Challenges in Combining Immunotherapy with Radiotherapy in Recurrent/Metastatic Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Biological Rational for Immunoradiotherapy Combination in R/M HNSCC
2.1. Resistance to Anti-PD-1 Therapy in R/M HNSCC
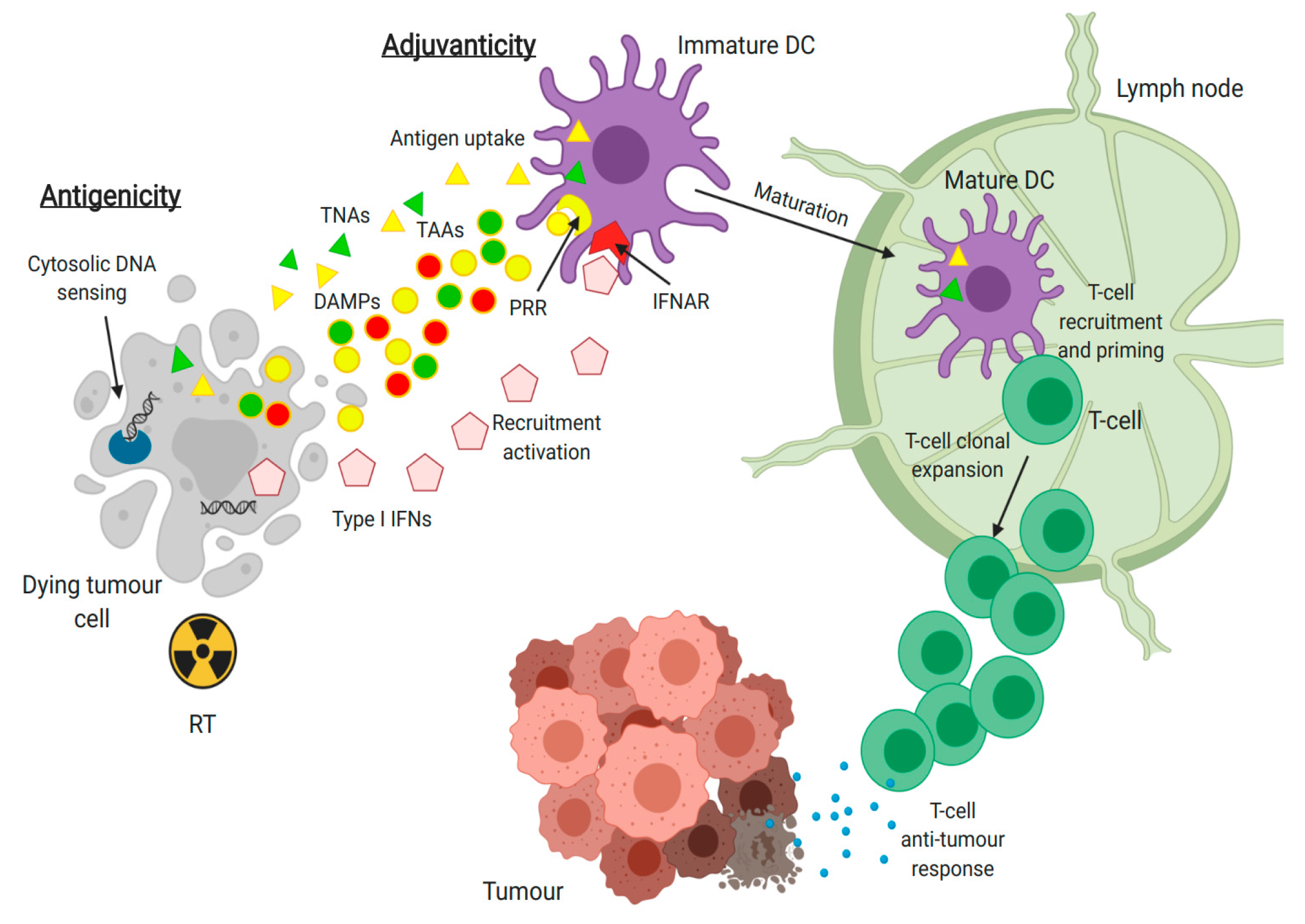
2.2. Immunomodulatory Effects of Radiotherapy
3. Clinical Challenges with Combination of RT and Anti-PD-1 in R/M HNSCC
3.1. Patient Selection
3.2. Fractionation and Dose Selection
3.3. Site and Number of Lesions
3.4. Timing
3.5. Field Selection and Dose Heterogeneity
3.6. Other Outcome Defining Factors and Evaluation of Immunoradiotherapy Efficacy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gatta, G.; Botta, L.; Sánchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Hackl, M.; Zielonke, N.; Oberaigner, W.; Van Eycken, E.; et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Klussmann, J.P. Head and Neck Cancer—New Insights into a Heterogeneous Disease. Oncol. Res. Treat. 2017, 40, 318–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, K.K.; Grønhøj, C.; Jensen, D.H.; Karnov, K.K.S.; Agander, T.K.; Specht, L.; von Buchwald, C. Increasing incidence and survival of head and neck cancers in Denmark: A nation-wide study from 1980 to 2014. Acta Oncol. 2018, 57, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Pastorino, R.; Arzani, D.; Bosetti, C.; Canova, C.; Garavello, W.; La Vecchia, C.; Maule, M.; et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015, 39, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Duprez, F.; Berwouts, D.; De Neve, W.; Bonte, K.; Boterberg, T.; Deron, P.; Huvenne, W.; Rottey, S.; Mareel, M. Distant metastases in head and neck cancer. Head Neck 2017, 39, 1733–1743. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Licitra, L.; Fayette, J.; Even, C.; Blumenschein, G.; Harrington, K.J.; Guigay, J.; Vokes, E.E.; Saba, N.F.; Haddad, R.; et al. Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin. Cancer Res. 2019, 25, 5221–5230. [Google Scholar] [CrossRef] [Green Version]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Nowicki, T.S.; Hu-Lieskovan, S.; Ribas, A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J. 2018, 24, 47–53. [Google Scholar] [CrossRef]
- Wang, H.; Mustafa, A.; Liu, S.; Liu, J.; Lv, D.; Yang, H.; Zou, J. Immune checkpoint inhibitor toxicity in head and neck cancer: From identification to management. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, K.; Jankowska, P.; Hingorani, M. Molecular Biology for the Radiation Oncologist: The 5Rs of Radiobiology meet the Hallmarks of Cancer. Clin. Oncol. 2007, 19, 561–571. [Google Scholar] [CrossRef]
- Wara, W.M. Immunosuppression associated with radiation therapy. Int. J. Radiat. Oncol. 1977, 2, 593–596. [Google Scholar] [CrossRef]
- Kabiljo, J.; Harpain, F.; Carotta, S.; Bergmann, M. Radiotherapy as a backbone for novel concepts in cancer immunotherapy. Cancers 2020, 12, 79. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, S.; Sherman, E.; Tsai, C.J.; Baxi, S.; Aghalar, J.; Eng, J.; Zhi, W.I.; McFarland, D.; Michel, L.S.; Young, R.; et al. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2020, 36, JCO.20.00290. [Google Scholar] [CrossRef]
- Ishida, Y. PD-1: Its Discovery, Involvement in Cancer Immunotherapy, and Beyond. Cells 2020, 9, 1376. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Barbari, C.; Fontaine, T.; Parajuli, P.; Lamichhane, N.; Jakubski, S.; Lamichhane, P.; Deshmukh, R.R. Immunotherapies and Combination Strategies for Immuno-Oncology. Int. J. Mol. Sci. 2020, 21, 5009. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.F.; Blumenschein, G.; Guigay, J.; Licitra, L.; Fayette, J.; Harrington, K.J.; Kiyota, N.; Gillison, M.L.; Ferris, R.L.; Jayaprakash, V.; et al. Nivolumab versus investigator’s choice in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: Efficacy and safety in CheckMate 141 by age. Oral Oncol. 2019, 96, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Wang, Y.-Q.; Lv, J.-W.; Li, Y.-Q.; Chua, M.L.K.; Le, Q.-T.; Lee, N.; Colevas, A.D.; Seiwert, T.; Hayes, D.N.; et al. Identification and validation of novel microenvironment-based immune molecular subgroups of head and neck squamous cell carcinoma: Implications for immunotherapy. Ann. Oncol. 2019, 30, 68–75. [Google Scholar] [CrossRef]
