Multilineage Dysplasia as Assessed by Immunophenotype in Acute Myeloid Leukemia: A Prognostic Tool in a Genetically Undefined Category
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Morphology
2.3. Karyotype
2.4. Molecular Genetics
2.5. Flow Cytometry
2.6. Definitions
2.7. Statistical Aspects
3. Results
3.1. Characteristics of Patients and Treatment Flow
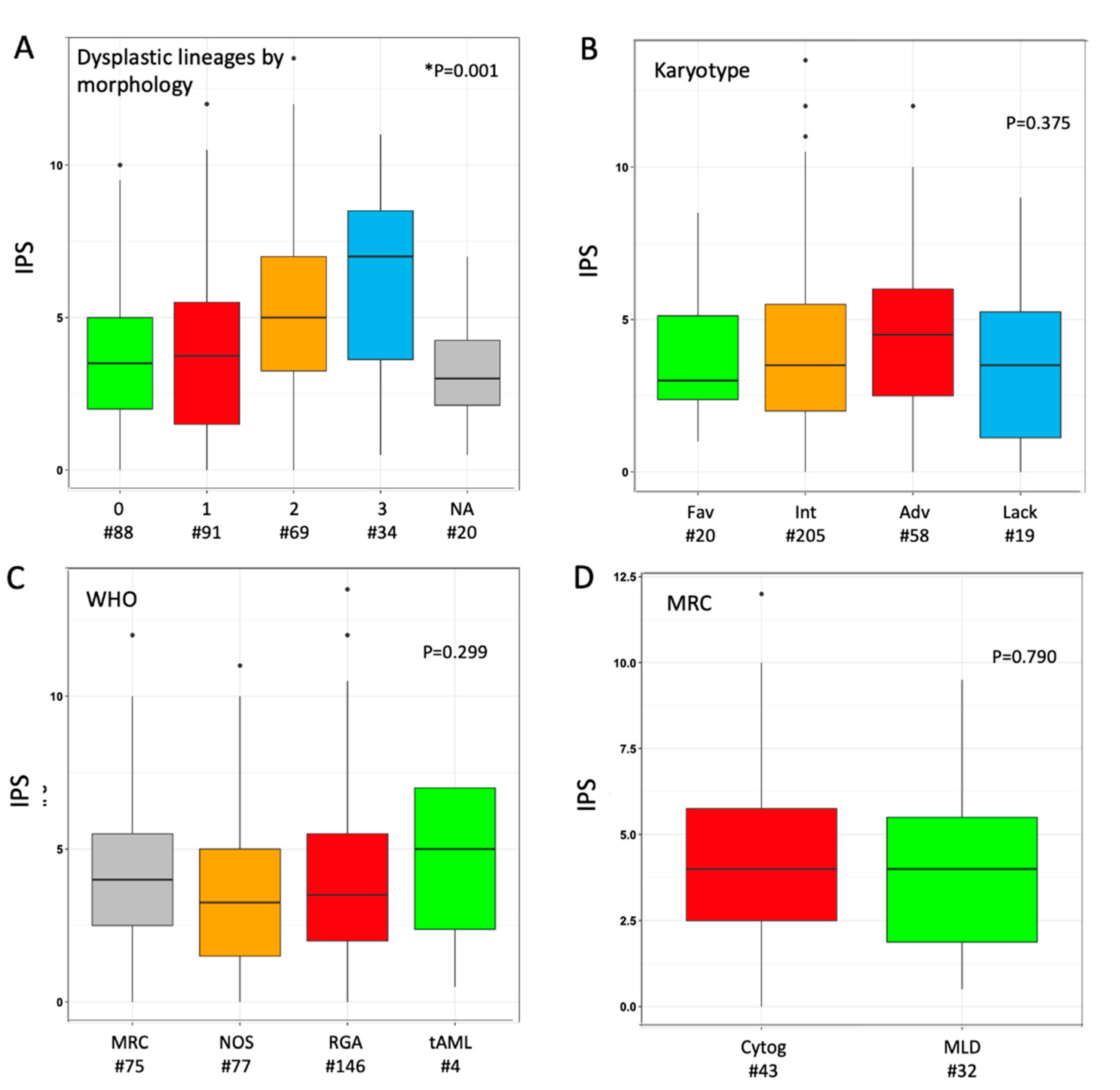
3.2. Study of MLD by Immuno-Phenotype
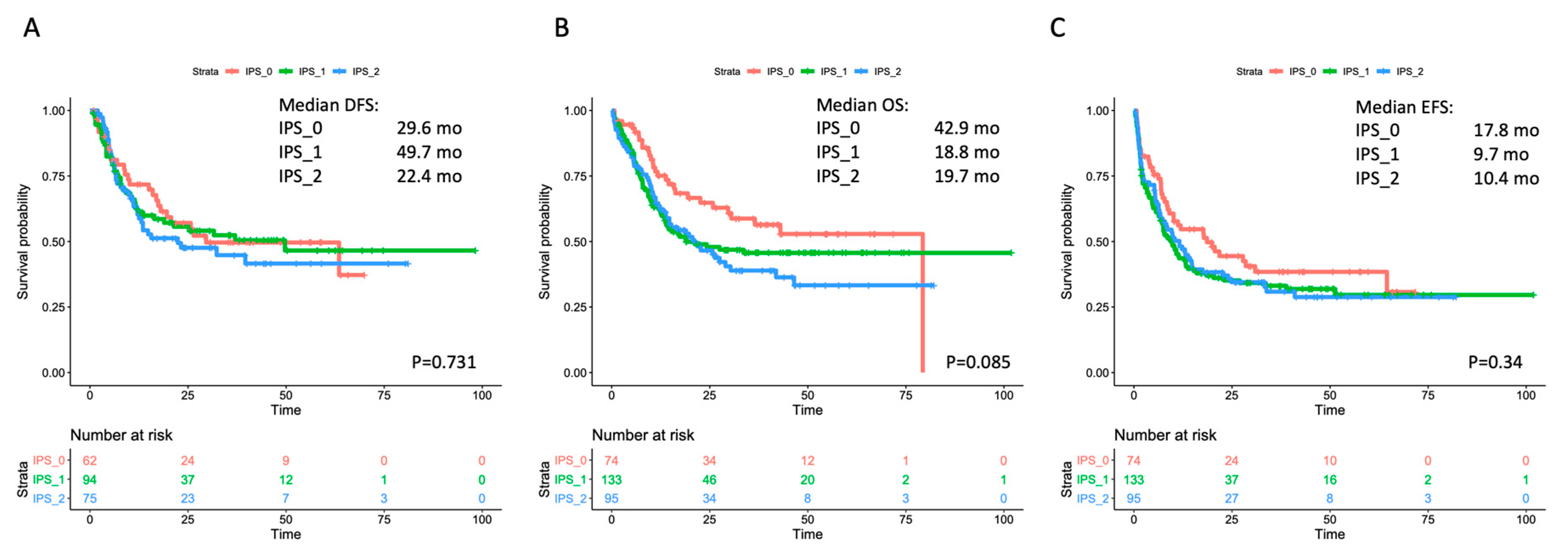
3.3. Correlation with Outcome in the Overall Cohort and within Selected Subgroups
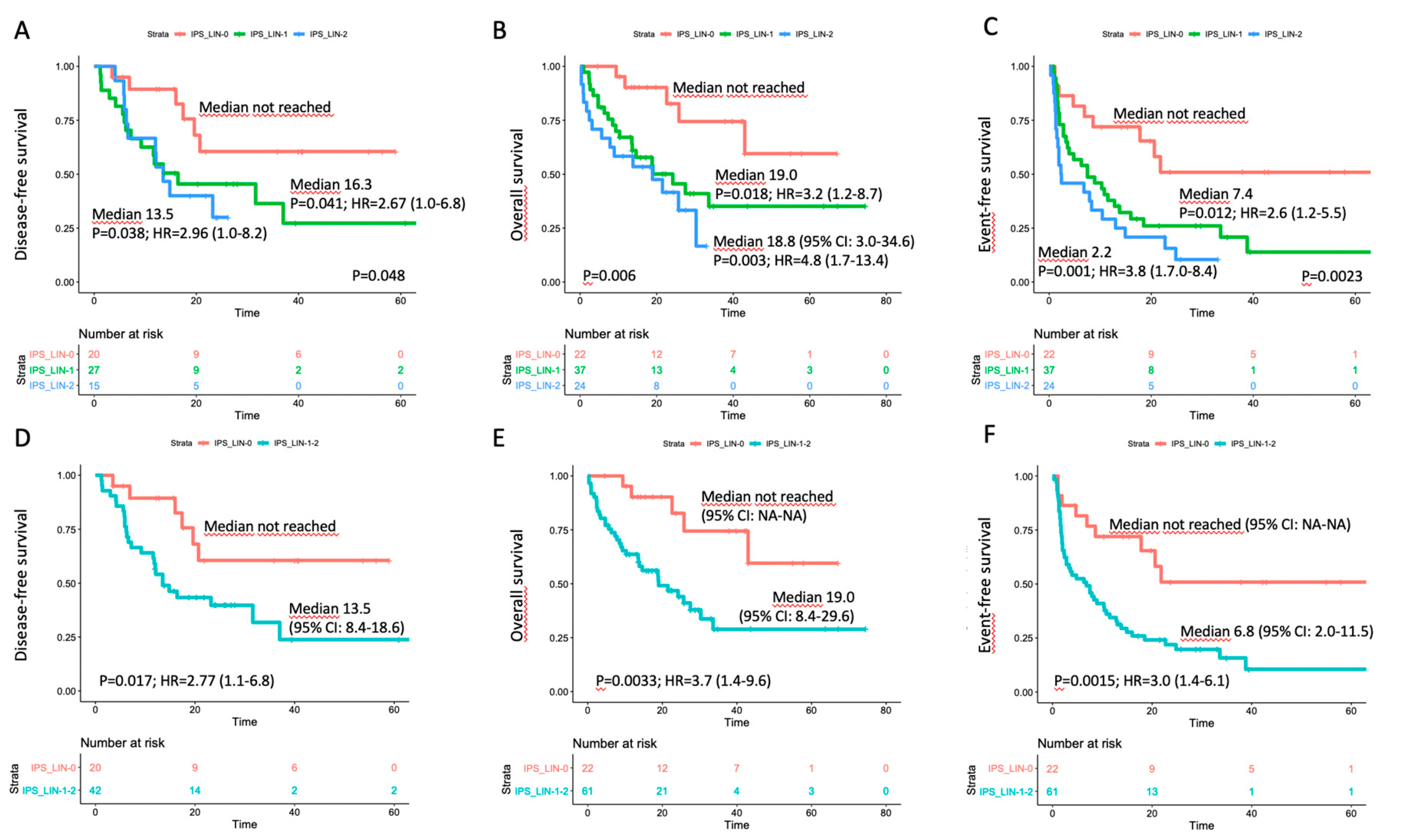
3.4. Correlation with Outcome in the Triple-Negative Subset
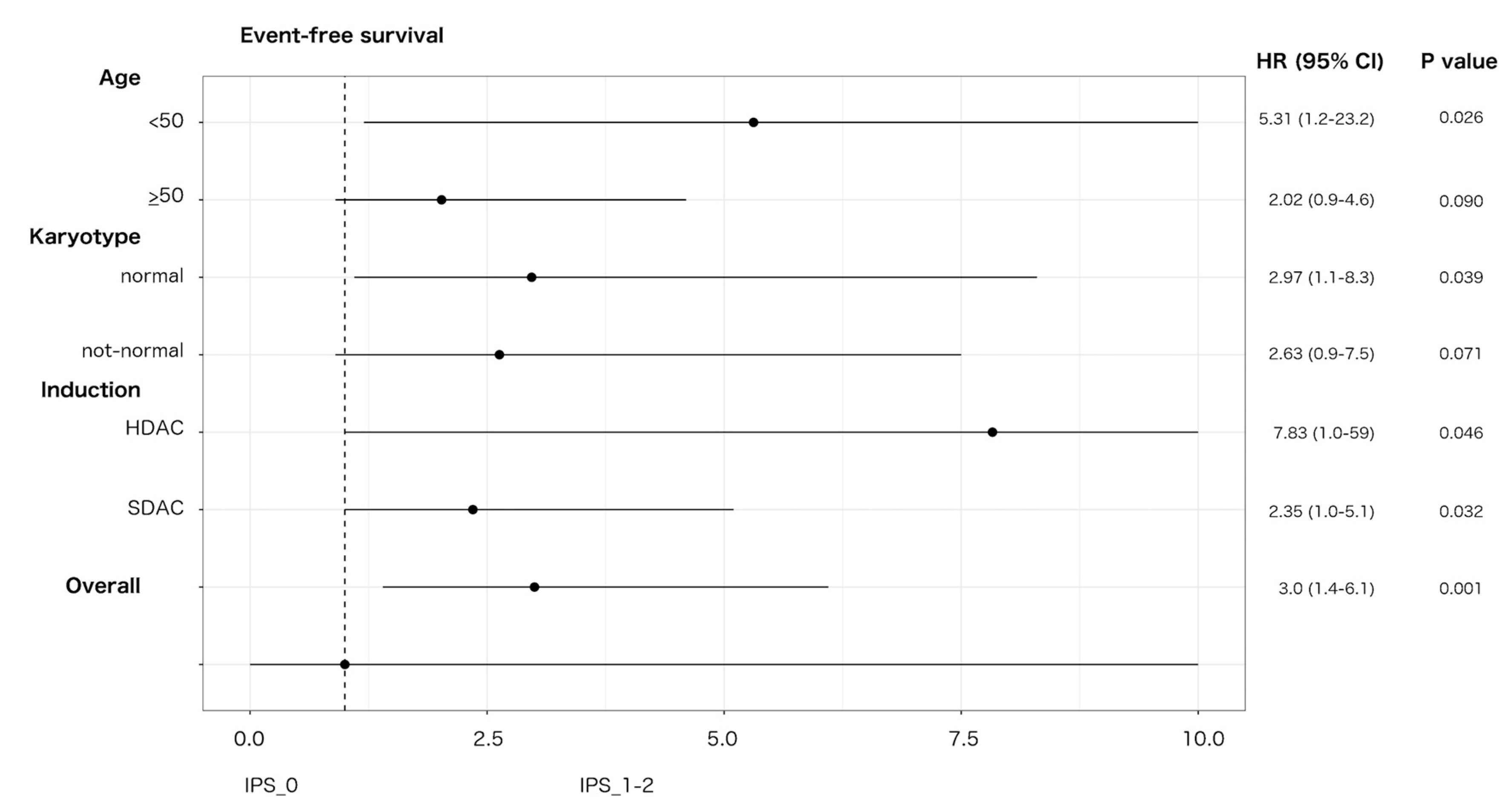
3.5. Interaction with Treatment Covariates in TN-AML
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.; Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Stein, A.S.; Carter, N.H.; Ikle, D.; Forman, S.J.; Slovak, M.L. Prognostic impact of acute myeloid leukemia classification. Importance of detection of recurring cytogenetic abnormalities and multilineage dysplasia on survival. Am. J. Clin. Pathol. 2003, 119, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Wandt, H.; Schäkel, U.; Kroschinsky, F.; Prange-Krex, G.; Mohr, B.; Thiede, C.; Pascheberg, U.; Soucek, S.; Schaich, M.; Ehninger, G. MLD according to the WHO classification in AML has no correlation with age and no independent prognostic relevance as analyzed in 1766 patients. Blood 2008, 111, 1855–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miesner, M.; Haferlach, C.; Bacher, U.; Weiss, T.; Macijewski, K.; Kohlmann, A.; Klein, H.-U.; Dugas, M.; Kern, W.; Schnittger, S.; et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: A comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood 2010, 116, 2742–2751. [Google Scholar] [PubMed]
