Unsupervised Hierarchical Clustering of Pancreatic Adenocarcinoma Dataset from TCGA Defines a Mucin Expression Profile that Impacts Overall Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Mucin Expression Patterns in Pancreatic Adenocarcinoma
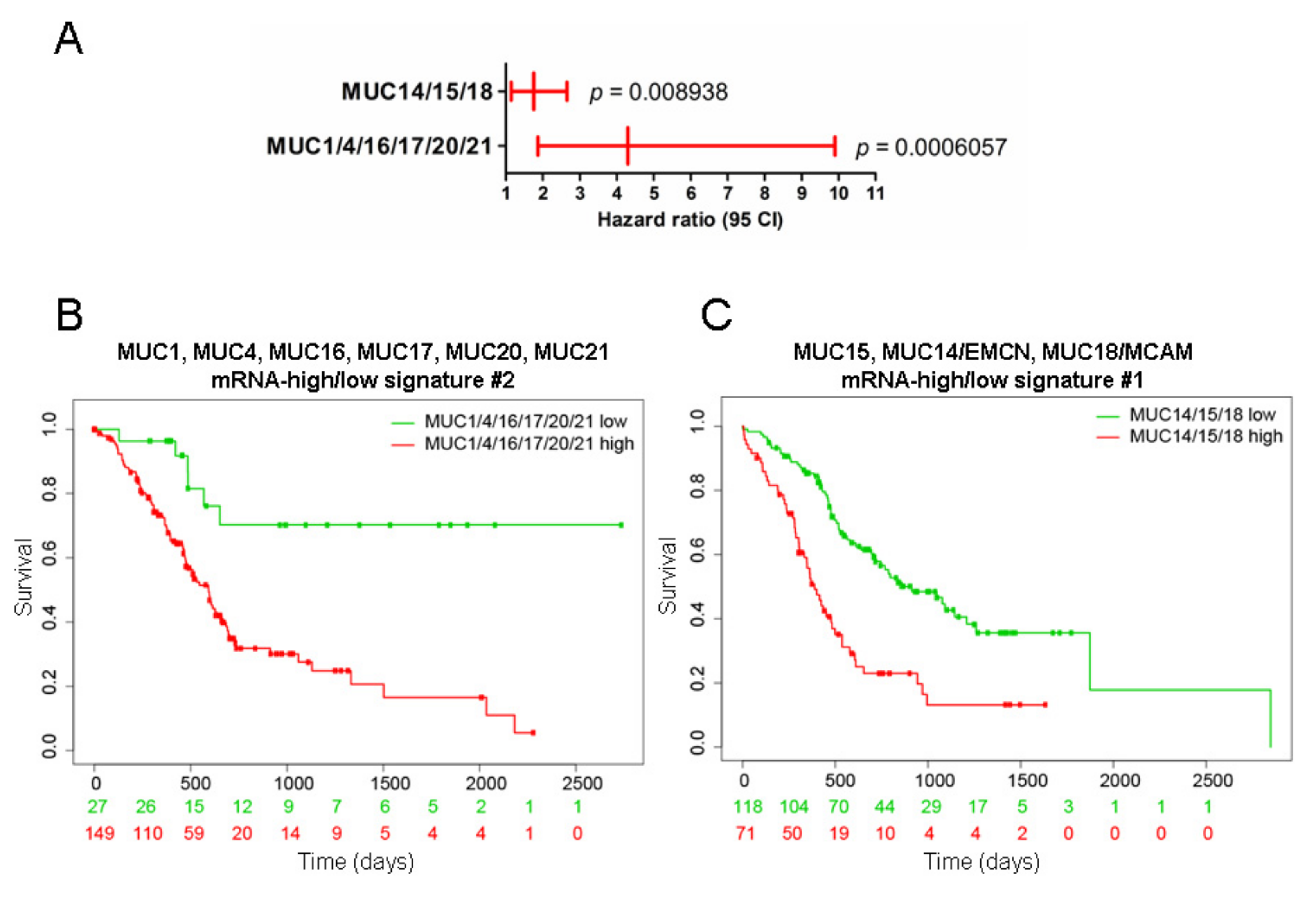
2.2. Mucins and Patient Survival
2.3. Mucin Genomic Alterations in Pancreatic Adenocarcinoma
2.4. Correlation among Mucin Gene Expression in Pancreatic Adenocarcinoma
2.5. Unsupervised Hierarchical Clustering of the PAAD Cohort
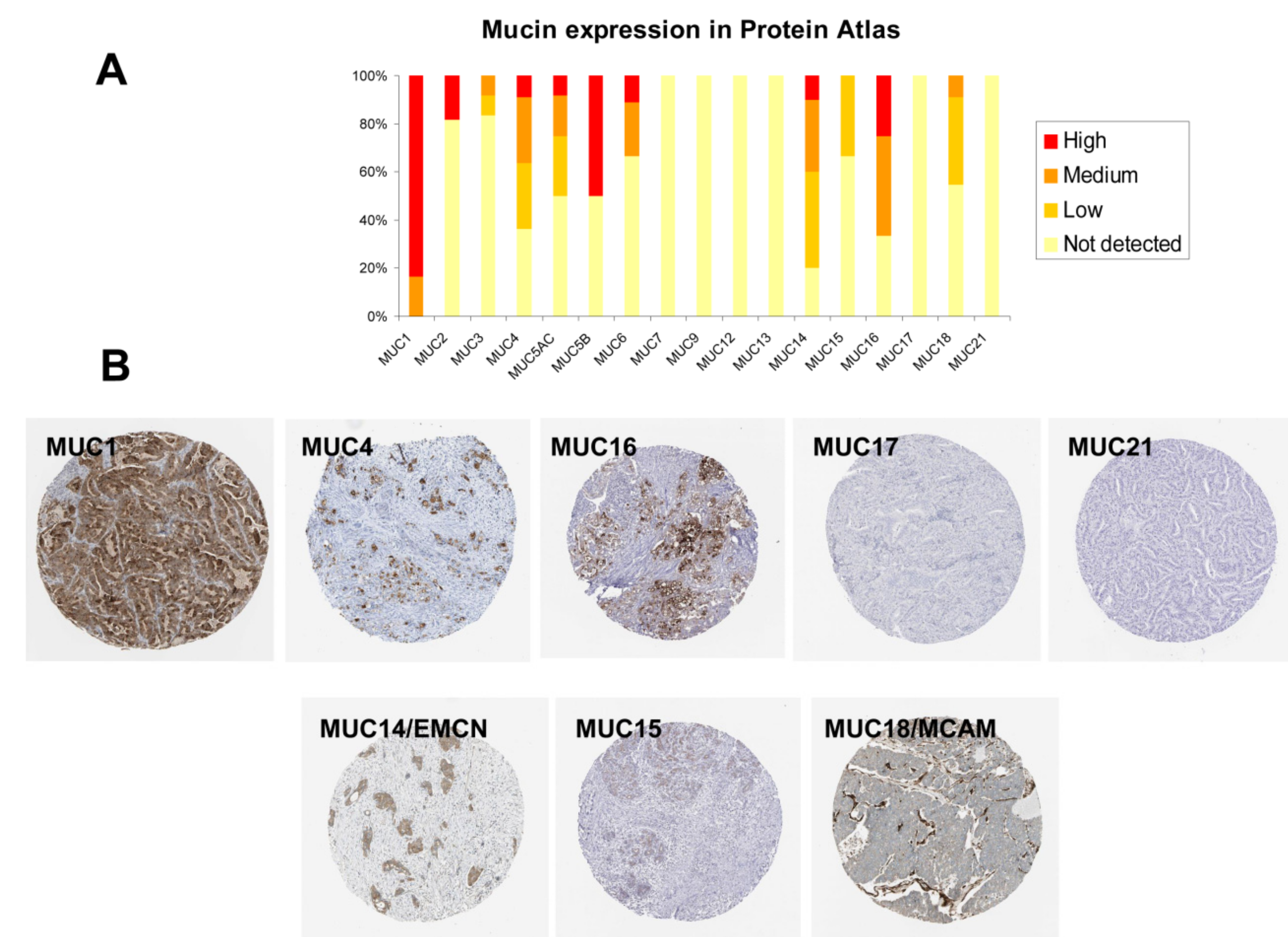
2.6. Immunohistochemistry (IHC) Analysis of Mucin Expression in Human PDAC Samples
3. Discussion
4. Materials and Methods
4.1. Expression Data Retrieval and Analysis
4.2. Survival Analysis
4.3. Immunohistochemistry (IHC)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H.; et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef]
- Puleo, F.; Nicolle, R.; Blum, Y.; Cros, J.; Marisa, L.; Demetter, P.; Quertinmont, E.; Svrcek, M.; Elarouci, N.; Iovanna, J.; et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology 2018, 155, 1999–2013.e1993. [Google Scholar] [CrossRef] [Green Version]
- Makohon-Moore, A.; Iacobuzio-Donahue, C.A. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat. Rev. Cancer 2016. [Google Scholar] [CrossRef] [Green Version]
- TCGA-Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e113. [Google Scholar] [CrossRef]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.S.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Van Seuningen, I.; Pigny, P.; Perrais, M.; Porchet, N.; Aubert, J.P. Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front. Biosci. 2001, 6, D1216–D1234. [Google Scholar] [PubMed]
- Jonckheere, N.; Van Seuningen, I. The membrane-bound mucins: How large O-glycoproteins play key roles in epithelial cancers and hold promise as biological tools for gene-based and immunotherapies. Crit. Rev. Oncog. 2008, 14, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, J.P.M.; Strijbis, K. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef]
- Jonckheere, N.; Skrypek, N.; Van Seuningen, I. Mucins and pancreatic cancer. Cancers 2010, 2, 1794–1812. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Rachagani, S.; Torres-Gonzalez, M.P.; Lakshmanan, I.; Majhi, P.D.; Smith, L.M.; Wagner, K.U.; Batra, S.K. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 2015, 6, 5772–5787. [Google Scholar] [CrossRef] [Green Version]
