Midline Meningiomas of the Anterior Skull Base: Surgical Outcomes and a Decision-Making Algorithm for Classic Skull Base Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinical Baseline Characteristics
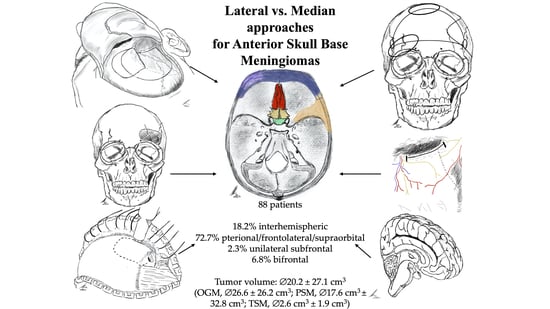
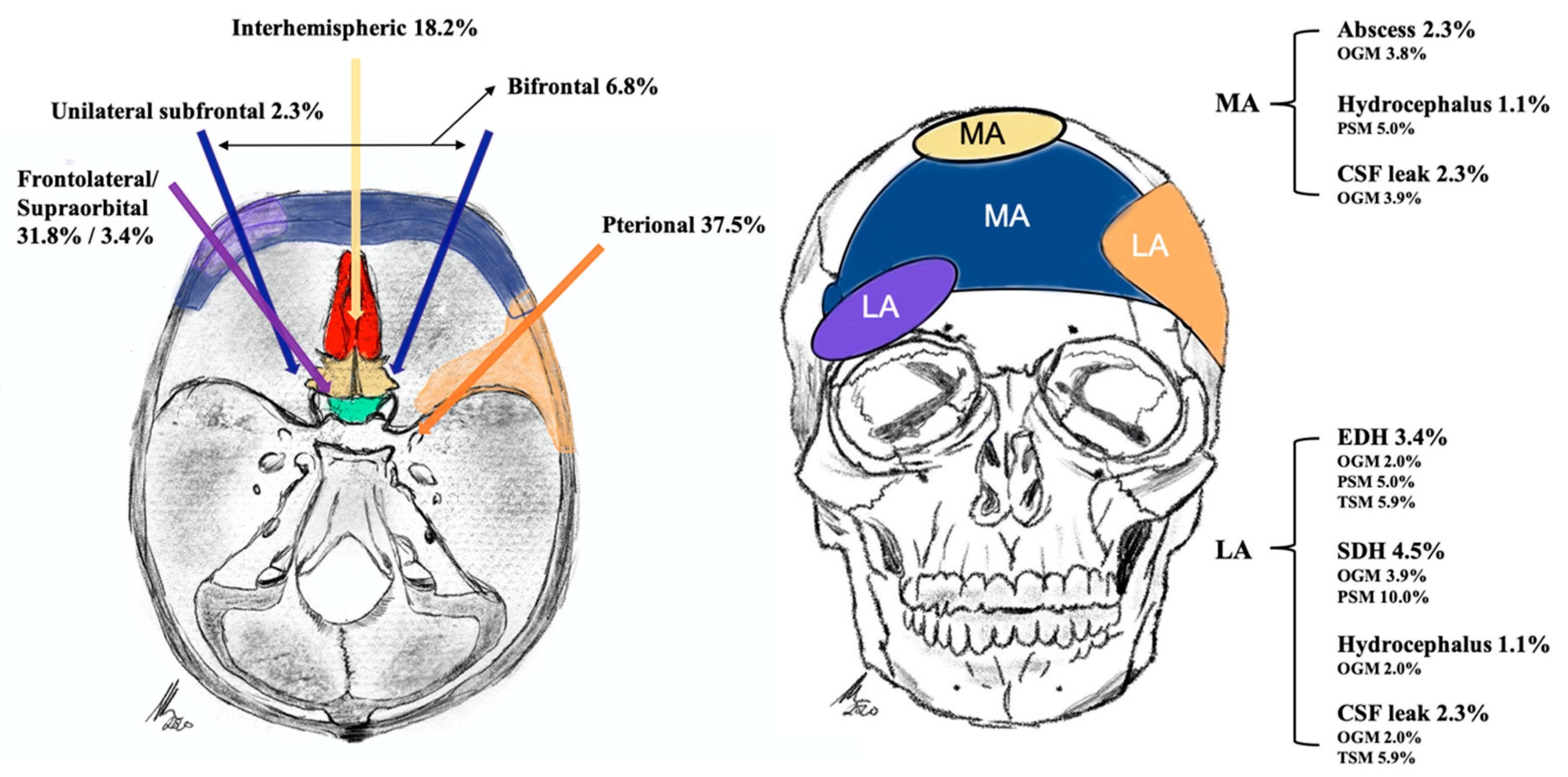
2.2. Tumor Characteristics and Approach-Related Findings
2.3. Functional Outcome
Adverse Events
2.4. Median vs. Lateral Approach Regarding Operative Characteristics
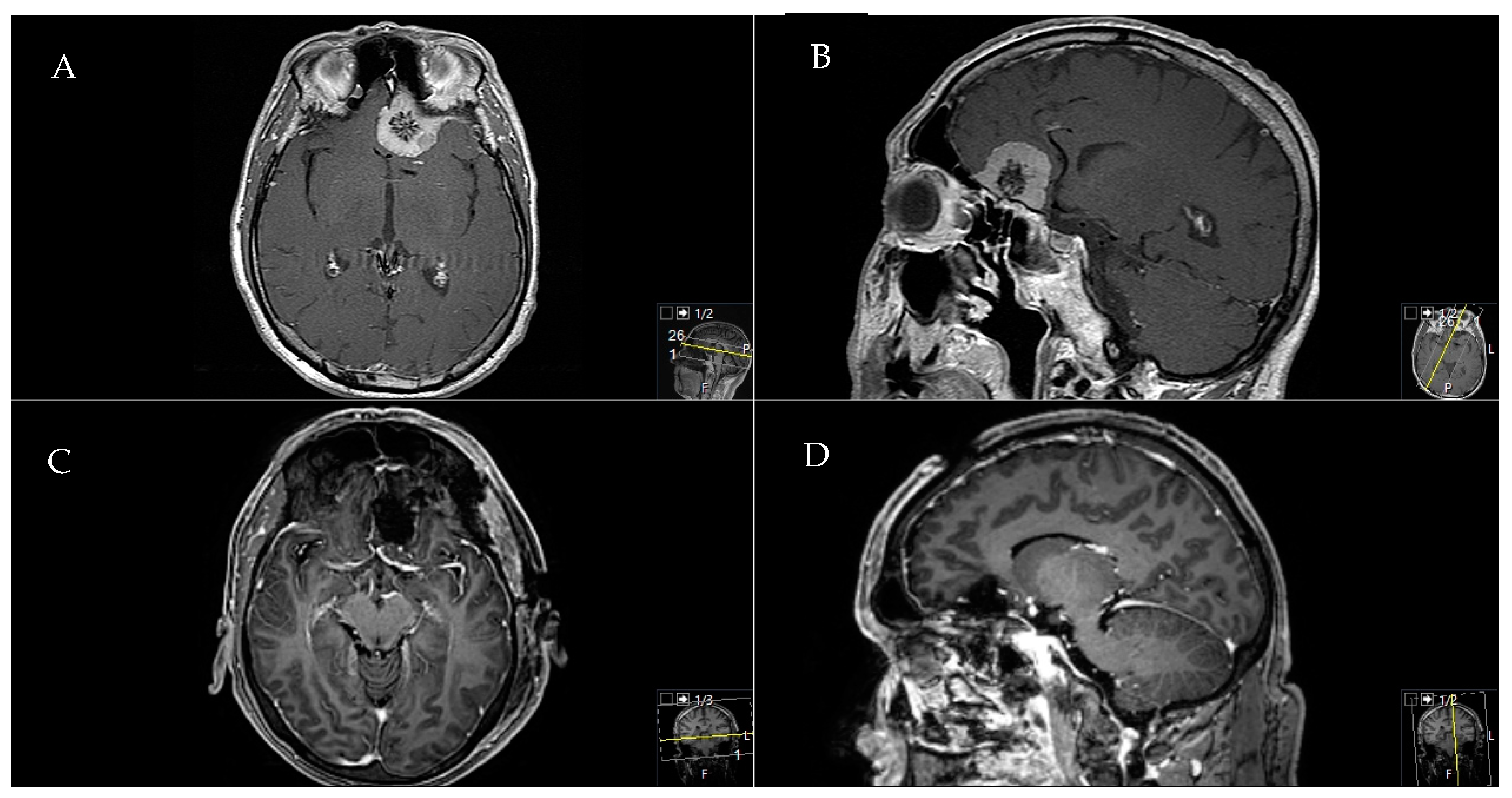
2.5. Exemplary Cases
2.5.1. Exemplary Case One
2.5.2. Exemplary Case Two
2.5.3. Exemplary Case Three
3. Discussion
3.1. The Median Approaches
3.2. The Lateral Approaches
3.3. Extent of Resection
3.4. Clinical Outcome
3.5. Adverse Events
3.6. Choosing a Suitable Approach
3.7. Study Limitations
4. Materials and Methods
4.1. Study Design
4.2. Outcome Parameters
4.3. Statistics
4.4. Ethics Approval
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mortazavi, M.M.; Brito da Silva, H.; Ferreira, M., Jr.; Barber, J.K.; Pridgeon, J.S.; Sekhar, L.N. Planum Sphenoidale and Tuberculum Sellae Meningiomas: Operative Nuances of a Modern Surgical Technique with Outcome and Proposal of a New Classification System. World Neurosurg. 2016, 86, 270–286. [Google Scholar] [CrossRef]
- Nanda, A.; Bir, S.C.; Maiti, T.K.; Konar, S.K.; Missios, S.; Guthikonda, B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J. Neurosurg. 2017, 126, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Komotar, R.J.; Starke, R.M.; Raper, D.M.; Anand, V.K.; Schwartz, T.H. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012, 77, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Maiti, T.K.; Bir, S.C.; Konar, S.K.; Guthikonda, B. Olfactory Groove Meningiomas: Comparison of Extent of Frontal Lobe Changes after Lateral and Bifrontal Approaches. World Neurosurg. 2016, 94, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Struck, M.; Roser, F.; Vorkapic, P.; Samii, M. Olfactory groove meningiomas: Clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 2008, 62, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Roser, F.; Struck, M.; Vorkapic, P.; Samii, M. Tuberculum sellae meningiomas: Clinical outcome considering different surgical approaches. Neurosurgery 2006, 59, 1019–1028, discussion 1019–1028. [Google Scholar] [CrossRef]