- Brooks, J.M.; Menezes, A.N.; Ibrahim, M.; Archer, L.; Lal, N.; Bagnall, C.J.; von Zeidler, S.V.; Valentine, H.R.; Spruce, R.J.; Batis, N.; et al. Development and Validation of a Combined Hypoxia and Immune Prognostic Classifier for Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 5315–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Keck, M.K.; Zuo, Z.; Khattri, A.; Stricker, T.P.; Brown, C.D.; Imanguli, M.; Rieke, D.; Endhardt, K.; Fang, P.; Bragelmann, J.; et al. Integrative Analysis of Head and Neck Cancer Identifies Two Biologically Distinct HPV and Three Non-HPV Subtypes. Clin. Cancer Res. 2015, 21, 870–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Allen, C.T. Mechanisms of resistance to T cell-based immunotherapy in head and neck cancer. Head Neck 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chikamatsu, K.; Takahashi, G.; Sakakura, K.; Ferrone, S.; Masuyama, K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck 2011, 33, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Chan, L.P.; Cho, S.F. Targeting the Immune Microenvironment in the Treatment of Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.E. Recent advances in antigen processing and presentation. Nat. Immunol. 2007, 8, 1041–1048. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [Green Version]
- López-Albaitero, A.; Nayak, J.V.; Ogino, T.; Machandia, A.; Gooding, W.; DeLeo, A.B.; Ferrone, S.; Ferris, R.L. Role of Antigen-Processing Machinery in the In Vitro Resistance of Squamous Cell Carcinoma of the Head and Neck Cells to Recognition by CTL. J. Immunol. 2006, 176, 3402–3409. [Google Scholar] [CrossRef] [Green Version]
- Meissner, M.; Reichert, T.E.; Kunkel, M.; Gooding, W.; Whiteside, T.L.; Ferrone, S.; Seliger, B. Defects in the human leukocyte antigen class I antigen-processing machinery in head and neck squamous cell carcinoma: Association with clinical outcome. Clin. Cancer Res. 2005, 11, 2552–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concha-Benavente, F.; Srivastava, R.; Ferrone, S.; Ferris, R.L. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016, 58, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restifo, N.P.; Marincola, F.M.; Kawakami, Y.; Taubenberger, J.; Yannelli, J.R.; Rosenberg, S.A. Loss of Functional Beta2-Microglobulin in Metastatic Melanomas From Five Patients Receiving Immunotherapy. JNCI J. Natl. Cancer Inst. 1996, 88, 100–108. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Fernández, M.A.; Ruiz-Cabello, F.; Oliva, M.R.; Cabrera, T.; Jimenez, P.; López Nevot, M.A.; Garrido, F. β2-microglobulin gene mutation is not a common mechanism of HLA class I total loss in human tumors. Int. J. Clin. Lab. Res. 2000, 30, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Hunt, J.L.; Ferrone, S. Human Leukocyte Antigen (HLA) Class I Defects in Head and Neck Cancer: Molecular Mechanisms and Clinical Significance. Immunol. Res. 2005, 33, 113–134. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Oweida, A.; Hararah, M.K.; Phan, A.; Binder, D.; Bhatia, S.; Lennon, S.; Bukkapatnam, S.; Van Court, B.; Uyanga, N.; Darragh, L.; et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin. Cancer Res. 2018, 24, 5368–5380. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef]
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 Positive Human T-Lymphocytes by an in Vitro Colony Formation Assay. Radiat. Res. 1990, 123, 224. [Google Scholar] [CrossRef]
- Drabsch, Y.; Ten Dijke, P. TGF-β signaling in breast cancer cell invasion and bone metastasis. J. Mammary Gland Biol. Neoplasia 2011, 16, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcellos-Hoff, M.H.; Derynck, R.; Tsang, M.L.S.; Weatherbee, J.A. Transforming growth factor-β activation in irradiated murine mammary gland. J. Clin. Investig. 1994, 93, 892–899. [Google Scholar] [CrossRef]
- Rödel, F.; Frey, B.; Capalbo, G.; Gaipl, U.; Keilholz, L.; Voll, R.; Hildebrandt, G.; Rödel, C. Discontinuous induction of X-linked inhibitor of apoptosis in EA.hy.926 endothelial cells is linked to NF-κB activation and mediates the anti-inflammatory properties of low-dose ionising-radiation. Radiother. Oncol. 2010, 97, 346–351. [Google Scholar] [CrossRef]
- Suwa, T.; Saio, M.; Umemura, N.; Yamashita, T.; Toida, M.; Shibata, T.; Takami, T. Preoperative radiotherapy contributes to induction of proliferative activity of CD8+ tumor-infiltrating T-cells in oral squamous cell carcinoma. Oncol. Rep. 2006, 15, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Arina, A.; Beckett, M.; Fernandez, C.; Zheng, W.; Pitroda, S.; Chmura, S.J.; Luke, J.J.; Forde, M.; Hou, Y.; Burnette, B.; et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.-I.; Cheng, P.; Cho, H.-I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Onishi, H.; Wada, J.; Yamasaki, A.; Tanaka, H.; Nakano, K.; Morisaki, T.; Katano, M. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur. J. Immunol. 2010, 40, 197–203. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef]
