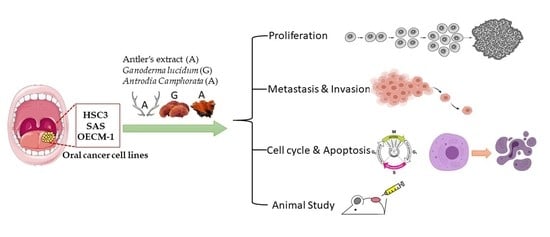
The Novel Herbal Cocktail AGA Alleviates Oral Cancer through Inducing Apoptosis, Inhibited Migration and Promotion of Cell Cycle Arrest at SubG1 Phase
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. AGA Extract Inhibits Oral Cancer Cell Viability and Colony-Forming Ability
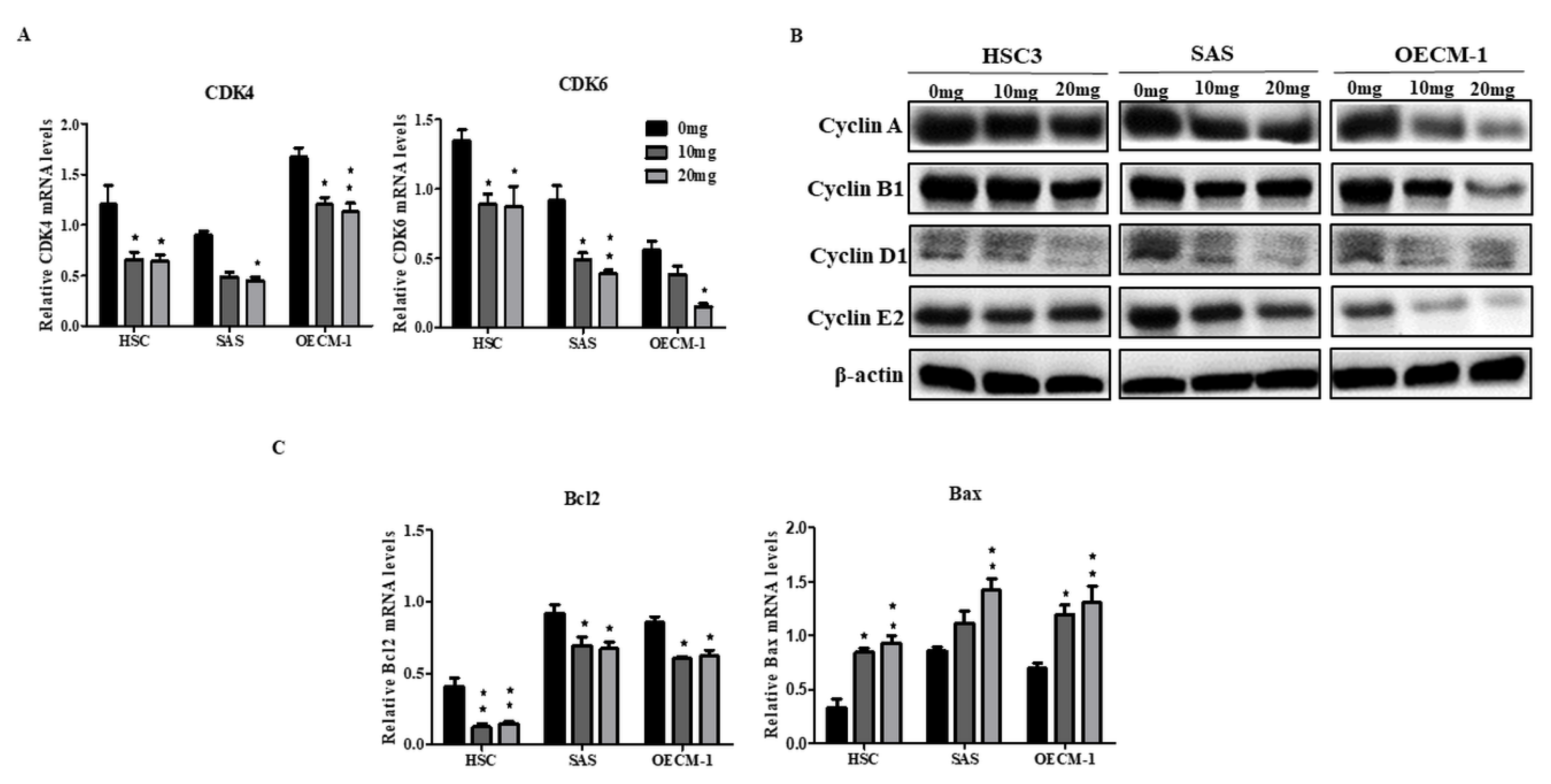
2.2. AGA Extract Impacts the Expression Levels of Cell Cycle and Apoptosis Regulators in Oral Cancer Cells
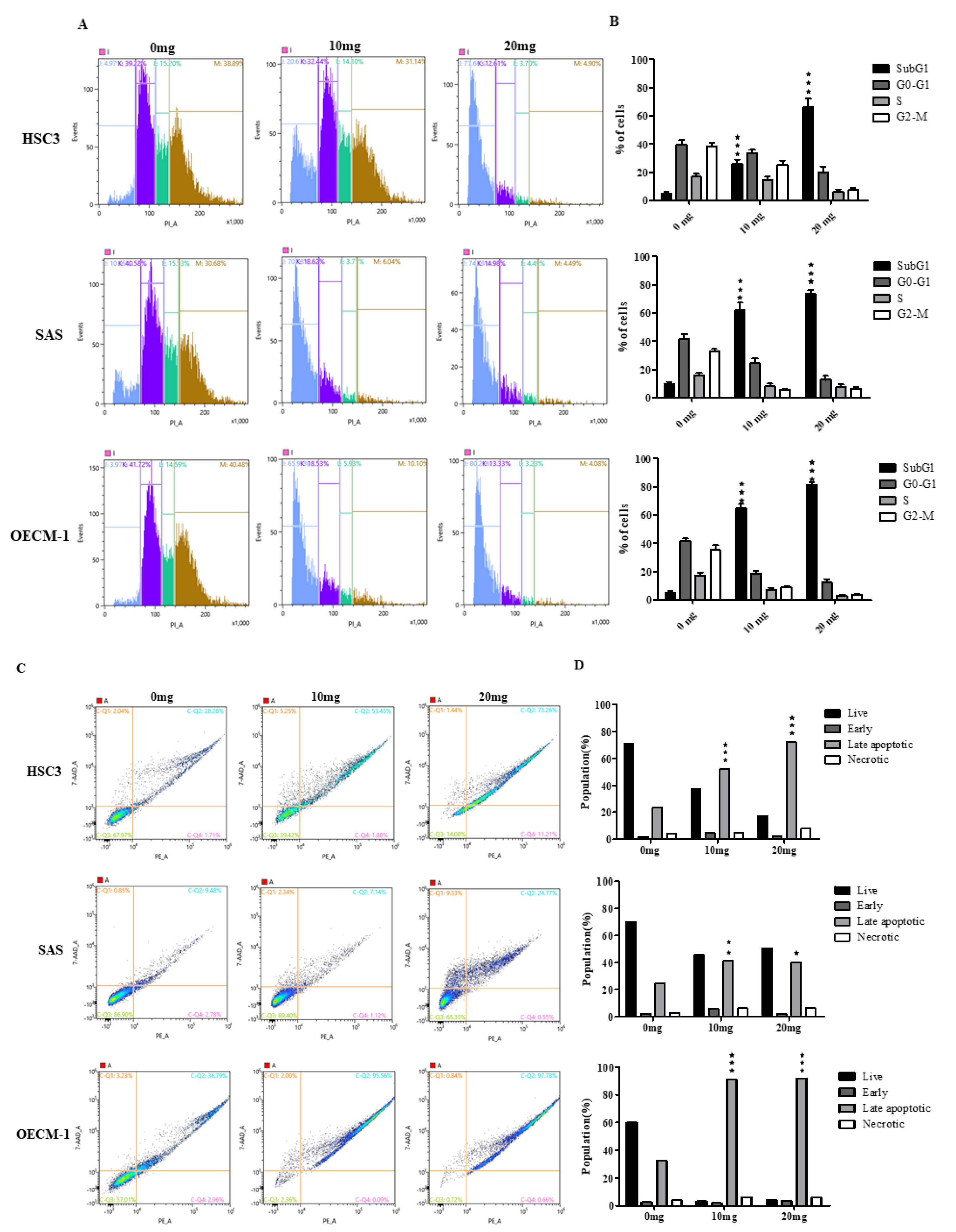
2.3. AGA Extract Influence the Phase of Cell Cycle Progression and Apoptosis in Oral Cancer Cells
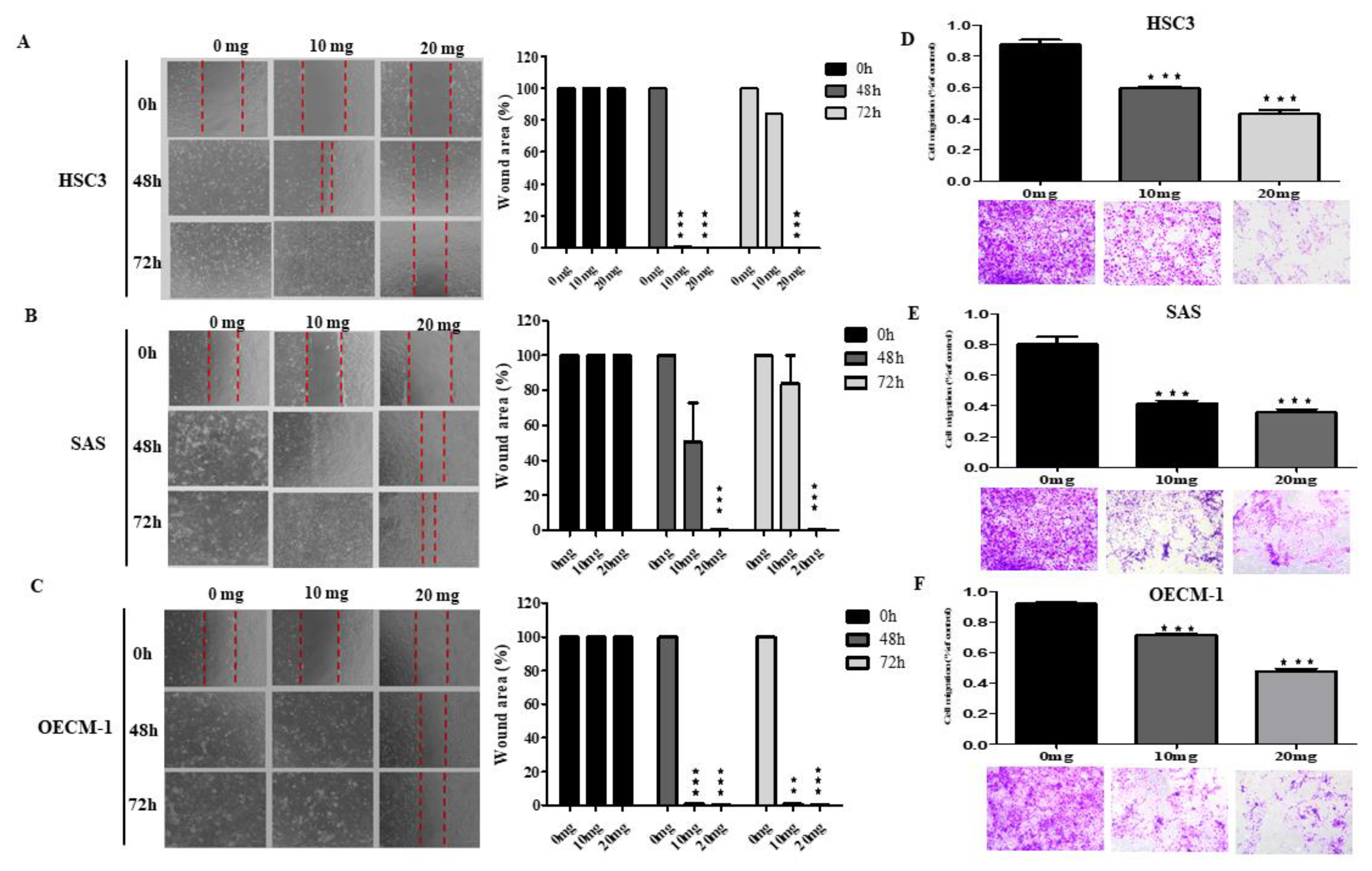
2.4. AGA Extract Inhibits Migration in Oral Cancer Cells
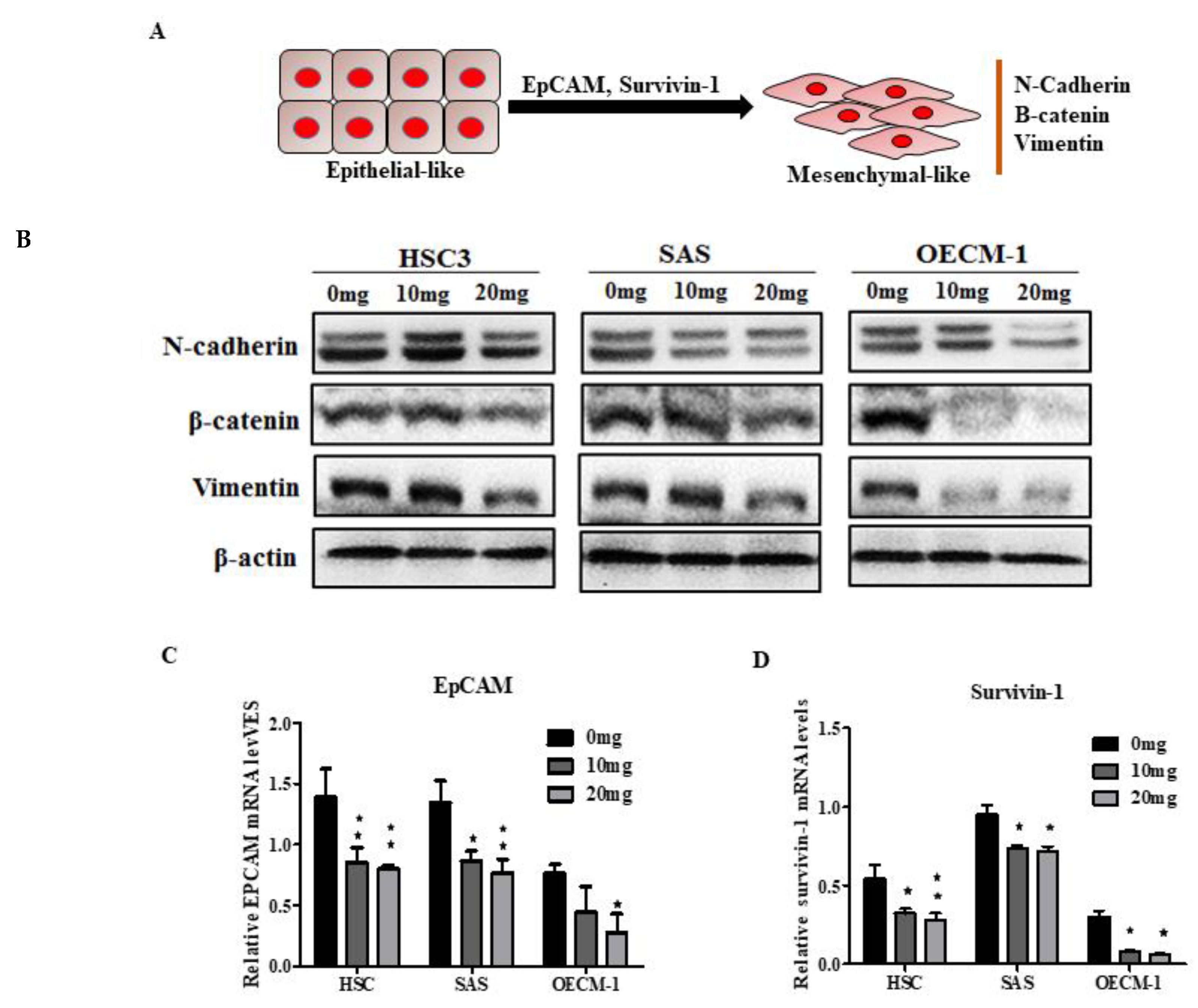
2.5. AGA Extract Influence EMT and its Regulatory Factors in Oral Cancer Cells
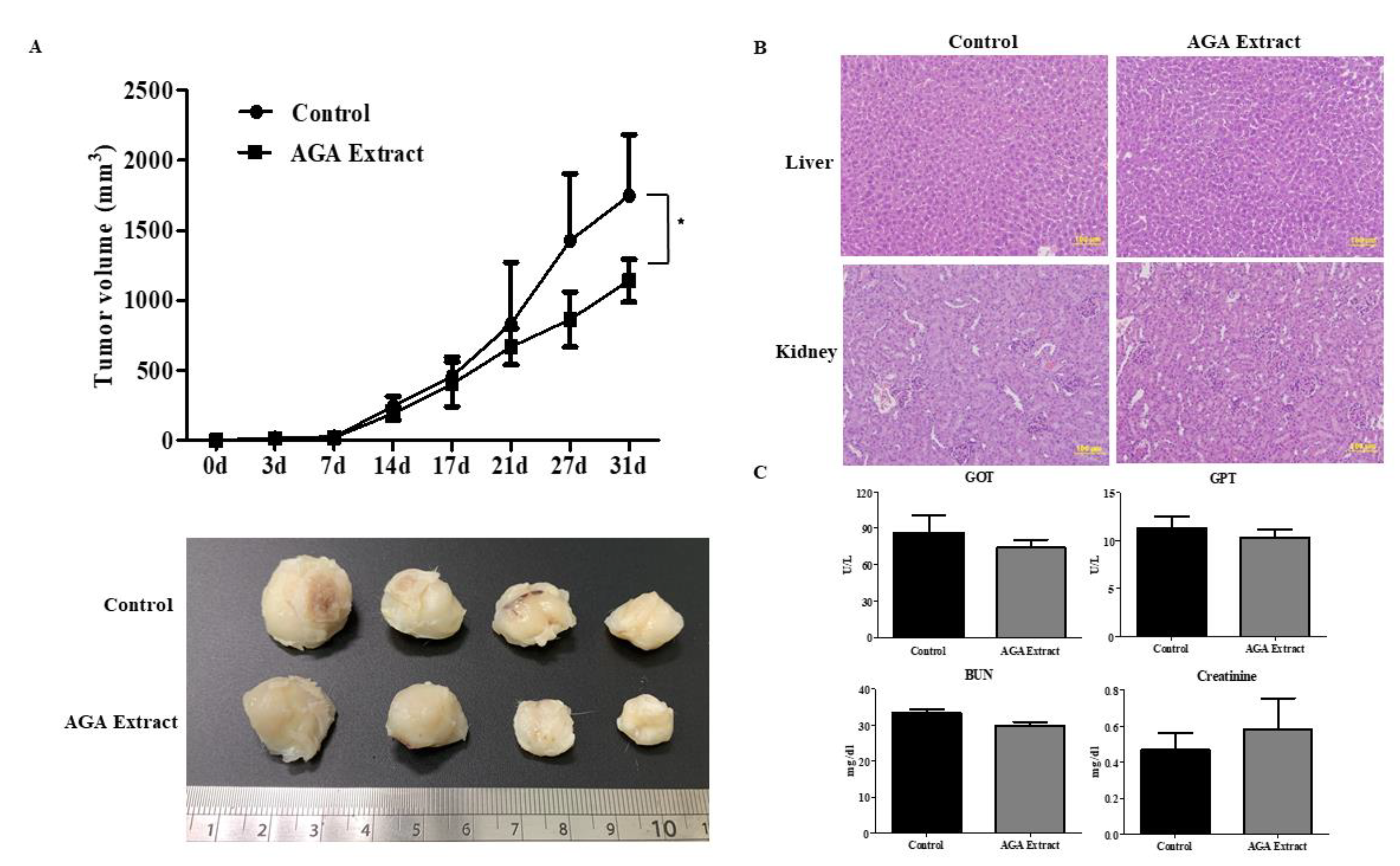
2.6. Inhibitory Effect of AGA on Tumorigenesis in Nude Mice
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Preparation of the Herbal Cocktail AGA
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Wound-Healing Assay
4.6. Transwell Migration and Invasion Assays
4.7. Cell-Cycle Analysis
4.8. Apoptosis Assay
4.9. RNA Extraction and Quantitative Real Time-PCR (qRT-PCR)
- β-actin-forward: 5′–AGAGCTACGAGCTGCCTGAC–3′
- β-actin-reverse: 5′–AGCACTGTGTTGGCGTACAG–3′
- EpCAM- forward: 5′– GCAGCTCAGGAAGAATGTG–3′
- EpCAM–reverse: 5′– CAGCCAGCTTTGAGCAAATGAC–3′
- Survivin-1-forward: 5′–AGGACCACCGACATGTCTACCT–3′
- Survivin-1-reverse: 5′–AAGTCTGGCTCGTTCTCAGTG–3′