- Obeid, F.; Al-Mefty, O. Recurrence of olfactory groove meningiomas. Neurosurgery 2003, 53, 534–542, discussion 533–542. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.N.; Nanda, A.; Sen, C.N.; Snyderman, C.N.; Janecka, I.P. The extended frontal approach to tumors of the anterior, middle, and posterior skull base. J. Neurosurg. 1992, 76, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Silva, N.A.; Sevak, I.A.; Eloy, J.A. Transbasal versus endoscopic endonasal versus combined approaches for olfactory groove meningiomas: Importance of approach selection. Neurosurg. Focus 2018, 44, E8. [Google Scholar] [CrossRef] [Green Version]
- Ottenhausen, M.; Rumalla, K.; Alalade, A.F.; Nair, P.; Corte, E.L.; Younus, I.; Forbes, J.A.; Nsir, A.B.; Banu, M.A.; Tsiouris, A.J.; et al. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg. Focus 2018, 44, E7. [Google Scholar] [CrossRef] [Green Version]
- Yaşargil, M.G. Microneurosurgery: Microsurgery of CNS Tumors: Instrumentation and Equipment, Laboratory, Training, Surgical Approaches, Strategies, Tactics and Techniques, Surgery and Results of Extrinsic and Intrinsic Tumors, Interventional Neuroradiology, Neuroanesthesia, Complications; Thieme: New York, NY, USA, 1996. [Google Scholar]
- Hassler, W.; Zentner, J. Pterional Approach for Surgical Treatment of Olfactory Groove Meningiomas. Neurosurgery 1989, 25, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Bitter, A.D.; Stavrinou, L.C.; Ntoulias, G.; Petridis, A.K.; Dukagjin, M.; Scholz, M.; Hassler, W. The Role of the Pterional Approach in the Surgical Treatment of Olfactory Groove Meningiomas: A 20-year Experience. J. Neurol. Surg. Part B Skull Base 2013, 74, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, P.H.; Tahara, A.; Almeida, A.N.; Simm, R.; Silva, A.N.; Maldaun, M.V.; Panagopoulos, A.T.; Zicarelli, C.A.; Silva, P.G. Olfactory groove meningiomas: Approaches and complications. J. Clin. Neurosci. 2009, 16, 1168–1173. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Holoubi, A.; Rifai, A.; Fox, J.L. Microsurgical removal of suprasellar meningiomas. Neurosurgery 1985, 16, 364–372. [Google Scholar] [CrossRef]