- Horikawa, N.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Baba, T.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; Konishi, I. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells. Clin. Cancer Res. 2017, 23, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Grapin, M.; Richard, C.; Limagne, E.; Boidot, R.; Morgand, V.; Bertaut, A.; Derangere, V.; Laurent, P.-A.; Thibaudin, M.; Fumet, J.D.; et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: A promising new combination. J. Immunother. Cancer 2019, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, J.; Li, R.; Yin, L.-M.; Deng, L.; Gui, J.; Chen, B.-Q.; Zhou, L.; Meng, M.-B.; Huang, Q.-R.; Mo, X.-M.; et al. Targeting Myeloid-derived Suppressor Cells and Programmed Death Ligand 1 Confers Therapeutic Advantage of Ablative Hypofractionated Radiation Therapy Compared With Conventional Fractionated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Filatenkov, A.; Baker, J.; Mueller, A.M.S.; Kenkel, J.; Ahn, G.O.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-Jones, S.; et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 2015, 21, 3727–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlashi, E.; Chen, A.M.; Boyrie, S.; Yu, G.; Nguyen, A.; Brower, P.A.; Hess, C.B.; Pajonk, F. Radiation-Induced Dedifferentiation of Head and Neck Cancer Cells Into Cancer Stem Cells Depends on Human Papillomavirus Status. Int. J. Radiat. Oncol. 2016, 94, 1198–1206. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Helen Barcellos-Hoff, M.; Formenti, S.C.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Kepp, O.; Kroemer, G. Decoding Cell Death Signals in Inflammation and Immunity. Cell 2010, 140, 798–804. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Paulete, A.R.; Teijeira, A.; Cueto, F.J.; Garasa, S.; Pérez-Gracia, J.L.; Sánchez-Arráez, A.; Sancho, D.; Melero, I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann. Oncol. 2017, 28, xii44–xii55. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Duan, J.; Bai, H.; Wang, Y.; Wan, R.; Wang, X.; Chen, S.; Tian, Y.; Wang, D.; Fei, K.; et al. TCR Repertoire Diversity of Peripheral PD-1 + CD8 + T Cells Predicts Clinical Outcomes after Immunotherapy in Patients with Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 146–154. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Formenti, S.C. Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Chiang, C.; Pauli, E.-K.; Biryukov, J.; Feister, K.F.; Meng, M.; White, E.A.; Münger, K.; Howley, P.M.; Meyers, C.; Gack, M.U. The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling. J. Virol. 2017, 92. [Google Scholar] [CrossRef] [Green Version]
- Lau, L.; Gray, E.E.; Brunette, R.L.; Stetson, D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 2015, 350, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Donnelly, C.R.; Gong, W.; Heath, B.R.; Hao, Y.; Donnelly, L.A.; Moghbeli, T.; Tan, Y.S.; Lin, X.; Bellile, E.; et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Investig. 2020, 130, 1635–1652. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.H.; Bortnik, V.; McMillan, N.A.; Idris, A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019, 132, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Yang, W.; Zhang, L.; Liu, S.; Zhao, M.; Zhou, G.; Wang, L.; Jin, S.; Zhang, Z.; Hu, J. Interferon-alpha promotes immunosuppression through IFNAR1/STAT1 signalling in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 317–330. [Google Scholar] [CrossRef]
- Jonna, S.; Vanderwalde, A.M.; Nieva, J.J.; Poorman, K.A.; Saul, M.; von Buttlar, X.; Hu, J.Y.; Liu, S.V. Impact of prior chemotherapy or radiation therapy on tumor mutation burden in NSCLC. J. Clin. Oncol. 2019, 37, 2627. [Google Scholar] [CrossRef]
- Giordano, F.A.; Veldwijk, M.R.; Herskind, C.; Wenz, F. Radiotherapy, tumor mutational burden, and immune checkpoint inhibitors: Time to do the math. Strahlenther. Onkol. 2018, 194, 873–875. [Google Scholar] [CrossRef] [Green Version]