- Ki67-forward: 5′ –GCCTGCTCGACCCTACAGA–3′
- Ki67-reverse: 5′–GCTTGTCAACTGCGGTTGC–3′
- PCNA-forward: 5′–CCTGCTGGGATATTAGCTCCA–3′
- PCNA-reverse: 5′–GCGGTAGGTGTCGAAGC–3′
- Bcl2-forward: 5′–CGGAGGCTGGGATGCCTTTG –3′
- Bcl2-reverse: 5′–TTTGGGGCAGGCATGTTGAC–3′
- Bax-forward: 5′-GCCCTTTTGCTTCAGGGTTT–3′
- Bax-reverse: 5′-TCCAATGTCCAGCCCATGAT–3′
- CDK4-forward: 5′– AAATCTTTGACCTGATTGGG–3′
- CDK4-reverse: 5′–CCTTATGTAGATAAGAGTGCTG–3′
- CDK6-forward: 5′–CTGAATGCTCTTGCTCCTTT–3′
- CDK6-reverse: 5′–AAAGTTTTGGTGGTCCTTGA–3′
4.10. Western Blot Analysis
4.11. Animal Studies
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hung, L.C.; Kung, P.T.; Lung, C.H.; Tsai, M.H.; Liu, S.A.; Chiu, L.T.; Huang, K.H.; Tsai, W.C. Assessment of the Risk of Oral Cancer Incidence in A High-Risk Population and Establishment of A Predictive Model for Oral Cancer Incidence Using A Population-Based Cohort in Taiwan. Int. J Environ. Res. Public Health 2020, 17, 665. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.H.; Dubey, N.K.; Li, W.S.; Liu, M.C.; Chiang, H.S.; Leu, S.J.; Shieh, Y.H.; Tsai, F.C.; Deng, W.P. Cordyceps militaris Treatment Preserves Renal Function in Type 2 Diabetic Nephropathy Mice. PLoS ONE 2016, 11, e0166342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.Y.; Huang, C.F.; Li, C.H.; Tsai, C.Y.; Chen, W.H.; Wei, H.J.; Wang, M.F.; Kuo, Y.H.; Cheong, M.L.; Deng, W.P. Osteoporosis Recovery by Antrodia camphorata Alcohol Extracts through Bone Regeneration in SAMP8 Mice. Evid Based Complement. Alternat. Med. 2016, 2016, 2617868. