] | 70% [52,126] |
| AKAP9-BRAF fusion | 1% [100,124] | 0–1% [115,117,124,125] | 0–11% [117,124,125] | 0% [52] |
| AGK-BRAF fusion | 0–0.2% [100,117,127,128] | 0–19% [115,117,119,121,129,130,131] | 0–4% [117,125,127] | ND |
| Novel BRAF fusions | 2% [100] | 0–4% [123,131] | 10% [125] | ND |
| RET/PTC1–3 fusions | 5–70% [100,105,132,133] | 0–37% [26,87,115,134] | 27–77% [87,125,133,135,136,137,138] | 6.5% [126] |
| Other RET fusions | 1–7% [100,133] | 2–7% [115,131] | 0–6% [125,139,140,141,142,143,144] | 3% [126] |
| ETV6-NTRK3 fusion | 1–5% [100,127,145] | 0–18% [115,117,120,127,131,146] | 6–14.5% [125,146,147] | 5%[126] |
| Other NTRK fusions | 1% [100] | 2–4% [115,131] | 3% [125] | 1.4% [126] |
| STRN-ALK fusion | 0–7% [100,127,148] | 0–6.5% [123,131,148] | 1.4–7% [125,126,139] | 1.4% [126] |
| PAX8-PPARγ fusion | 0–5% [100,149,150,151,152] | 0–9% [113,119,122,123,129] | 4% [117] | ND |
| RAS mutations | 1–20% [100,105,108,153] | 0–7% [111,113,119,120,123,134] | 0–9% [26,125] | 0% [52] |
| TERT promoter mutation (C250T, C228T) | 2–82% [100,108,154,155,156,157] | 0–4% [115,131,134,158,159,160] | ND | 0% [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-Pozzo, A.; Sisdelli, L.; Cordioli, M.I.V.; Vaisman, F.; Caria, P.; Mai, S.; Cerutti, J.M. Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations. Cancers 2020, 12, 3146. https://doi.org/10.3390/cancers12113146
Rangel-Pozzo A, Sisdelli L, Cordioli MIV, Vaisman F, Caria P, Mai S, Cerutti JM. Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations. Cancers. 2020; 12(11):3146. https://doi.org/10.3390/cancers12113146
Chicago/Turabian StyleRangel-Pozzo, Aline, Luiza Sisdelli, Maria Isabel V. Cordioli, Fernanda Vaisman, Paola Caria, Sabine Mai, and Janete M. Cerutti. 2020. "Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations" Cancers 12, no. 11: 3146. https://doi.org/10.3390/cancers12113146
APA StyleRangel-Pozzo, A., Sisdelli, L., Cordioli, M. I. V., Vaisman, F., Caria, P., Mai, S., & Cerutti, J. M. (2020). Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations. Cancers, 12(11), 3146. https://doi.org/10.3390/cancers12113146