- Punnanitinont, A.; Kannisto, E.D.; Matsuzaki, J.; Odunsi, K.; Yendamuri, S.; Singh, A.K.; Patnaik, S.K. Sublethal Radiation Affects Antigen Processing and Presentation Genes to Enhance Immunogenicity of Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manukian, G.; Bar-Ad, V.; Lu, B.; Argiris, A.; Johnson, J.M. Combining radiation and immune checkpoint blockade in the treatment of head and neck squamous cell carcinoma. Front. Oncol. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Chen, M.; Hong, L.; Zhao, H.; Chen, Q. Crosstalk between PD-1/PD-L1 blockade and its combinatorial therapies in tumor immune microenvironment: A focus on HNSCC. Front. Oncol. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Dunn, L. Immunotherapy Mythbusters in Head and Neck Cancer: The Abscopal Effect and Pseudoprogression. Am. Soc. Clin. Oncol. Educ. B 2019, 352–363. [Google Scholar] [CrossRef]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef] [Green Version]
- Leidner, R.; Bell, R.B.; Young, K.; Curti, B.; Couey, M.; Patel, A.; Watters, A.; Xiao, H.; Bifulco, C.; Piening, B.; et al. Abstract CT182: Neoadjuvant immuno-radiotherapy (NIRT) in head and neck cancer: Phase I/Ib study of combined PD-1/SBRT prior to surgical resection. In Proceedings of the American Association for Cancer Research Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019. CT182. [Google Scholar]
- Tubin, S.; Popper, H.H.; Brcic, L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): Improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiat. Oncol. 2019, 14, 21. [Google Scholar] [CrossRef]
- Babiker, H.M.; Brana, I.; Mahadevan, D.; Owonikoko, T.K.; Calvo, E.; Rischin, D.; Moreno, V.; Papadopoulos, K.P.; Crittenden, M.; Formenti, S.; et al. Phase I expansion cohort results of cemiplimab, a human PD-1 monoclonal antibody, in combination with radiotherapy (RT), cyclophosphamide and GM-CSF, in patients (pts) with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann. Oncol. 2018, 29, X27. [Google Scholar] [CrossRef]
- Faraji, F.; Eisele, D.W.; Fakhry, C. Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2017, 2, 10–18. [Google Scholar] [CrossRef]
- Nguyen-Tan, P.F.; Zhang, Q.; Ang, K.K.; Weber, R.S.; Rosenthal, D.I.; Soulieres, D.; Kim, H.; Silverman, C.; Raben, A.; Galloway, T.J.; et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination With Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. J. Clin. Oncol. 2014, 32, 3858–3867. [Google Scholar] [CrossRef]
- Sun, X.S.; Michel, C.; Babin, E.; De Raucourt, D.; Péchery, A.; Gherga, E.; Géry, B.; Florescu, C.; Bourhis, J.; Thariat, J. Approach to oligometastatic disease in head and neck cancer, on behalf of the GORTEC. Futur. Oncol. 2018, 14, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.-J.; Wang, H.; Liu, H.-H.; Dong, Z.-Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 13404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Patel, J.J.; Levy, D.A.; Nguyen, S.A.; Knochelmann, H.M.; Day, T.A. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma—Systematic review and meta-analysis. Head Neck 2020, 42, 774–786. [Google Scholar] [CrossRef]
- Bol, V.; Grégoire, V. Biological Basis for Increased Sensitivity to Radiation Therapy in HPV-Positive Head and Neck Cancers. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Winter, H.; Meimarakis, G.; Hoffmann, G.; Hummel, M.; Rüttinger, D.; Zilbauer, A.; Stelter, K.; Spelsberg, F.; Jauch, K.-W.; Hatz, R.; et al. Does Surgical Resection of Pulmonary Metastases of Head and Neck Cancer Improve Survival? Ann. Surg. Oncol. 2008, 15, 2915–2926. [Google Scholar] [CrossRef]
- Vengaloor Thomas, T.; Packianathan, S.; Bhanat, E.; Albert, A.; Abraham, A.; Gordy, X.; Kanakamedala, M.; Mehta, D.; Vijayakumar, S. Oligometastatic head and neck cancer: Comprehensive review. Head Neck 2020, 42, 2194–2201. [Google Scholar] [CrossRef]
- Ordoñez, R.; Otero, A.; Jerez, I.; Medina, J.A.; Lupiañez-Pérez, Y.; Gomez-Millan, J. Role of radiotherapy in the treatment of metastatic head and neck cancer. Oncol. Targets. Ther. 2019, 12, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, H.; Wong, W.-L.; McConkey, C.C.; Rahman, J.K.; Robinson, M.; Hartley, A.G.J.; Nutting, C.; Powell, N.; Al-Booz, H.; Robinson, M.; et al. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N. Engl. J. Med. 2016, 374, 1444–1454. [Google Scholar] [CrossRef]