- Weinberg, O.K.; Seetharam, M.; Ren, L.; Seo, K.; Ma, L.; Merker, J.D.; Gotlib, J.; Zehnder, J.L.; Arber, D.A. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood 2009, 113, 1906–1908. [Google Scholar] [CrossRef] [PubMed]
- Rozman, M.; Navarro, J.-T.; Arenillas, L.; Aventín, A.; Giménez, T.; Alonso, E.; Perea, G.; Camós, M.; Navarrete, M.; Tuset, E.; et al. Multilineage dysplasia is associated with a poorer prognosis in patients with de novo acute myeloid leukemia with intermediate-risk cytogenetics and wild-type NPM. Ann. Hematol. 2014, 93, 1695–1703. [Google Scholar] [CrossRef]
- Falini, B.; Macijewski, K.; Weiss, T.; Bacher, U.; Schnittger, S.; Kern, W.; Kohlmann, A.; Klein, H.-U.U.; Vignetti, M.; Piciocchi, A.; et al. Multilineage dysplasia has no impact on biologic, clinicopathologic, and prognostic features of AML with mutated nucleophosmin (NPM1). Blood 2010, 115, 3776–3786. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Beyá, M.; Rozman, M.; Pratcorona, M.; Torrebadell, M.; Camós, M.; Aguilar, J.L.; Esteve, J. The prognostic value of multilineage dysplasia in de novo acute myeloid leukemia patients with intermediate-risk cytogenetics is dependent on NPM1 mutational status. Blood 2010, 116, 6147–6148. [Google Scholar] [CrossRef]
- Bacher, U.; Schnittger, S.; Macijewski, K.; Grossmann, V.; Kohlmann, A.; Alpermann, T.; Kowarsch, A.; Nadarajah, N.; Kern, W.; Haferlach, C.; et al. Multilineage dysplasia does not influence prognosis in CEBPA-mutated AML, supporting the WHO proposal to classify these patients as a unique entity. Blood 2012, 119, 4719–4722. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, F.; Ponziani, V.; Bonetti, M.; Bencini, S.; Cutini, I.; Gianfaldoni, G.; Scappini, B.; Pancani, F.; Rondelli, T.; Benelli, M.; et al. Multilineage dysplasia as assessed by immunophenotype has no impact on clinical-biological features and outcome of NPM1-mutated acute myeloid leukemia. Exp. Hematol. 2015, 43, 869–879.e22. [Google Scholar] [CrossRef]