- Schick, U.; Hassler, W. Surgical management of tuberculum sellae meningiomas: Involvement of the optic canal and visual outcome. J. Neurol. Neurosurg. Psychiatry 2005, 76, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Struck, M.; Roser, F.; Vorkapic, P.; Samii, M. Olfactory groove meningiomas: Clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 2007, 60, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thakur, B.; Corns, R.; Connor, S.; Bhangoo, R.; Ashkan, K.; Gullan, R. Resection of olfactory groove meningioma—A review of complications and prognostic factors. Br. J. Neurosurg. 2015, 29, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, H.; Asgari, S.; Stolke, D. Olfactory groove meningiomas: Functional outcome in a series treated microsurgically. Acta Neurochir. (Wien) 2007, 149, 109–121, discussion 121. [Google Scholar] [CrossRef]
- Kempe, L.G.; VanderArk, G.D. Anterior communicating artery aneurysms. Gyrus rectus approach. Neurochirurgia (Stuttg) 1971, 14, 63–70. [Google Scholar] [CrossRef]
- Feiz-Erfan, I.; Spetzler, R.F.; Horn, E.M.; Porter, R.W.; Beals, S.P.; Lettieri, S.C.; Joganic, E.F.; Demonte, F. Proposed classification for the transbasal approach and its modifications. Skull Base 2008, 18, 29–47. [Google Scholar] [CrossRef] [Green Version]
- Spektor, S.; Valarezo, J.; Fliss, D.M.; Gil, Z.; Cohen, J.; Goldman, J.; Umansky, F. Olfactory groove meningiomas from neurosurgical and ear, nose, and throat perspectives: Approaches, techniques, and outcomes. Neurosurgery 2005, 57, 268–280, discussion 268–280. [Google Scholar] [CrossRef]
- Dehdashti, A.R.; de Tribolet, N. Frontobasal interhemispheric trans-lamina terminalis approach for suprasellar lesions. Neurosurgery 2008, 62, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Raveh, J.; Turk, J.B.; Ladrach, K.; Seiler, R.; Godoy, N.; Chen, J.; Paladino, J.; Virag, M.; Leibinger, K. Extended anterior subcranial approach for skull base tumors: Long-term results. J. Neurosurg. 1995, 82, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Kurtsoy, A.; Menkü, A.; Tucer, B.; Oktem, I.; Akdemir, H.; Koç, R. Transbasal approaches: Surgical details, pitfalls and avoidances. Neurosurg. Rev. 2004, 27, 267–273. [Google Scholar] [CrossRef]
- Nanda, A.; Patra, D.P.; Savardekar, A.R.; Mohammed, N.; Narayan, V.; Bir, S.C. Surgery of Tuberculum Sellae Meningioma: A Technical Purview on Pterional Approach. J. Neurol. Surg. B Skull Base 2018, 79, S265–S266. [Google Scholar] [CrossRef] [Green Version]