- Thariat, J.; Ang, K.K.; Allen, P.K.; Ahamad, A.; Williams, M.D.; Myers, J.N.; El-Naggar, A.K.; Ginsberg, L.E.; Rosenthal, D.I.; Glisson, B.S.; et al. Prediction of Neck Dissection Requirement After Definitive Radiotherapy for Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2012, 82, e367–e374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoir, M.; Schmitz, S.; Suarez, C.; Strojan, P.; Hutcheson, K.A.; Rodrigo, J.P.; Mendenhall, W.M.; Simo, R.; Saba, N.F.; D’Cruz, A.K.; et al. The current role of salvage surgery in recurrent head and neck squamous cell carcinoma. Cancers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Strojan, P.; Corry, J.; Eisbruch, A.; Vermorken, J.B.; Mendenhall, W.M.; Lee, A.W.M.; Haigentz, M.; Beitler, J.J.; De Bree, R.; Takes, R.P.; et al. Recurrent and second primary squamous cell carcinoma of the head and neck: When and how to reirradiate. Head Neck 2015, 37, 134–150. [Google Scholar] [CrossRef] [Green Version]
- Saleh, K.; Daste, A.; Martin, N.; Pons-Tostivint, E.; Auperin, A.; Herrera-Gomez, R.G.; Baste-Rotllan, N.; Bidault, F.; Guigay, J.; Le Tourneau, C.; et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur. J. Cancer 2019, 121, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, T.; Rordorf, T.; Ikenberg, K.; Huber, G.F.; Guckenberger, M.; Garcia Schueler, H.I. Radiotherapy-induced anti-tumor immune response and immune-related adverse events in a case of recurrent nasopharyngeal carcinoma undergoing anti-PD-1 immunotherapy. BMC Cancer 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, P.; Lucidi, S.; Desideri, I.; Scotti, V.; Casati, M.; Palomba, A.; Ciabatti, C.; Garlatti, P.; Massi, D.; Gallo, O.; et al. Re-irradiation for oligoprogression under Nivolumab in recurrent head and neck squamous cell carcinoma: A case report. Clin. Transl. Radiat. Oncol. 2020, 23, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Mezi, S.; Pomati, G.; Sciattella, P.; Cerbelli, B.; Roberto, M.; Mammone, G.; Cirillo, A.; Cassano, A.; Di Dio, C.; et al. The impact of locoregional treatment on response to nivolumab in advanced platinum refractory head and neck cancer: The need trial. Vaccines 2020, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Patel, R.R.; Verma, V.; Ramapriyan, R.; Barsoumian, H.B.; Cortez, M.A.; Welsh, J.W. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother. Oncol. 2020. [Google Scholar] [CrossRef]
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1306–1310. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Mick, R.; Huang, A.C.; George, S.M.; Farwell, M.D.; Lukens, J.N.; Berman, A.T.; Mitchell, T.C.; Bauml, J.; Schuchter, L.M.; et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br. J. Cancer 2018, 119, 1200–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gengenbacher, N.; Singhal, M.; Augustin, H.G. Preclinical mouse solid tumour models: Status quo, challenges and perspectives. Nat. Rev. Cancer 2017, 17, 751–765. [Google Scholar] [CrossRef]
- Koontz, B.F.; Verhaegen, F.; De Ruysscher, D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol. 2017, 90, 20160441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crittenden, M.R.; Zebertavage, L.; Kramer, G.; Bambina, S.; Friedman, D.; Troesch, V.; Blair, T.; Baird, J.R.; Alice, A.; Gough, M.J. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- de Jong, E.E.C.; Guckenberger, M.; Andratschke, N.; Dieckmann, K.; Hoogeman, M.S.; Milder, M.; Møller, D.S.; Nyeng, T.B.; Tanadini-Lang, S.; Lartigau, E.; et al. Variation in current prescription practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer: Recommendations for prescribing and recording according to the ACROP guideline and ICRU report 91. Radiother. Oncol. 2020, 142, 217–223. [Google Scholar] [CrossRef]
- Price, J.G.; Idoyaga, J.; Salmon, H.; Hogstad, B.; Bigarella, C.L.; Ghaffari, S.; Leboeuf, M.; Merad, M. CDKN1A regulates Langerhans cell survival and promotes treg cell generation upon exposure to ionizing irradiation. Nat. Immunol. 2015, 16, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Stafford, M.; Kaczmar, J. The neoadjuvant paradigm reinvigorated: A review of pre-surgical immunotherapy in HNSCC. Cancers Head Neck 2020, 5, 4. [Google Scholar] [CrossRef]
- Melchardt, T.; Magnes, T.; Hufnagl, C.; Thorner, A.R.; Ducar, M.; Neureiter, D.; Tränkenschuh, W.; Klieser, E.; Gaggl, A.; Rösch, S.; et al. Clonal evolution and heterogeneity in metastatic head and neck cancer—An analysis of the Austrian Study Group of Medical Tumour Therapy study group. Eur. J. Cancer 2018, 93, 69–78. [Google Scholar] [CrossRef]