- Mannelli, F.; Ponziani, V.; Bencini, S.; Bonetti, M.I.; Benelli, M.; Cutini, I.; Gianfaldoni, G.; Scappini, B.; Pancani, F.; Piccini, M.; et al. CEBPA–double-mutated acute myeloid leukemia displays a unique phenotypic profile: A reliable screening method and insight into biological features. Haematologica 2016, 102, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalban-Bravo, G.; Kanagal-Shamanna, R.; Class, C.A.; Sasaki, K.; Ravandi, F.; Cortes, J.E.; Daver, N.; Takahashi, K.; Short, N.J.; DiNardo, C.D.; et al. Outcomes of acute myeloid leukemia with myelodysplasia related changes depend on diagnostic criteria and therapy. Am. J. Hematol. 2020, 95, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Matarraz, S.; López, A.; Barrena, S.; Fernandez, C.; Jensen, E.; Flores-Montero, J.; Rasillo, A.; Sayagues, J.M.; Sánchez, M.L.; Bárcena, P.; et al. Bone marrow cells from myelodysplastic syndromes show altered immunophenotypic profiles that may contribute to the diagnosis and prognostic stratification of the disease: A pilot study on a series of 56 patients. Cytom. Part B Clin. Cytom. 2010, 78, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Van de Loosdrecht, A.A.; Alhan, C.; Béné, M.C.; Porta, M.G.D.; Dräger, A.M.; Feuillard, J.; Font, P.; Germing, U.; Haase, D.; Homburg, C.H.; et al. Standardization of flow cytometry in myelodysplastic syndromes: Report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica 2009, 94, 1124–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, K.; Kishikawa, Y.; Satoh, C.; Tamura, H.; Dan, K.; Hayashi, A. Diagnostic