- Muniyan, S.; Haridas, D.; Chugh, S.; Rachagani, S.; Lakshmanan, I.; Gupta, S.; Seshacharyulu, P.; Smith, L.M.; Ponnusamy, M.P.; Batra, S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer 2016, 7, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Streppel, M.M.; Vincent, A.; Mukherjee, R.; Campbell, N.R.; Chen, S.H.; Konstantopoulos, K.; Goggins, M.G.; Van Seuningen, I.; Maitra, A.; Montgomery, E.A. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum. Pathol. 2012, 43, 1755–1763. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, D.P.; Sandanayake, N.S.; Jenkinson, C.; Gentry-Maharaj, A.; Apostolidou, S.; Fourkala, E.O.; Camuzeaux, S.; Blyuss, O.; Gunu, R.; Dawnay, A.; et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: Implications for early disease detection. Clin. Cancer Res. 2015, 21, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogendorf, P.; Skulimowski, A.; Durczynski, A.; Kumor, A.; Poznanska, G.; Olesna, A.; Rut, J.; Strzelczyk, J. A Panel of CA19-9, Ca125, and Ca15-3 as the Enhanced Test for the Differential Diagnosis of the Pancreatic Lesion. Dis. Markers 2017, 2017, 8629712. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Smith, L.M.; Patel, A.; Menning, M.; Watley, D.C.; Malik, S.S.; Krishn, S.R.; Mallya, K.; Aithal, A.; Sasson, A.R.; et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am. J. Gastroenterol. 2017, 112, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonckheere, N.; Van Seuningen, I. Integrative analysis of the cancer genome atlas and cancer cell lines encyclopedia large-scale genomic databases: MUC4/MUC16/MUC20 signature is associated with poor survival in human carcinomas. J. Transl. Med. 2018, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Nakamura, T.; Ihara, M.; Haraguchi, T.; Hiraoka, Y.; Tashiro, K.; Noda, M. Identification of human endomucin-1 and -2 as membrane-bound O-sialoglycoproteins with anti-adhesive activity. FEBS Lett. 2001, 499, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.P.; Rummel, M.M.; Rothbacher, U.; Sers, C. MUC18: A cell adhesion molecule with a potential role in tumor growth and tumor cell dissemination. Curr. Top. Microbiol. Immunol. 1996, 213, 95–105. [Google Scholar] [PubMed]
- Dai, W.; Liu, J.; Liu, B.; Li, Q.; Sang, Q.; Li, Y.Y. Systematical Analysis of the Cancer Genome Atlas Database Reveals EMCN/MUC15 Combination as a Prognostic Signature for Gastric Cancer. Front. Mol. Biosci. 2020, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Ohuchida, K.; Chijiiwa, Y.; Zhao, M.; Mizuuchi, Y.; Cui, L.; Horioka, K.; Ohtsuka, T.; Mizumoto, K.; Oda, Y.; et al. CD146 attenuation in cancer-associated fibroblasts promotes pancreatic cancer progression. Mol. Carcinog. 2016, 55, 1560–1572. [Google Scholar] [CrossRef]