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.I.; Cortez, M.A.; Erasmus, J.J.; De Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- McGee, H.M.; Daly, M.E.; Azghadi, S.; Stewart, S.L.; Oesterich, L.; Schlom, J.; Donahue, R.; Schoenfeld, J.D.; Chen, Q.; Rao, S.; et al. Stereotactic Ablative Radiation Therapy Induces Systemic Differences in Peripheral Blood Immunophenotype Dependent on Irradiated Site. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; De Stefani, A.; Trevisan, F.; Parati, C.; Inno, A.; Merelli, B.; Ghidini, M.; Bruschieri, L.; Vitali, E.; Cabiddu, M.; et al. Combination of radiotherapy and immunotherapy for brain metastases: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 144, 102830. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Zagar, T.M.; Deal, A.; Moschos, S.J.; Ewend, M.G.; Sasaki-Adams, D.; Lee, C.B.; Collichio, F.A.; Fried, D.; Marks, L.B.; et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: The potential impact of immunotherapy. Anti Cancer Drugs 2017, 28, 669–675. [Google Scholar] [CrossRef]
- Tang, C.; Welsh, J.W.; De Groot, P.; Massarelli, E.; Chang, J.Y.; Hess, K.R.; Basu, S.; Curran, M.A.; Cabanillas, M.E.; Subbiah, V.; et al. Ipilimumab with stereotactic ablative radiation therapy: Phase i results and immunologic correlates from peripheral T cells. Clin. Cancer Res. 2017, 23, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Ahern, E.; Harjunpää, H.; O’Donnell, J.S.; Allen, S.; Dougall, W.C.; Teng, M.W.L.; Smyth, M.J. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology 2018, 7, e1431088. [Google Scholar] [CrossRef] [Green Version]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Samstein, R.; Rimner, A.; Barker, C.A.; Yamada, Y. Combined Immune Checkpoint Blockade and Radiation Therapy: Timing and Dose Fractionation Associated with Greatest Survival Duration Among Over 750 Treated Patients. Int. J. Radiat. Oncol. 2017, 99, S129–S130. [Google Scholar] [CrossRef]
- Haque, S.; Yellu, M.; Randhawa, J.; Hashemi-Sadraei, N. Profile of pembrolizumab in the treatment of head and neck squamous cell carcinoma: Design development and place in therapy. Drug Des. Devel. Ther. 2017, 11, 2537–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Ortega Franco, A.; Plana, M.; Braña, I.; Taberna Sanz, M.; Oliva Bernal, M.; Vázquez, S.; Domenech Vinyolas, M.; Berenguer, G.; Vilajosana, E.; Bergamino, M.; et al. Does hyper-progression exist among head and neck cancer patients treated with immunotherapy? Ann. Oncol. 2017, 28, v379. [Google Scholar] [CrossRef]
- Alfieri, S.; Ferrara, R.; Calareso, G.; Cavalieri, S.; Platini, F.; Mancinelli, M.; Resteghini, C.; Orlandi, E.; Iacovelli, N.A.; Ferella, L.; et al. Hyperprogressive disease (HPD) in head and neck squamous cell carcinoma (HNSCC) patients treated with immune checkpoint inhibitors (ICI). J. Clin. Oncol. 2019, 37, 6029. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—A systematic review and meta-analysis. Radiother. Oncol. 2011, 100, 22–32. [Google Scholar] [CrossRef]
- Vito, A.; El-Sayes, N.; Mossman, K. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, Y.-J.; Griffin, R.J.; Park, I.; Koonce, N.A.; Hui, S.; Kim, M.-S.; Dusenbery, K.E.; Sperduto, P.W.; Cho, L.C. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. Int. J. Radiat. Oncol. 2015, 93, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Wan, X.-L.; Deng, L.; Xue, J.-X.; Wang, L.-S.; Meng, M.-B.; Ling, H.; Zhang, X.; Mo, X.-M.; Lu, Y. Ablative Hypofractionated Radiotherapy Normalizes Tumor Vasculature in Lewis Lung Carcinoma Mice Model. Radiat. Res. 2013, 179, 458–464. [Google Scholar] [CrossRef]
- Song, C.W.; Griffin, R.J.; Lee, Y.J.; Cho, H.; Seo, J.; Park, I.; Kim, H.K.; Kim, D.H.; Kim, M.S.; Dusenbery, K.E.; et al. Reoxygenation and Repopulation of Tumor Cells after Ablative Hypofractionated Radiotherapy (SBRT and SRS) in Murine Tumors. Radiat. Res. 2019, 192, 159–168. [Google Scholar] [CrossRef]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef]
- Leontovich, A.A.; Dronca, R.S.; Suman, V.J.; Ashdown, M.L.; Nevala, W.K.; Thompson, M.A.; Robinson, A.; Kottschade, L.A.; Kaur, J.S.; McWilliams, R.R.; et al. Fluctuation of systemic immunity in melanoma and implications for timing of therapy. Front. Biosci. 2012, E4, 958–975. [Google Scholar] [CrossRef] [Green Version]
- Coventry, B.J.; Ashdown, M.L.; Quinn, M.A.; Markovic, S.N.; Yatomi-Clarke, S.L.; Robinson, A.P. CRP identifies homeostatic immune oscillations in cancer patients: A potential treatment targeting tool? J. Transl. Med. 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubin, S.; Ashdown, M.; Jeremic, B. Time-synchronized immune-guided SBRT partial bulky tumor irradiation targeting hypoxic segment while sparing the peritumoral immune microenvironment. Radiat. Oncol. 2019, 14, 220. [Google Scholar] [CrossRef]