application of flow cytometric characteristics of CD34+ cells in low-grade myelodysplastic syndromes. Blood 2006, 108, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, M.G.; Picone, C.; Pascutto, C.; Malcovati, L.; Tamura, H.; Handa, H.; Czader, M.; Freeman, S.; Vyas, P.; Porwit, A.; et al. Multicenter validation of a reproducible flow cytometric score for the diagnosis of low-grade myelodysplastic syndromes: Results of a European LeukemiaNET study. Haematologica 2012, 97, 1209–1217. [Google Scholar] [CrossRef]
- Mitelman, F. (Ed.) ISCN 1995: An International System for Human Cytogenetic Nomenclature (1995): Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature; Karger Medical and Scientific Publishers: Memphis, TN, USA, 1994. [Google Scholar]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K.; Group, N. On behalf of the Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; Starza, R.; Diverio, D.; Colombo, E.; Santucci, A.; et al. Cytoplasmic Nucleophosmin in Acute Myelogenous Leukemia with a Normal Karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef]
- Noguera, N.; Ammatuna, E.; Zangrilli, D.; Lavorgna, S.; Divona, M.; Buccisano, F.; Amadori, S.; Mecucci, C.; Falini, B.; Lo-Coco, F. Simultaneous detection of NPM1 and FLT3-ITD mutations by capillary electrophoresis in acute myeloid leukemia. Leukemia 2005, 19, 1479–1482. [Google Scholar] [CrossRef]
- Kottaridis, P.; Gale, R.; Frew, M.; Harrison, G.; Langabeer, S.; Belton, A.; Walker, H.; Wheatley, K.; Bowen, D.; Burnett, A.; et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001, 98, 1752–1759. [Google Scholar]