- Chandler, J.P.; Silva, F.E. Extended transbasal approach to skull base tumors. Technical nuances and review of the literature. Oncology (Williston Park) 2005, 19, 913–919, discussion 915–923, 929. [Google Scholar]
- Fliss, D.M.; Zucker, G.; Cohen, A.; Amir, A.; Sagi, A.; Rosenberg, L.; Leiberman, A.; Gatot, A.; Reichenthal, E. Early outcome and complications of the extended subcranial approach to the anterior skull base. Laryngoscope 1999, 109, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ganna, A.; Dehdashti, A.R.; Karabatsou, K.; Gentili, F. Fronto-basal interhemispheric approach for tuberculum sellae meningiomas; long-term visual outcome. Br. J. Neurosurg. 2009, 23, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, A.R.; de Tribolet, N. Frontobasal interhemispheric trans-lamina terminalis approach for suprasellar lesions. Neurosurgery 2005, 56, 418–424. [Google Scholar] [CrossRef]
- Suzuki, J.; Mizoi, K.; Yoshimoto, T. Bifrontal interhemispheric approach to aneurysms of the anterior communicating artery. J. Neurosurg. 1986, 64, 183–190. [Google Scholar] [CrossRef]
- Shibuya, M.; Takayasu, M.; Suzuki, Y.; Saito, K.; Sugita, K. Bifrontal basal interhemispheric approach to craniopharyngioma resection with or without division of the anterior communicating artery. J. Neurosurg. 1996, 84, 951–956. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Dias, S.F.; Burkhardt, J.-K.; Fierstra, J.; Serra, C.; Bozinov, O.; Regli, L. Selection Strategy for Optimal Keyhole Approaches for Middle Cerebral Artery Aneurysms: Lateral Supraorbital Versus Minipterional Craniotomy. World Neurosurg. 2019, 122, e349–e357. [Google Scholar] [CrossRef]
- Banu, M.A.; Mehta, A.; Ottenhausen, M.; Fraser, J.F.; Patel, K.S.; Szentirmai, O.; Anand, V.K.; Tsiouris, A.J.; Schwartz, T.H. Endoscope-assisted endonasal versus supraorbital keyhole resection of olfactory groove meningiomas: Comparison and combination of 2 minimally invasive approaches. J. Neurosurg. 2016, 124, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Bhaganagare, A.; Shah, A.; Kaswa, A.; Rai, S.; Dharurkar, P.; Gore, S. Olfactory groove meningiomas: An analysis based on surgical experience with 129 cases. Neurol. India 2018, 66, 1081–1086. [Google Scholar] [CrossRef]