- Kaanders, J.H.A.M.; van den Bosch, S.; Dijkema, T.; Al-Mamgani, A.; Raaijmakers, C.P.J.; Vogel, W.V. Advances in cancer imaging require renewed radiotherapy dose and target volume concepts. Radiother. Oncol. 2020, 148, 140–142. [Google Scholar] [CrossRef]
- Takeshima, T.; Chamoto, K.; Wakita, D.; Ohkuri, T.; Togashi, Y.; Shirato, H.; Kitamura, H.; Nishimura, T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: Its potentiation by combination with TH1 cell therapy. Cancer Res. 2010, 70, 2697–2706. [Google Scholar] [CrossRef] [Green Version]
- Markovsky, E.; Budhu, S.; Samstein, R.M.; Li, H.; Russell, J.; Zhang, Z.; Drill, E.; Bodden, C.; Chen, Q.; Powell, S.N.; et al. An Antitumor Immune Response Is Evoked by Partial-Volume Single-Dose Radiation in 2 Murine Models. Int. J. Radiat. Oncol. 2019, 103, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.M.; Luke, J.J.; Janisch, L.; Hseu, R.; Melotek, J.M.; Chmura, S.J. The ADscopal Effect? Control of Partially Irradiated Versus Completely Irradiated Tumors on a Prospective Trial of Pembrolizumab and SBRT Per NRG-BR001. Int. J. Radiat. Oncol. 2017, 99, S87. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS+/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallahan, D.; Kuchibhotla, J.; Wyble, C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996, 56, 5150–5155. [Google Scholar]
- Savage, T.; Pandey, S.; Guha, C. Postablation modulation after single high-dose radiation therapy improves tumor control via enhanced immunomodulation. Clin. Cancer Res. 2020, 26, 910–921. [Google Scholar] [CrossRef]
- Yamazaki, H.; Ogita, M.; Himei, K.; Nakamura, S.; Kotsuma, T.; Yoshida, K.; Yoshioka, Y. Carotid blowout syndrome in pharyngeal cancer patients treated by hypofractionated stereotactic re-irradiation using CyberKnife: A multi-institutional matched-cohort analysis. Radiother. Oncol. 2015, 115, 67–71. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Boivin, G.; Kalambaden, P.; Faget, J.; Rusakiewicz, S.; Montay-Gruel, P.; Meylan, E.; Bourhis, J.; Lesec, G.; Vozenin, M.-C. Cellular Composition and Contribution of Tertiary Lymphoid Structures to Tumor Immune Infiltration and Modulation by Radiation Therapy. Front. Oncol. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Ladányi, A.; Kiss, J.; Somlai, B.; Gilde, K.; Fejős, Z.; Mohos, A.; Gaudi, I.; Tímár, J. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 2007, 56, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Hori, R.; Shinohara, S.; Kojima, T.; Kagoshima, H.; Kitamura, M.; Tateya, I.; Tamaki, H.; Kumabe, Y.; Asato, R.; Harada, H.; et al. Real-World Outcomes and Prognostic Factors in Patients Receiving Nivolumab Therapy for Recurrent or Metastatic Head and Neck Carcinoma. Cancers 2019, 11, 1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kwon, M.; Kim, B.; Jung, H.A.; Sun, J.-M.; Lee, S.-H.; Park, K.; Ahn, M.-J. Clinical outcomes of immune checkpoint inhibitors for patients with recurrent or metastatic head and neck cancer: Real-world data in Korea. BMC Cancer 2020, 20, 727. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Elkrief, A.; Derosa, L.; Zitvogel, L.; Kroemer, G.; Routy, B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes 2019, 10, 424–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenclares, P.; Bhide, S.A.; Sandoval-Insausti, H.; Pialat, P.; Gunn, L.; Melcher, A.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur. J. Cancer 2020, 131, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-de-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2019, 130, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Joseph, N.T.; Shankar, S.R.; Narasimhamurthy, R.K.; Rao, S.B.S.; Mumbrekar, K.D. Bi-Directional interactions between microbiota and ionizing radiation in head and neck and pelvic radiotherapy – clinical relevance. Int. J. Radiat. Biol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Yamanouchi, K.; Tsujiguchi, T.; Sakamoto, Y.; Ito, K. Short-term follow-up of intestinal flora in radiation-exposed mice. J. Radiat. Res. 2019, 60, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Paulos, C.M.; Wrzesinski, C.; Kaiser, A.; Hinrichs, C.S.; Chieppa, M.; Cassard, L.; Palmer, D.C.; Boni, A.; Muranski, P.; Yu, Z.; et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Investig. 2007, 117, 2197–2204. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Pang, J.; Gold, K.A.; Gutkind, J.S.; Califano, J.A.; Mell, L.K.; Cohen, E.E.W.; Sharabi, A.B. Immune modulation of head and neck squamous cell carcinoma and the tumor microenvironment by conventional therapeutics. Clin. Cancer Res. 2019, 25, 4211–4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, R.; Firat, E.; Gaedicke, S.; Guffart, E.; Watanabe, T.; Niedermann, G. Cisplatin facilitates radiation-induced abscopal effects in conjunction with PD-1 checkpoint blockade through CXCR3/CXCL10-mediated T-cell recruitment. Clin. Cancer Res. 2019, 25, 7243–7255. [Google Scholar] [CrossRef] [Green Version]