- Pabst, T.; Mueller, B.U.; Zhang, P.; Radomska, H.S.; Narravula, S.; Schnittger, S.; Behre, G.; Hiddemann, W.; Tenen, D.G. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-α (C/EBPα), in acute myeloid leukemia. Nat. Genet. 2001, 27, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, A.T.; et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Freeman, S.; Kjeldsen, L.; Hunter, A.E.; Yin, J.; Craddock, C.F.; Dufva, I.H.; et al. Addition of Gemtuzumab Ozogamicin to Induction Chemotherapy Improves Survival in Older Patients with Acute Myeloid Leukemia. J. Clin. Oncol. 2012, 30, 3924–3931. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef] [Green Version]
- Lancet, J.E.; Cortes, J.E.; Hogge, D.E.; Tallman, M.S.; Kovacsovics, T.J.; Damon, L.E.; Komrokji, R.; Solomon, S.R.; Kolitz, J.E.; Cooper, M.; et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 2014, 123, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2016, 34, 2684–2692. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-S.; Kim, H.-J.; Kim, Y.-K.; Jung, S.-H.; Yang, D.-H.; Lee, J.-J.; Kim, N.Y.; Choi, S.H.; Jung, C.W.; Jang, J.-H.; et al. Transplant outcomes of the triple-negative NPM1/FLT3-ITD/CEBPA mutation subgroup are equivalent to those of the favourable ELN risk group, but significantly better than the intermediate-I risk group after allogeneic transplant in normal-karyotype AML. Ann. Hematol. 2016, 95, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Labopin, M.; Socié, G.; Daguindau, E.; Volin, L.; Huynh, A.; Bourhis, J.H.; Milpied, N.; Cornelissen, J.; Chevallier, P.; et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood 2015, 126, 2062–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidrich, K.; Thiede, C.; Schäfer-Eckart, K.; Schmitz, N.; Aulitzky, W.E.; Krämer, A.; Rösler, W.; Hänel, M.; Einsele, H.; Baldus, C.D.; et al. Allogeneic hematopoietic cell transplantation in intermediate risk acute myeloid leukemia negative forFLT3-ITD, NPM1- or biallelic CEBPA mutations. Ann. Oncol. 2017, 28, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