- Goel, A.; Muzumdar, D.; Desai, K.I. Tuberculum sellae meningioma: A report on management on the basis of a surgical experience with 70 patients. Neurosurgery 2002, 51, 1358–1363, discussion 1354–1363. [Google Scholar] [CrossRef]
- Chokyu, I.; Goto, T.; Ishibashi, K.; Nagata, T.; Ohata, K. Bilateral subfrontal approach for tuberculum sellae meningiomas in long-term postoperative visual outcome. J. Neurosurg. 2011, 115, 802–810. [Google Scholar] [CrossRef]
- Ohta, K.; Yasuo, K.; Morikawa, M.; Nagashima, T.; Tamaki, N. Treatment of tuberculum sellae meningiomas: A long-term follow-up study. J. Clin. Neurosci. 2001, 8 (Suppl. 1), 26–31. [Google Scholar] [CrossRef]
- Arai, H.; Sato, K.; Okuda, O.; Miyajima, M.; Hishii, M.; Nakanishi, H.; Ishii, H. Transcranial transsphenoidal approach for tuberculum sellae meningiomas. Acta Neurochir. (Wien) 2000, 142, 751–756, discussion 756–757. [Google Scholar] [CrossRef]
- Liu, J.K.; Watanabe, K. Modified One-Piece Extended Transbasal Approach for Endoscopic-Assisted Microsurgical Resection of Tuberculum Sellae Meningioma: Operative Video and Technical Nuances. J. Neurol. Surg. B Skull Base 2018, 79, S213–S214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effendi, S.T.; Rao, V.Y.; Momin, E.N.; Cruz-Navarro, J.; Duckworth, E.A. The 1-piece transbasal approach: Operative technique and anatomical study. J. Neurosurg. 2014, 121, 1446–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasello, F.; Angileri, F.F.; Grasso, G.; Granata, F.; De Ponte, F.S.; Alafaci, C. Giant olfactory groove meningiomas: Extent of frontal lobes damage and long-term outcome after the pterional approach. World Neurosurg. 2011, 76, 311–317. [Google Scholar] [CrossRef]
- Lu, Z.F. Resection of suprasellar meningioma through interhemispheric approach. J. Craniofac. Surg. 2014, 25, 1302–1304. [Google Scholar] [CrossRef]
- Joseph, V.; Chacko, A.G. Suprabrow minicraniotomy for suprasellar tumours. Br. J. Neurosurg. 2005, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Balanzar, G.G. Giant Olfactory Groove Meningiomas: Extent of Frontal Lobes Damage and Long-Term Outcome after the Pterional Approach. World Neurosurg. 2011, 76, 255–256. [Google Scholar] [CrossRef]