- Jonckheere, N.; Skrypek, N.; Merlin, J.; Dessein, A.F.; Dumont, P.; Leteurtre, E.; Harris, A.; Desseyn, J.L.; Susini, C.; Frenois, F.; et al. The mucin MUC4 and its membrane partner ErbB2 regulate biological properties of human CAPAN-2 pancreatic cancer cells via different signalling pathways. PLoS ONE 2012, 7, e32232. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, P.; Singh, A.P.; Moniaux, N.; Senapati, S.; Chakraborty, S.; Meza, J.L.; Batra, S.K. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol. Cancer Res. 2007, 5, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, M.; Goto, M.; Horinouchi, M.; Tamada, S.; Nagata, K.; Hamada, T.; Osako, M.; Takao, S.; Batra, S.K.; Aikou, T.; et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J. Clin. Pathol. 2005, 58, 845–852. [Google Scholar] [CrossRef]
- Yokoyama, S.; Higashi, M.; Kitamoto, S.; Oeldorf, M.; Knippschild, U.; Kornmann, M.; Maemura, K.; Kurahara, H.; Wiest, E.; Hamada, T.; et al. Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget 2016, 7, 42553–42565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezawa, S.; Higashi, M.; Yamada, N.; Yokoyama, S.; Goto, M. Significance of mucin expression in pancreatobiliary neoplasms. J. Hepatobiliary Pancreat. Sci. 2010, 17, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Kuo, T.C.; Liao, Y.Y.; Lin, M.C.; Tien, Y.W.; Huang, M.C. Silencing of MUC20 suppresses the malignant character of pancreatic ductal adenocarcinoma cells through inhibition of the HGF/MET pathway. Oncogene 2018, 37, 6041–6053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirono, S.; Yamaue, H.; Hoshikawa, Y.; Ina, S.; Tani, M.; Kawai, M.; Ushijima, M.; Matsuura, M.; Saiki, Y.; Saiura, A.; et al. Molecular markers associated with lymph node metastasis in pancreatic ductal adenocarcinoma by genome-wide expression profiling. Cancer Sci. 2010, 101, 259–266. [Google Scholar] [CrossRef]
- Itoh, Y.; Kamata-Sakurai, M.; Denda-Nagai, K.; Nagai, S.; Tsuiji, M.; Ishii-Schrade, K.; Okada, K.; Goto, A.; Fukayama, M.; Irimura, T. Identification and expression of human epiglycanin/MUC21: A novel transmembrane mucin. Glycobiology 2008, 18, 74–83. [Google Scholar] [CrossRef]