| Overall n = 302 | |
| Age, median (range) | 56 (18–75) |
| WBC, ×109/L, median (range) | 13.9 (0.6–435.0) |
| Hb, g/dL, median (range) | 9.0 (3.4–14.9) |
| Plt, ×109/L, median (range) | 50 (3–373) |
| Bone marrow blasts, %, median (range) | 90 (20–100) |
| Karyotype, n (%) | |
| Favorable | 20 (6.6) |
| t(8;21)/RUNX1-RUNX1T1 | 11 (3.6) |
| inv(16)/CBF-MYH11 | 9 (3.0) |
| Normal | 174 (57.6) |
| Intermediate, non-normal | 31 (10.3) |
| Adverse | 58 (19.2) |
| complex | 38 (12.6) |
| chromosomes 5/7 abnormalities | 14 (4.6) |
| chromosome 3 abnormalities | 4 (1.3) |
| t(6;9) | 2 (0.7) |
| Lack of growth | 19 (6.3) |
| Molecular genetics, n (%) | |
| NPM1-mutated | 115 (38.0) |
| FLT3-ITD | 73 (24.2) |
| FLT3-TKD | 7 (2.3) |
| CEBPA-DM | 13 (4.3) |
| WHO Classification, n (%) | |
| Recurrent genetic abnormalities | 146 (48.3) |
| Therapy-related | 4 (1.3) |
| Myelodisplasia-related changes | 75 (24.8) |
| Not otherwise classified | 77 (25.6) |
| ELN 2010 risk groups, n (%) | |
| Favorable | 90 (29.8) |
| Intermediate-1 | 129 (42.7) |
| Intermediate-2 | 25 (8.3) |
| Adverse | 58 (19.2) |
| IPS_0 n = 22 (26.5%) | IPS_1-2 n = 61 (73.5%) | p Value | |
|---|---|---|---|
| Age, median (range) | 56.5 (35–72) | 56 (22–73) | 0.958 |
| WBC, x109/L, median (range) | 2.2 (0.6–36.9) | 5.7 (1.0–220) | 0.018 |
| Hb, g/dL, median (range) | 9.5 (4.8–13.3) | 9.0 (4.6–14.9) | 0.350 |
| Plt, x109/L, median (range) | 72 (16–281) | 54 (3–271) | 0.070 |
| Bone marrow blasts, %, median (range) | 85 (20–100) | 80 (20–100) | 0.910 |
| Karyotype, n (%) | |||
| Normal | 11 (50.0) | 46 (75.4) | 0.034 |
| Intermediate, non-normal | 8 (36.4) | 12 (19.7) | 0.148 |