- Dedeciusova, M.; Svoboda, N.; Benes, V.; Astl, J.; Netuka, D. Olfaction in Olfactory Groove Meningiomas. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 310–317. [Google Scholar] [CrossRef]
- Mielke, D.; Mayfrank, L.; Psychogios, M.N.; Rohde, V. The anterior interhemispheric approach: A safe and effective approach to anterior skull base lesions. Acta Neurochir. (Wien) 2014, 156, 689–696. [Google Scholar] [CrossRef]
- Curey, S.; Derrey, S.; Hannequin, P.; Hannequin, D.; Freger, P.; Muraine, M.; Castel, H.; Proust, F. Validation of the superior interhemispheric approach for tuberculum sellae meningioma: Clinical article. J. Neurosurg. 2012, 117, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Georgantopoulou, A.; Hodgkinson, P.D.; Gerber, C.J. Cranial-base surgery: A reconstructive algorithm. Br. J. Plast. Surg. 2003, 56, 10–13. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]



| OGM (51) | PSM (20) | TSM (17) | Total (88) | |||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR), Mean (SD), N (%) | p | p | p | |||||
| Age (years) | 60 (54–74) | 62 (49–76) | 53 (44–60) | 60 (50–73) | ||||
| Sex | ♂ | 19 (37.3%) | 5 (25.0%) | 2 (11.8%) | 26 (29.5%) | |||
| ♀ | 32 (62.7%) | 15 (75.0%) | 15 (88.2%) | 62 (70.4%) | ||||
| Preop. volume (cm3) | 27 (±27) | 0. 011 | 18 (±34) | 3 (±2) | 20 (±27) | |||
| Preop. KPSS (%) | 80 (80–90) | 90 (80–95) | 90 (90–100) | 0.027 | 90 (80–90) | |||
| Postop. KPSS (%) | 90 (80–90) | 90 (80–100) | 100 (90–100) | 0.016 | 90 (70–90) | |||
| Hyposmia/ Anosmia | 51 (100.0%) | <0.01 | 2 (10.0%) | 0 | 53 (60.2%) | |||
| Visual affection | 13 (25.5%) | 8 (40.0%) | 10 (58.8%) | 0.036 | 31 (35.2%) | |||
| Amaurosis | 0 | 0 | 3 (17.6%) | 3 (3.4%) | ||||
| Psychomot. decline | 17 (34.0%) | 0.012 | 1 (5.0%) | 0 | 18 (20.5%) | |||
| Vertigo | 5 (9.8%) | 0 | 0 | 5 (5.7%) | ||||
| Seizure | 9 (17.6%) | 0 | 0 | 9 (10.2) | ||||
| Hemiparesis | 2 (3.9%) | 0 | 0 | 2 (2.3%) | ||||
| OGM (51) | p | PSM (20) | p | TSM (17) | p | Total (88) | |
|---|---|---|---|---|---|---|---|
| Calcification | 14 (27.5%) | 5 (25.0%) | 2 (11.8%) | 21 (23.9%) | |||
| Perifocal edema | 30 (58.8%) | <0.01 | 7 (35.0%) | 0 | 37 (42.0%) | ||
| Ethmoid bone/cribrose plate infiltration | 26 (51.0%) | 0.001 | 1 (5.0%) | 2 (11.8%) | 29 (33.0%) | ||
