Epithelial Transfer of the Tyrosine Kinase Inhibitors Erlotinib, Gefitinib, Afatinib, Crizotinib, Sorafenib, Sunitinib, and Dasatinib: Implications for Clinical Resistance
Simple Summary
Abstract
1. Introduction
2. Results
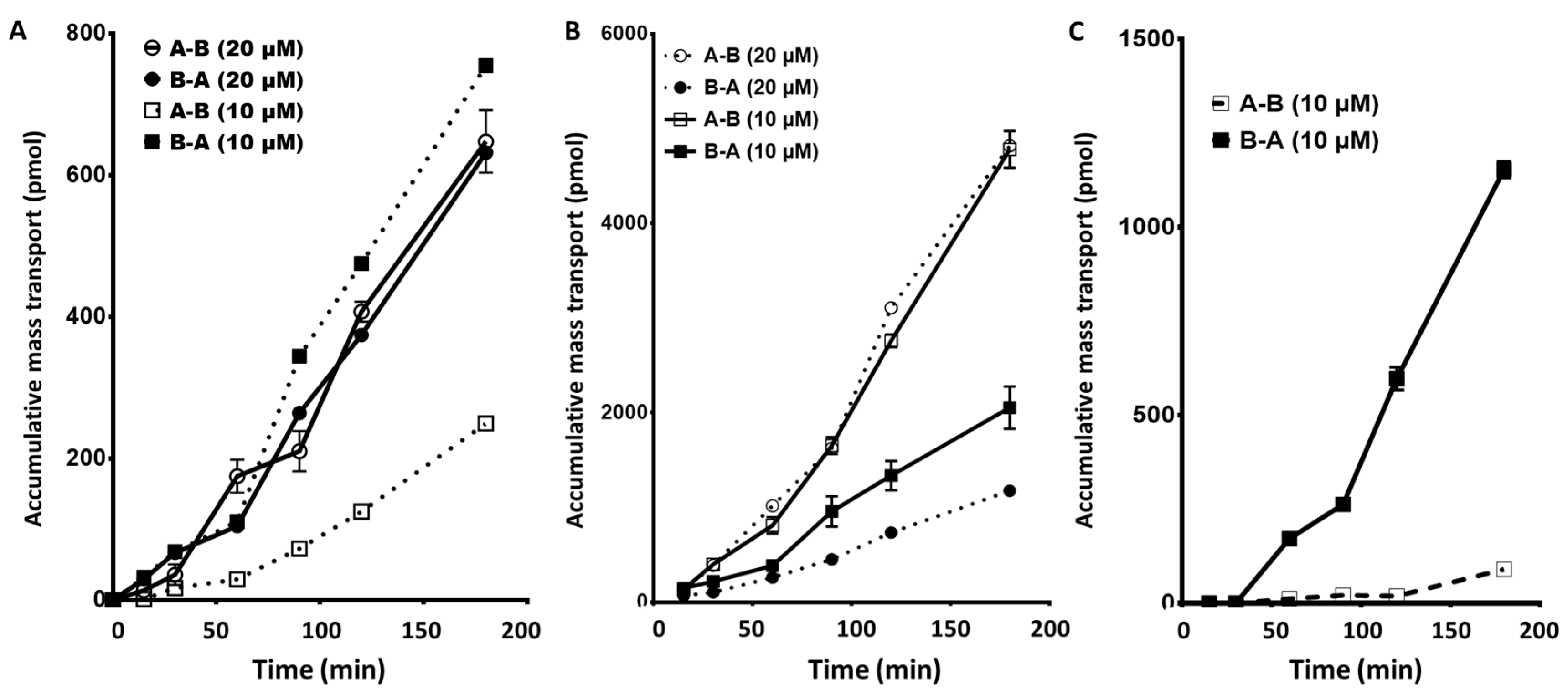