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Zhang, L.; Huo, Y.; Zhang, Y. Bioactive components of velvet antlers and their pharmacological properties. J. Pharm. Biomed. Anal. 2014, 87, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Mo, E.; Yang, Z.; Fang, Z.; Sun, B.; Wang, C.; Zhu, X.; Bao, J.; Sung, C. Effects of Red Deer Antlers on Cutaneous Wound Healing in Full-thickness Rat Models. Asian Australas J. Anim. Sci. 2008, 21, 277–290. [Google Scholar] [CrossRef]
- Chen, J.C.; Hsiang, C.Y.; Lin, Y.C.; Ho, T.Y. Deer Antler Extract Improves Fatigue Effect through Altering the Expression of Genes Related to Muscle Strength in Skeletal Muscle of Mice. Evid. Based Complement. Alternat. Med. 2014, 2014, 540580. [Google Scholar] [CrossRef]
- Dai, T.Y.; Wang, C.H.; Chen, K.N.; Huang, I.N.; Hong, W.S.; Wang, S.Y.; Chen, Y.P.; Kuo, C.Y.; Chen, M.J. The Antiinfective Effects of Velvet Antler of Formosan Sambar Deer (Cervus unicolor swinhoei) on Staphylococcus aureus-Infected Mice. Evid. Based Complement. Alternat. Med. 2011, 2011, 534069. [Google Scholar] [CrossRef] [Green Version]
- Cor, D.; Knez, Z.; Knez Hrncic, M. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: A Review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Geethangili, M.; Tzeng, Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. Based Complement. Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [Green Version]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sanchez-Acuna, G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e174–e179. [Google Scholar] [CrossRef]