| Pituitary stalk deviation/affection | 6 (11.8%) | 0 | 0 | 6 (6.8%) | |||
| Frontal sinus/planum/sphenoid destruction | 9 (17.6%) | 3 (15.0%) | 5 (29.4%) | 0.037 | 17 (19.3%) | ||
| Orbit/optic nerve affection | 11 (21.6%) | 9 (45.0%) | 16 (94.1%) | <0.01 | 36 (40.9%) | ||
| ACOMA/MCA/ACA involvement | 12 (23.5%) | 6 (30.0%) | 5 (29.4%) | 23 (26.1%) | |||
| Anterior clinoid infiltration | 0 | 2 (10.0%) | 10 (58.8%) | <0.01 | 12 (13.6%) |
| OGM (51) | PSM (20) | TSM (17) | Total (88) | |
|---|---|---|---|---|
| Postoperative new visual deficits | 4 (7.8%) | 2 (10.0%) | 0 | 6 (6.8%) |
| Visual improvement | 15 (29.4%) | 5 (25.0%) | 10 (58.8%) | 30 (34.1%) |
| EDH | 1 (2.0%) | 1 (5.0%) | 1 (5.9%) | 3 (3.4%) |
| SDH | 2 (3.9%) | 2 (10.0%) | 0 | 4 (4.5%) |
| Hydrocephalus | 1 (2.0%) | 1 (5.0%) | 0 | 2 (2.3%) |
| CSF leak | 3 (5.9%) | 0 | 1 (5.9%) | 4 (4.5%) |
| Abscess | 2 (3.9%) | 0 | 0 | 2 (2.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aftahy, A.K.; Barz, M.; Krauss, P.; Wagner, A.; Lange, N.; Hijazi, A.; Wiestler, B.; Meyer, B.; Negwer, C.; Gempt, J. Midline Meningiomas of the Anterior Skull Base: Surgical Outcomes and a Decision-Making Algorithm for Classic Skull Base Approaches. Cancers 2020, 12, 3243. https://doi.org/10.3390/cancers12113243
Aftahy AK, Barz M, Krauss P, Wagner A, Lange N, Hijazi A, Wiestler B, Meyer B, Negwer C, Gempt J. Midline Meningiomas of the Anterior Skull Base: Surgical Outcomes and a Decision-Making Algorithm for Classic Skull Base Approaches. Cancers. 2020; 12(11):3243. https://doi.org/10.3390/cancers12113243
Chicago/Turabian StyleAftahy, Amir Kaywan, Melanie Barz, Philipp Krauss, Arthur Wagner, Nicole Lange, Alaa Hijazi, Benedikt Wiestler, Bernhard Meyer, Chiara Negwer, and Jens Gempt. 2020. "Midline Meningiomas of the Anterior Skull Base: Surgical Outcomes and a Decision-Making Algorithm for Classic Skull Base Approaches" Cancers 12, no. 11: 3243. https://doi.org/10.3390/cancers12113243
APA StyleAftahy, A. K., Barz, M., Krauss, P., Wagner, A., Lange, N., Hijazi, A., Wiestler, B., Meyer, B., Negwer, C., & Gempt, J. (2020). Midline Meningiomas of the Anterior Skull Base: Surgical Outcomes and a Decision-Making Algorithm for Classic Skull Base Approaches. Cancers, 12(11), 3243. https://doi.org/10.3390/cancers12113243