2.1. EGFR Inhibitors: Gefitinib, Erlotinib, and Afatinib: Apical to Basolateral and the Reverse
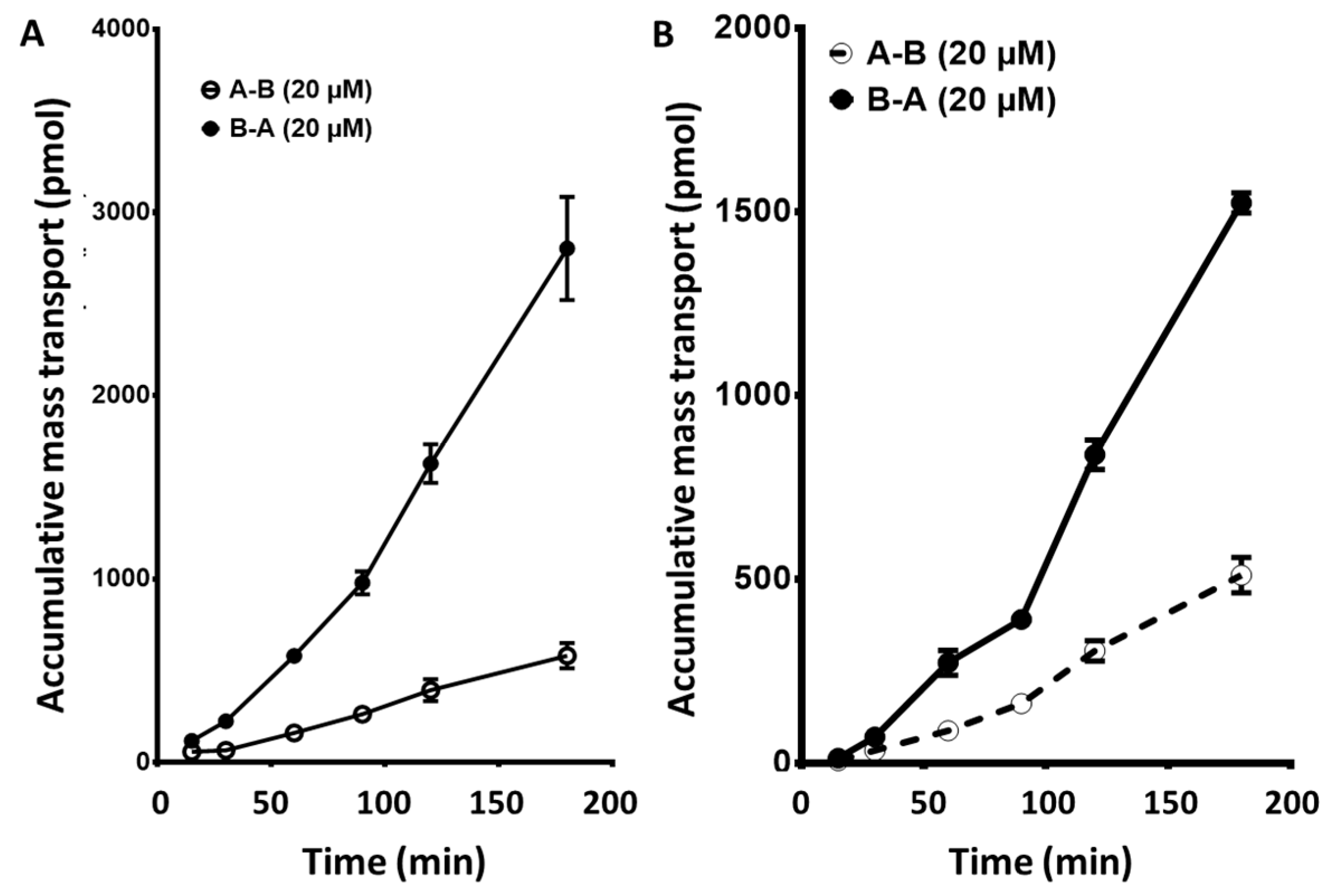
2.2. Sunitinib, a Vascular Endothelial Growth Factor Receptor (VEGFR) Inhibitor, and Sorafenib, a Platelet-Derived Growth Factor