- Yang, F.; Ma, J.; Wan, J.; Ha, W.; Fang, C.; Lu, H.; Zhang, W. Epithelial-mesenchymal transition of circulating tumor cells in prostate cancer is promoted by survivin. J. Int. Med. Res. 2020, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.T.; Osmulski, P.; Wang, Y.; Huang, Y.W.; Liu, L.; Ruan, J.; Jin, V.X.; Kirma, N.B.; Gaczynska, M.E.; Huang, T.H. EpCAM-Regulated Transcription Exerts Influences on Nanomechanical Properties of Endometrial Cancer Cells That Promote Epithelial-to-Mesenchymal Transition. Cancer Res. 2016, 76, 6171–6182. [Google Scholar] [CrossRef] [Green Version]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Grand Challenges in Oral Cancers. Front. Oral. Health 2020, 1. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, S.; Li, L.; Mao, M.; Wang, J.; Li, Y.; Wang, Z.; Ye, F.; Huang, L. The Effect of Velvet Antler Proteins on Cardiac Microvascular Endothelial Cells Challenged with Ischemia-Hypoxia. Front. Pharmacol. 2017, 8, 601. [Google Scholar] [CrossRef]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef]
- Lam, F.F.; Ko, I.W.; Ng, E.S.; Tam, L.S.; Leung, P.C.; Li, E.K. Analgesic and anti-arthritic effects of Lingzhi and San Miao San supplementation in a rat model of arthritis induced by Freund’s complete adjuvant. J. Ethnopharmacol. 2008, 120, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Imaizumi, T.; Akiba, M.; Kinoshita, K.; Takahashi, K.; Suzuki, A.; Yano, S.; Horie, S.; Watanabe, K.; Naoi, Y. Antinociceptive components of Ganoderma lucidum. Planta. Med. 1997, 63, 224–227. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Gao, X.; Ma, Z.; Song, Z. L-arginine reduces cell proliferation and ornithine decarboxylase activity in patients with colorectal adenoma and adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 7407–7412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mycielska, M.E.; Mohr, M.T.J.; Schmidt, K.; Drexler, K.; Rümmele, P.; Haferkamp, S.; Schlitt, H.J.; Gaumann, A.; Adamski, J.; Geissler, E.K. Potential Use of Gluconate in Cancer Therapy. Front. Oncol. 2019, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- So, E.Y.; Ouchi, M.; Cuesta-Sancho, S.; Olson, S.L.; Reif, D.; Shimomura, K.; Ouchi, T. Tumor suppression by resistant maltodextrin, Fibersol-2. Cancer Biol. Ther. 2015, 16, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Wang, H.; Wang, Z.; Fu, Y.; Yin, J. Expression of PCNA, Ki-67 and COX-2 in breast cancer based on DCE-MRI image information. J. Infect. Public Health 1876, S1876-0341(19)30212-6. [Google Scholar] [CrossRef]
- Jurikova, M.; Danihel, L.; Polak, S.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Qiu, X.; Mei, J.; Yin, J.; Wang, H.; Wang, J.; Xie, M. Correlation analysis between expression of PCNA, Ki-67 and COX-2 and X-ray features in mammography in breast cancer. Oncol. Lett. 2017, 14, 2912–2918. [Google Scholar] [CrossRef] [Green Version]
- Ben-Izhak, O.; Bar-Chana, M.; Sussman, L.; Dobiner, V.; Sandbank, J.; Cagnano, M.; Cohen, H.; Sabo, E. Ki67 antigen and PCNA proliferation markers predict survival in anorectal malignant melanoma. Histopathology 2002, 41, 519–525. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [Green Version]