- Bafna, S.; Kaur, S.; Momi, N.; Batra, S.K. Pancreatic cancer cells resistance to gemcitabine: The role of MUC4 mucin. Br. J. Cancer 2009, 101, 1155–1161. [Google Scholar] [CrossRef]
- Nath, S.; Daneshvar, K.; Roy, L.D.; Grover, P.; Kidiyoor, A.; Mosley, L.; Sahraei, M.; Mukherjee, P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013, 2, e51. [Google Scholar] [CrossRef] [Green Version]
- Skrypek, N.; Duchene, B.; Hebbar, M.; Leteurtre, E.; van Seuningen, I.; Jonckheere, N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene 2013, 32, 1714–1723. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, V.P.; Luksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Senbabaoglu, Y.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017, 551, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, J.; Ming, Y.; Chen, Z.; Yu, J.; Mao, R.; Chen, H.; Zhou, G.; Fan, Y. Orchestrating a biomarker panel with lncRNAs and mRNAs for predicting survival in pancreatic ductal adenocarcinoma. J. Cell Biochem. 2018, 119, 7696–7706. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.; Ji, H.; Xu, Y.; Wang, J.; Ma, Z.; Zhang, C.; Wang, K.; Zhou, Y. RREB1-induced upregulation of the lncRNA AGAP2-AS1 regulates the proliferation and migration of pancreatic cancer partly through suppressing ANKRD1 and ANGPTL4. Cell Death Dis. 2019, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- McWilliams, R.R.; Wieben, E.D.; Rabe, K.G.; Pedersen, K.S.; Wu, Y.; Sicotte, H.; Petersen, G.M. Prevalence of CDKN2A mutations in pancreatic cancer patients: Implications for genetic counseling. Eur. J. Hum. Genet. 2011, 19, 472–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, S.; Naitoh, Y.; Mino-Kenudson, M.; Sakurai, T.; Kuroda, M.; Koyama, I.; Lauwers, G.Y.; Shimizu, M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Its histopathologic difference between 2 major types. Am. J. Surg. Pathol. 2006, 30, 1561–1569. [Google Scholar] [CrossRef]
- Krishn, S.R.; Ganguly, K.; Kaur, S.; Batra, S.K. Ramifications of secreted mucin MUC5AC in malignant journey: A holistic view. Carcinogenesis 2018, 39, 633–651. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.H.; Jeong, D.G.; Kim, J.H. DUSP28 links regulation of Mucin 5B and Mucin 16 to migration and survival of AsPC-1 human pancreatic cancer cells. Tumour Biol. 2016, 37, 12193–12202. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hamada, T.; Higashi, M.; Matsuo, K.; Maemura, K.; Kurahara, H.; Horinouchi, M.; Hiraki, T.; Sugimoto, T.; Akahane, T.; et al. Predicted Prognosis of Patients with Pancreatic Cancer by Machine Learning. Clin. Cancer Res. 2020, 26, 2411–2421. [Google Scholar] [CrossRef] [Green Version]