Receptor (PDGFR): Apical to Basolateral and the Reverse
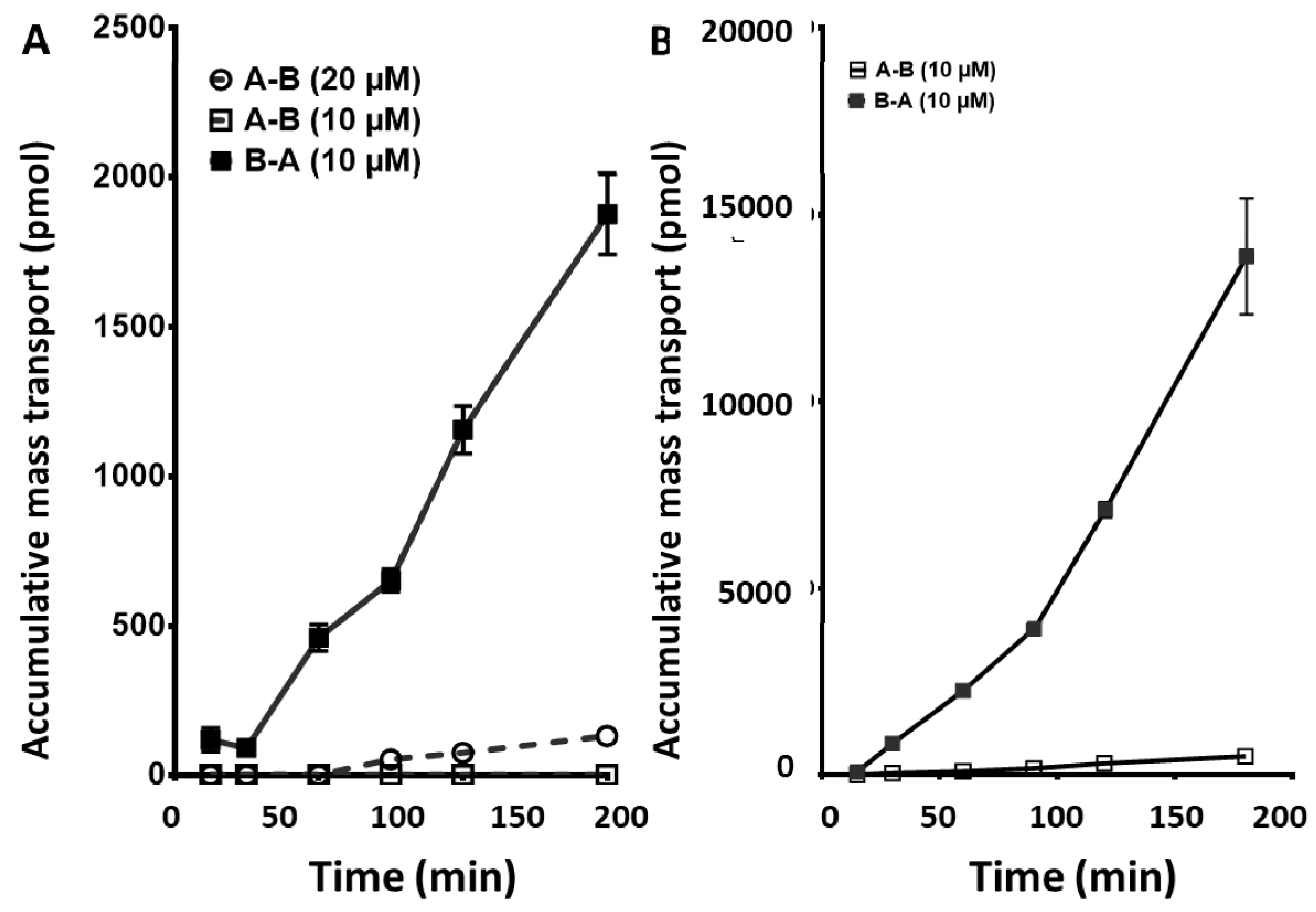
2.3. Crizotinib, an EML4-ALK and c-MET Inhibitor, and Dasatinib, a PDGFR and a BCR-Abl Inhibitor: Apical to Basolateral and the Reverse
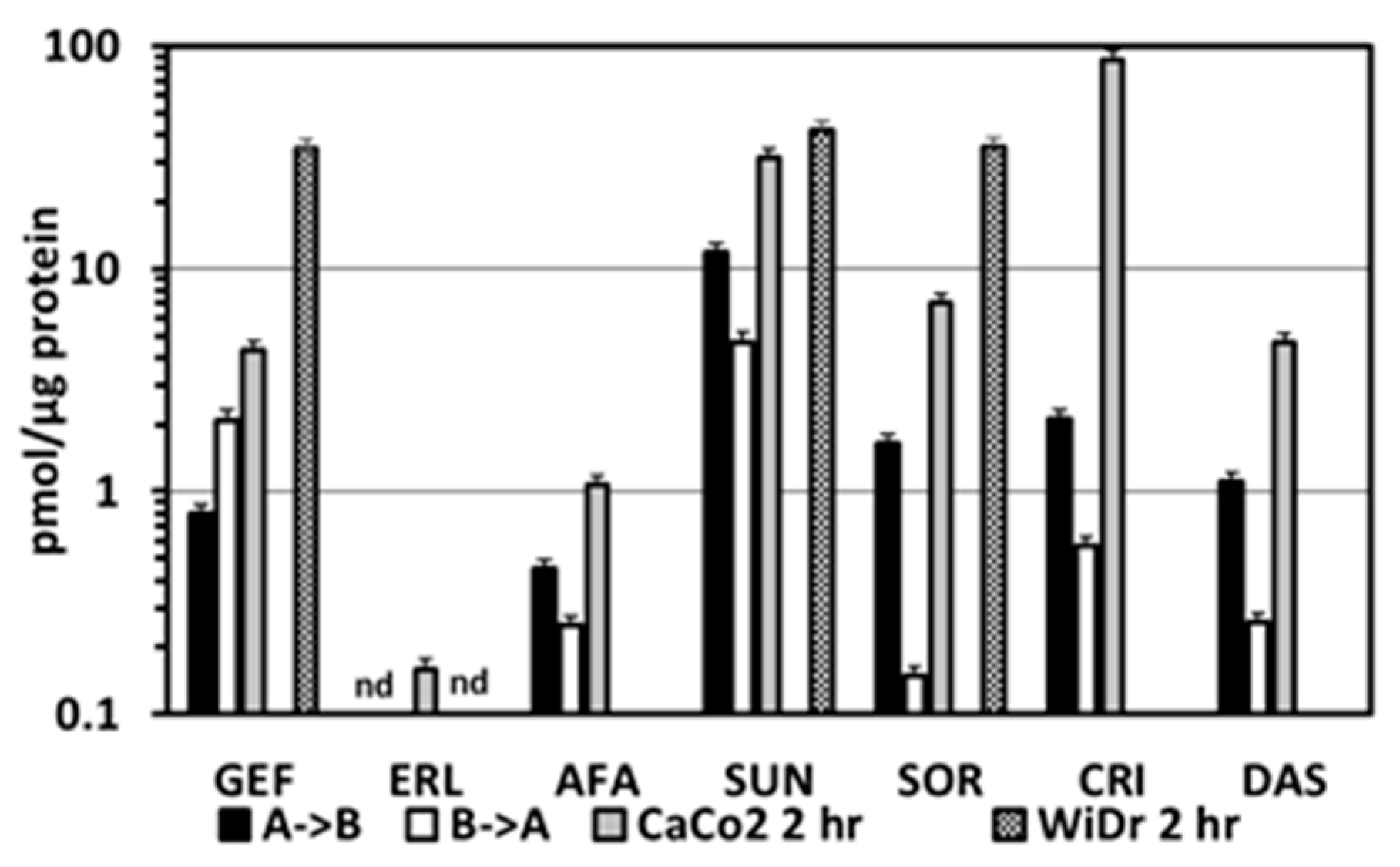