- Ruan, W.; Wei, Y.; Popovich, D.G. Distinct Responses of Cytotoxic Ganoderma lucidum Triterpenoids in Human Carcinoma Cells. Phytother. Res. 2015, 29, 1744–1752. [Google Scholar] [CrossRef]
- Wang, T.; Xie, Z.P.; Huang, Z.S.; Li, H.; Wei, A.Y.; Di, J.M.; Xiao, H.J.; Zhang, Z.G.; Cai, L.H.; Tao, X.; et al. Total triterpenoids from Ganoderma Lucidum suppresses prostate cancer cell growth by inducing growth arrest and apoptosis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2015, 35, 736–741. [Google Scholar] [CrossRef]
- Gaumer, S.; Guenal, I.; Brun, S.; Theodore, L.; Mignotte, B. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Differ. 2000, 7, 804–814. [Google Scholar] [CrossRef] [Green Version]
- Paul-Samojedny, M.; Kokocinska, D.; Samojedny, A.; Mazurek, U.; Partyka, R.; Lorenz, Z.; Wilczok, T. Expression of cell survival/death genes: Bcl-2 and Bax at the rate of colon cancer prognosis. Biochim. Biophys. Acta 2005, 1741, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Sawa, H.; Morikawa, J.; Zhang, W.; Shiku, H. Bax induction activates apoptotic cascade via mitochondrial cytochrome c release and Bax overexpression enhances apoptosis induced by chemotherapeutic agents in DLD-1 colon cancer cells. Jpn. J. Cancer Res. 2000, 91, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ren, D.; Guo, W.; Huang, S.; Wang, Z.; Li, Q.; Du, H.; Song, L.; Peng, X. N-cadherin promotes epithelial-mesenchymal transition and cancer stem cell-like traits via ErbB signaling in prostate cancer cells. Int. J. Oncol. 