- Andrianifahanana, M.; Moniaux, N.; Batra, S.K. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 2006, 1765, 189–222. [Google Scholar] [CrossRef]
- Van Seuningen, I.; Vincent, A. Mucins: A new family of epigenetic biomarkers in epithelial cancers. Expert Opin. Med. Diagn. 2009, 3, 411–427. [Google Scholar] [CrossRef]
- Liang, C.; Qin, Y.; Zhang, B.; Ji, S.; Shi, S.; Xu, W.; Liu, J.; Xiang, J.; Liang, D.; Hu, Q.; et al. Oncogenic KRAS Targets MUC16/CA125 in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Res. 2017, 15, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasseur, R.; Skrypek, N.; Duchene, B.; Renaud, F.; Martinez-Maqueda, D.; Vincent, A.; Porchet, N.; Van Seuningen, I.; Jonckheere, N. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-kappaB and RalB signaling pathways. Biochim. Biophys. Acta 2015, 1849, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Coppin, L.; Vincent, A.; Frenois, F.; Duchene, B.; Lahdaoui, F.; Stechly, L.; Renaud, F.; Villenet, C.; Van Seuningen, I.; Leteurtre, E.; et al. Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci. Rep. 2017, 7, 43927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Gamboa, R.; Gomez-Rueda, H.; Martinez-Ledesma, E.; Martinez-Torteya, A.; Chacolla-Huaringa, R.; Rodriguez-Barrientos, A.; Tamez-Pena, J.G.; Trevino, V. SurvExpress: An online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS ONE 2013, 8, e74250. [Google Scholar] [CrossRef] [Green Version]
- Goswami, C.P.; Nakshatri, H. PROGgene: Gene expression based survival analysis web application for multiple cancers. J. Clin. Bioinform. 2013, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.T.; Davis-Yadley, A.H.; Huang, P.Y.; Husain, K.; Centeno, B.A.; Permuth-Wey, J.; Pimiento, J.M.; Malafa, M. Prognostic Fifteen-Gene Signature for Early Stage Pancreatic Ductal Adenocarcinoma. PLoS ONE 2015, 10, e0133562. [Google Scholar] [CrossRef]






| Positive Correlation | |||
|---|---|---|---|
| Gene A | Gene B | Pearson r | p-Value |
| MUC12 | MUC2 | 0.85 | <0.00001 |
| MUC12 | MUC13 | 0.73 | <0.00001 |
| MUC13 | MUC2 | 0.72 | <0.00001 |
| MUC1 | MUC20 | 0.50 | <0.00001 |
| MUC13 | MUC5B | 0.50 | <0.00001 |
| MUC12 | MUC5B | 0.49 | <0.00001 |
| MUC6 | MUC7 | 0.49 | <0.00001 |
| MUC5B | MUC7 | 0.48 | <0.00001 |
| EMCN | MCAM | 0.44 | <0.00001 |
| MUC5B | MUC6 | 0.43 | <0.00001 |
| MUC2 | MUC5B | 0.42 | <0.00001 |