- Kroon, P.; Frijlink, E.; Iglesias-Guimarais, V.; Volkov, A.; van Buuren, M.M.; Schumacher, T.N.; Verheij, M.; Borst, J.; Verbrugge, I. Radiotherapy and Cisplatin Increase Immunotherapy Efficacy by Enabling Local and Systemic Intratumoral T-cell Activity. Cancer Immunol. Res. 2019, 7, 670–682. [Google Scholar] [CrossRef]
- Chen, D.; Verma, V.; Patel, R.R.; Barsoumian, H.B.; Cortez, M.A.; Welsh, J.W. Absolute Lymphocyte Count Predicts Abscopal Responses and Outcomes in Patients Receiving Combined Immunotherapy and Radiotherapy: A prospective-retrospective analysis of 3 phase I/II Trials. Int. J. Radiat. Oncol. 2020, 108, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Marciscano, A.E.; Ghasemzadeh, A.; Nirschl, T.R.; Theodros, D.; Kochel, C.M.; Francica, B.J.; Muroyama, Y.; Anders, R.A.; Sharabi, A.B.; Velarde, E.; et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin. Cancer Res. 2018, 24, 5058–5071. [Google Scholar] [CrossRef] [Green Version]


| Name | Study Phase | (Planned) Number of Participants | Immunotherapy | RT fx | RT Technique | RT Target | Timing of RT and ICI | Primary Endpoint |
|---|---|---|---|---|---|---|---|---|
| CONFRONT, NCT03844763 | I–II | 71 | Avelumab and cyclophosphamide | 1 × 8 Gy | “Highly conformal” | 1 lesion | ICI 1. day and Q2W; RT 8. day | Toxicity and ORR |
| NCT03283605 | I–II | 35 | Durvalumab and tremelimumab | 30–50 Gy in 3–5 fx | SBRT | 2–5 lesions | RT between 2. and 3. ICI cycle | PFS |
| NCT03539198 | I | 91 | Nivolumab | 3–5 fx (various doses) | Proton SBRT | 1 lesion | RT between 2. and 3. ICI cycle | ORR |
| REPORT, NCT03317327 | I–II | 20 | Nivolumab | 60 Gy in 1.5 Gy fx BID | NA | LR recurrence or 2. primary | RT starts with the 2. ICI cycle | Toxicity |
| NCT03522584 | I–II | 20 | Durvalumab and tremelimumab | 3 fx (dose unknown) | HIGRT or SBRT | 1–5 lesions | RT during week 3 of ICI | Toxicity |
| NCT02684253 | II, randomized | 65 | Nivolumab | 3 × 9 Gy (randomized to nivolumab +/− RT) | SBRT | 1 lesion | RT between 1. and 2. cycle of ICI | ORR |
| NCT03521570 | II | 51 | Nivolumab | Unknown (completed in 6–6.5 weeks) | IMRT | LR recurrence or 2. primary | RT starts with the 2. ICI cycle | PFS |
| NCT02289209 | II | 48 | Pembrolizumab | 60 Gy in 1.2 Gy fx BID | NA | LR recurrence or 2. primary | RT starts with the 1. ICI cycle | PFS |
| NCT03085719 | II | 26 | Pembrolizumab | High dose in 3 fx and low dose in 2 fx | NA | Minimum 1 lesion | NA | ORR |
| KEYSTROKE, RTOG 3507, NCT03546582 | II, randomized | 102 | Pembrolizumab | NA (over 2 weeks; randomized to SBRT+/− pembrolizumab) | SBRT | LR recurrence or 2. primary | SBRT and then ICI | PFS |
| Keynote-717, IMPORTANCE, NCT03386357 | II, randomized | 130 | Pembrolizumab | 12 × 3 Gy (randomized to pembrolizumab +/− RT) | NA | 1–3 lesions | ICI on the 3. day of RT | ORR |
| NCT04454489 | II | 15 | Pembrolizumab | Quad-shot RT | NA | At least 1 lesion in the head and neck region | RT starts between ICI cycles 2 and 3 | ORR |
| NCT04399785 | II | 34 | Camrelizumab | NA | SBRT | NA | NA | ORR |
| Parameter | Recommendation | Explanation |
|---|---|---|
| RT regimen | SBRT (multiple fractions of 6–15 Gy) | |
| Number of lesions | Majority of lesions | |
| Timing of RT | Concurrent or close to ICI | |
| Selection of RT field | Tumor-only |
|
| Dose heterogeneity | Consider delivering high dose to partial tumor volume |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavc, G.; Jesenko, T.; Oražem, M.; Strojan, P. Challenges in Combining Immunotherapy with Radiotherapy in Recurrent/Metastatic Head and Neck Cancer. Cancers 2020, 12, 3197. https://doi.org/10.3390/cancers12113197
Plavc G, Jesenko T, Oražem M, Strojan P. Challenges in Combining Immunotherapy with Radiotherapy in Recurrent/Metastatic Head and Neck Cancer. Cancers. 2020; 12(11):3197. https://doi.org/10.3390/cancers12113197
Chicago/Turabian StylePlavc, Gaber, Tanja Jesenko, Miha Oražem, and Primož Strojan. 2020. "Challenges in Combining Immunotherapy with Radiotherapy in Recurrent/Metastatic Head and Neck Cancer" Cancers 12, no. 11: 3197. https://doi.org/10.3390/cancers12113197