2016, 48, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.Y.; Chen, H.; Liu, C.; Wang, H.Q.; Kang, J.; Li, Y.; Chen, R.Y. Triterpenoids of Ganoderma theaecolum and their hepatoprotective activities. Fitoterapia 2014, 98, 254–259. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Wu, S.-P.; Fang, L.-W.; Hwang, T.-S. Effects of Antrodia camphorata extracts on anti-oxidation, anti-mutagenesis and protection of DNA against hydroxyl radical damage. BMC Complement. Altern. Med. 2015, 15, 237. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.W.; Xia, Y.J.; Liu, X.F.; Wang, G.Q.; Xiong, Z.Q.; Ai, L.Z. Antrodin A from mycelium of Antrodia camphorata alleviates acute alcoholic liver injury and modulates intestinal flora dysbiosis in mice. J. Ethnopharmacol. 2020, 254, 112681. [Google Scholar] [CrossRef]
- Wu, G.; Qian, Z.; Guo, J.; Hu, D.; Bao, J.; Xie, J.; Xu, W.; Lu, J.; Chen, X.; Wang, Y. Ganoderma lucidum extract induces G1 cell cycle arrest, and apoptosis in human breast cancer cells. Am. J. Chin. Med. 2012, 40, 631–642. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef]
- Wei, H.J.; Wu, A.T.; Hsu, C.H.; Lin, Y.P.; Cheng, W.F.; Su, C.H.; Chiu, W.T.; Whang-Peng, J.; Douglas, F.L.; Deng, W.P. The development of a novel cancer immunotherapeutic platform using tumor-targeting mesenchymal stem cells and a protein vaccine. Mol. Ther. 2011, 19, 2249–2257. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.