| MUC16 | MUC21 | 0.40 | <0.00001 |
| MUC4 | MUC16 | 0.38 | <0.00001 |
| MUC20 | MUC21 | 0.37 | <0.00001 |
| MUC1 | MUC17 | 0.32 | 0.00003 |
| MUC4 | MUC21 | 0.29 | 0.00016 |
| MUC15 | EMCN | 0.24 | 0.00170 |
| MUC4 | MUC20 | 0.24 | 0.00202 |
| MUC13 | MUC17 | 0.21 | 0.00579 |
| MUC1 | MUC4 | 0.21 | 0.00623 |
| MUC1 | MUC21 | 0.19 | 0.01578 |
| MUC1 | MUC13 | 0.18 | 0.01768 |
| MUC17 | MUC20 | 0.18 | 0.02016 |
| MUC16 | MUC20 | 0.17 | 0.02416 |
| MUC13 | MUC20 | 0.17 | 0.02598 |
| MUC6 | EMCN | 0.17 | 0.02798 |
| Negative correlation | |||
| MUC1 | EMCN | −0.46 | <0.00001 |
| MUC1 | MCAM | −0.31 | 0.00004 |
| MUC20 | EMCN | −0.29 | 0.00018 |
| MUC4 | EMCN | −0.28 | 0.00029 |
| MUC13 | EMCN | −0.25 | 0.00087 |
| MUC16 | EMCN | −0.23 | 0.00272 |
| OVGP1 | MUC15 | −0.19 | 0.01240 |
| MUC13 | MCAM | −0.18 | 0.01819 |
| MUC2 | MCAM | −0.18 | 0.01860 |
| MUC20 | MCAM | −0.18 | 0.01888 |
| MUC17 | EMCN | −0.17 | 0.02811 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonckheere, N.; Auwercx, J.; Hadj Bachir, E.; Coppin, L.; Boukrout, N.; Vincent, A.; Neve, B.; Gautier, M.; Treviño, V.; Van Seuningen, I. Unsupervised Hierarchical Clustering of Pancreatic Adenocarcinoma Dataset from TCGA Defines a Mucin Expression Profile that Impacts Overall Survival. Cancers 2020, 12, 3309. https://doi.org/10.3390/cancers12113309
Jonckheere N, Auwercx J, Hadj Bachir E, Coppin L, Boukrout N, Vincent A, Neve B, Gautier M, Treviño V, Van Seuningen I. Unsupervised Hierarchical Clustering of Pancreatic Adenocarcinoma Dataset from TCGA Defines a Mucin Expression Profile that Impacts Overall Survival. Cancers. 2020; 12(11):3309. https://doi.org/10.3390/cancers12113309
Chicago/Turabian StyleJonckheere, Nicolas, Julie Auwercx, Elsa Hadj Bachir, Lucie Coppin, Nihad Boukrout, Audrey Vincent, Bernadette Neve, Mathieu Gautier, Victor Treviño, and Isabelle Van Seuningen. 2020. "Unsupervised Hierarchical Clustering of Pancreatic Adenocarcinoma Dataset from TCGA Defines a Mucin Expression Profile that Impacts Overall Survival" Cancers 12, no. 11: 3309. https://doi.org/10.3390/cancers12113309
APA StyleJonckheere, N., Auwercx, J., Hadj Bachir, E., Coppin, L., Boukrout, N., Vincent, A., Neve, B., Gautier, M., Treviño, V., & Van Seuningen, I. (2020). Unsupervised Hierarchical Clustering of Pancreatic Adenocarcinoma Dataset from TCGA Defines a Mucin Expression Profile that Impacts Overall Survival. Cancers, 12(11), 3309. https://doi.org/10.3390/cancers12113309