| Lack of growth | 3 (13.6) | 3 (4.9) | 0.186 |
| MULTIVARIATE ANALYSIS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | ||||||
| Disease-free survival | ||||||||||
| Age (</≥50 y) | 0.62 (0.25–1.57) | 0.31 | 0.64 (0.26–1.56) | 0.33 | 0.65 (0.27–1.58) | 0.34 | - | - | - | - |
| WBC (cont.) | 1.00 (1.00–1.00) | 0.19 | 1.00 (1.00–1.00) | 0.19 | 1.00 (1.00–1.00) | 0.18 | 1.00 (1.00–1.00) | 0.21 | - | - |
| Karyotype (normal vs. not) | 1.42 (0.53–3.78) | 0.48 | 1.32 (0.58–3.03) | 0.51 | - | - | - | - | - | - |
| Induction (SDAC vs. HDAC) | 0.88 (0.35–2.23) | 0.79 | - | - | - | - | - | - | - | - |
| HSCT (T-dep.) | 0.18 (0.06–0.52) | 0.002 | 0.18 (0.06–0.52) | 0.002 | 0.18 (0.06–0.53) | 0.002 | 0.22 (0.08–0.59) | 0.002 | 0.24 (0.09–0.63) | 0.046 |
| IPS (_0 vs. _1–2) | 3.04 (1.04–8.90) | 0.042 | 2.89 (1.07–7.80) | 0.036 | 2.53 (1.01–6.23) | 0.046 | 2.43 (0.98–6.00) | 0.046 | 2.21 (0.89–5.45) | 0.003 |
| Overall survival | ||||||||||
| Age (</≥50 y) | 0.97 (0.45–2.10) | 0.95 | - | - | - | - | - | - | - | - |
| WBC (cont.) | 1.00 (1.00–1.00) | 0.70 | 1.00 (1.00–1.00) | 0.70 | 1.00 (1.00–1.00) | 0.73 | - | - | - | - |
| Karyotype (normal vs. not) | 2.07 (0.92–4.63) | 0.08 | 2.06 (0.93–4.60) | 0.08 | 1.93 (0.97–3.84) | 0.06 | 1.94 (0.98–3.86) | 0.06 | 1.76 (0.91–3.42) | 0.095 |
| Induction (SDAC vs. HDAC) | 0.87 (0.38–2.00) | 0.75 | 0.88 (0.40–1.96) | 0.75 | - | - | - | - | - | - |
| HSCT (T-dep.) | 0.59 (0.25–1.40) | 0.23 | 0.59 (0.26–1.34) | 0.21 | 0.58 (0.26–1.29) | 0.18 | 0.58 (0.26–1.30) | 0.19 | - | - |
| IPS (_0 vs. _1-2) | 4.95 (1.73–14.1) | 0.003 | 4.93 (1.74–14.0) | 0.003 | 4.69 (1.74–12.6) | 0.002 | 4.60 (1.72–12.3) | 0.002 | 4.61 (1.74–12.2) | 0.002 |
| Event-free survival | ||||||||||
| Age (</≥50 y) | 0.61 (.33–1.13) | 0.12 | 0.62 (0.34–1.14) | 0.12 | 0.66 (0.37–1.18) | 0.16 | 0.69 (0.39–1.22) | 0.20 | - | - |