-H.; Chou, Y.-R.; Deng, Y.-H.; Huang, M.-S.; Chien, S.-T.; Quynh, B.T.N.; Wu, C.-Y.; Peláez Achtmann, E.A.; Cheng, H.-C.; Dubey, N.K.; et al. The Novel Herbal Cocktail AGA Alleviates Oral Cancer through Inducing Apoptosis, Inhibited Migration and Promotion of Cell Cycle Arrest at SubG1 Phase. Cancers 2020, 12, 3214. https://doi.org/10.3390/cancers12113214
Lu J-H, Chou Y-R, Deng Y-H, Huang M-S, Chien S-T, Quynh BTN, Wu C-Y, Peláez Achtmann EA, Cheng H-C, Dubey NK, et al. The Novel Herbal Cocktail AGA Alleviates Oral Cancer through Inducing Apoptosis, Inhibited Migration and Promotion of Cell Cycle Arrest at SubG1 Phase. Cancers. 2020; 12(11):3214. https://doi.org/10.3390/cancers12113214
Chicago/Turabian StyleLu, Jui-Hua, Yen-Ru Chou, Yue-Hua Deng, Mao-Suan Huang, Shaw-Ting Chien, Bach Thi Nhu Quynh, Chia-Yu Wu, Edlin Anahi Peláez Achtmann, Hsin-Chung Cheng, Navneet Kumar Dubey, and et al. 2020. "The Novel Herbal Cocktail AGA Alleviates Oral Cancer through Inducing Apoptosis, Inhibited Migration and Promotion of Cell Cycle Arrest at SubG1 Phase" Cancers 12, no. 11: 3214. https://doi.org/10.3390/cancers12113214
APA StyleLu, J.-H., Chou, Y.-R., Deng, Y.-H., Huang, M.-S., Chien, S.-T., Quynh, B. T. N., Wu, C.-Y., Peláez Achtmann, E. A., Cheng, H.-C., Dubey, N. K., & Deng, W.-P. (2020). The Novel Herbal Cocktail AGA Alleviates Oral Cancer through Inducing Apoptosis, Inhibited Migration and Promotion of Cell Cycle Arrest at SubG1 Phase. Cancers, 12(11), 3214. https://doi.org/10.3390/cancers12113214