| WBC (cont.) | 1.00 (1.00–1.00) | 0.34 | 1.00 (1.00–1.00) | 0.35 | 1.00 (1.00–1.00) | 0.41 | - | - | - | - |
| Karyotype (normal vs. not) | 1.17 (0.60–2.27) | 0.65 | - | - | - | - | - | - | - | - |
| Induction (SDAC vs. HDAC) | 0.76 (0.39–1.46) | 0.41 | 0.81 (0.45–1.47) | 0.48 | - | - | - | - | - | - |
| HSCT (T-dep.) | 0.21 (0.07–0.60) | 0.003 | 0.21 (0.08–0.61) | 0.004 | 0.21 (0.07–0.59) | 0.003 | 0.22 (0.08–0.61) | 0.004 | 0.26 (0.09–0.69) | 0.007 |
| IPS (_0 vs. _1–2) | 2.87 (1.31-6.28) | 0.009 | 2.69 (1.29–5.58) | 0.008 | 2.62 (1.27–5.41) | 0.009 | 2.51 (1.21–5.16) | 0.013 | 2.50 (1.22–5.14) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannelli, F.; Bencini, S.; Piccini, M.; Gianfaldoni, G.; Bonetti, M.I.; Peruzzi, B.; Caporale, R.; Scappini, B.; Pancani, F.; Ponziani, V.; et al. Multilineage Dysplasia as Assessed by Immunophenotype in Acute Myeloid Leukemia: A Prognostic Tool in a Genetically Undefined Category. Cancers 2020, 12, 3196. https://doi.org/10.3390/cancers12113196
Mannelli F, Bencini S, Piccini M, Gianfaldoni G, Bonetti MI, Peruzzi B, Caporale R, Scappini B, Pancani F, Ponziani V, et al. Multilineage Dysplasia as Assessed by Immunophenotype in Acute Myeloid Leukemia: A Prognostic Tool in a Genetically Undefined Category. Cancers. 2020; 12(11):3196. https://doi.org/10.3390/cancers12113196
Chicago/Turabian StyleMannelli, Francesco, Sara Bencini, Matteo Piccini, Giacomo Gianfaldoni, Maria Ida Bonetti, Benedetta Peruzzi, Roberto Caporale, Barbara Scappini, Fabiana Pancani, Vanessa Ponziani, and et al. 2020. "Multilineage Dysplasia as Assessed by Immunophenotype in Acute Myeloid Leukemia: A Prognostic Tool in a Genetically Undefined Category" Cancers 12, no. 11: 3196. https://doi.org/10.3390/cancers12113196