2.4. Intracellular Accumulation of TKIs in the CaCo2 Transwell Cells and in Various Colon Cancer Cell Lines
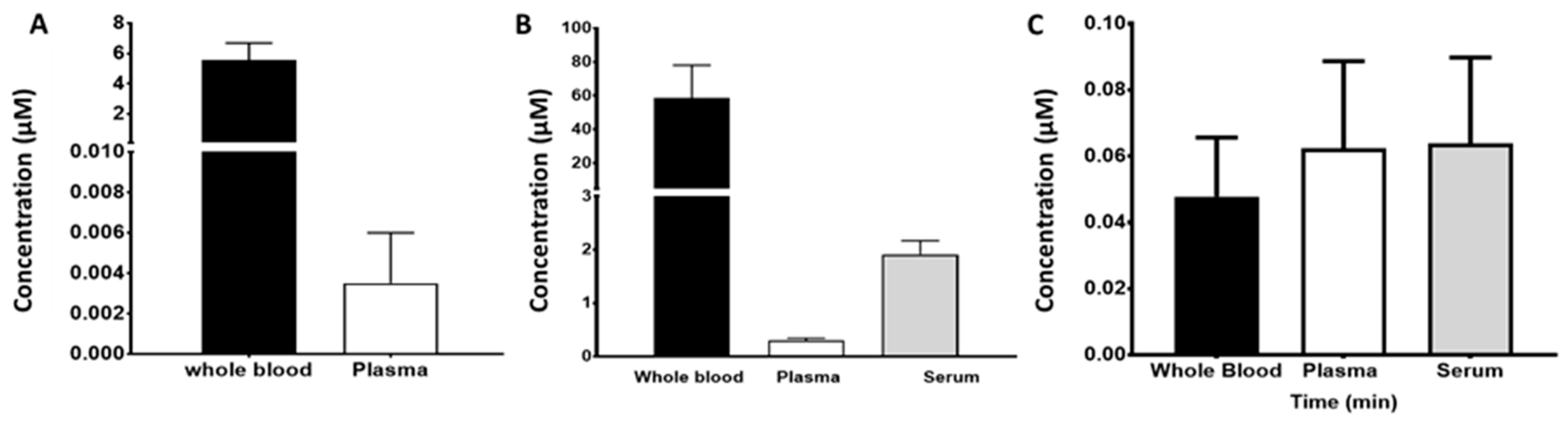
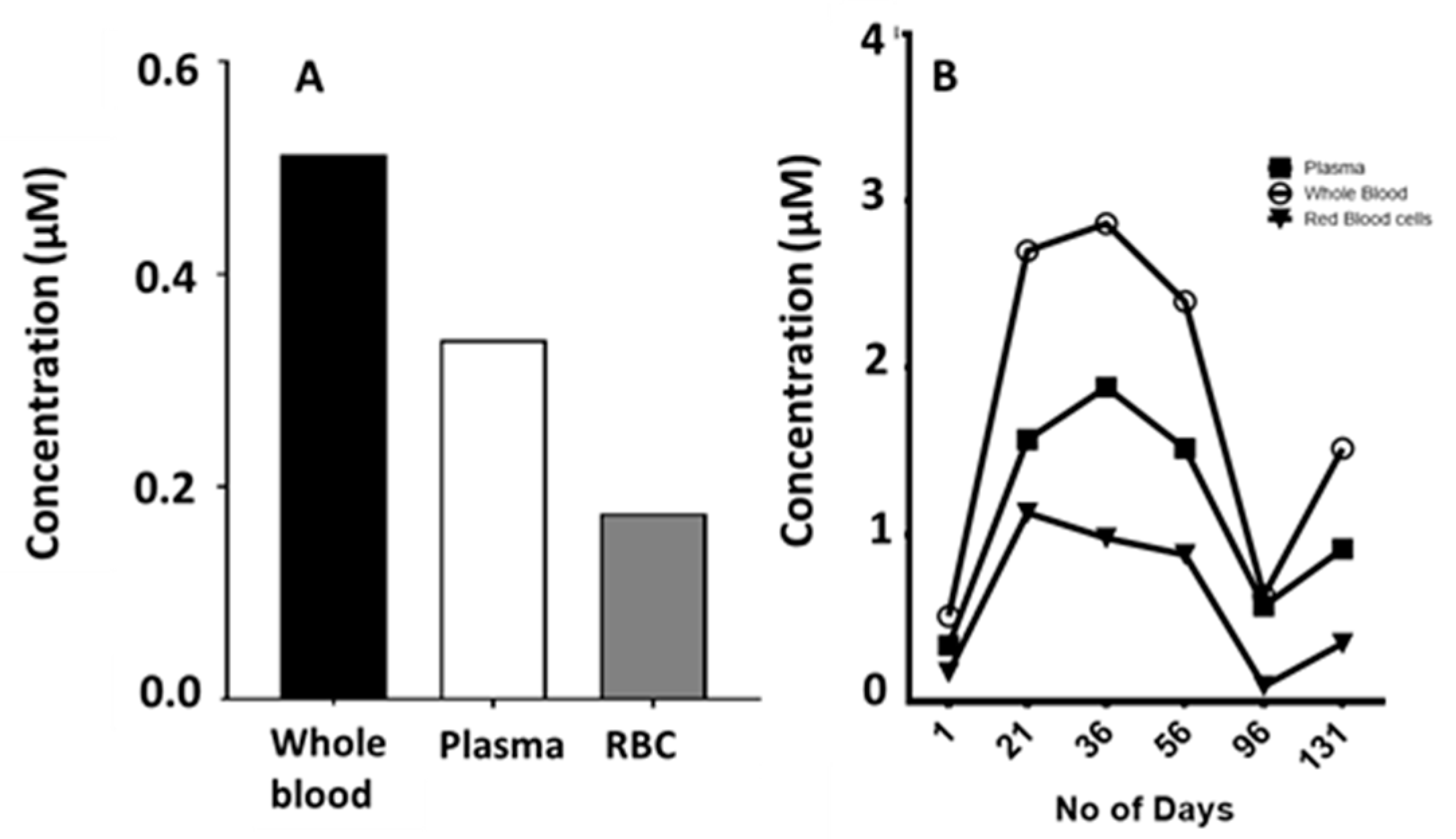
2.5. Blood Compartments
3. Discussion
4. Materials and Methods
4.1. Materials, Equipment and Analysis
4.2. Cell Culture Models and the CaCo2 Transwell Model
4.3. Calculations and Statistics
4.4. Blood Pharmacokinetics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broekman, F.; Giovannetti, E.; Peters, G.J. Tyrosine Kinase Inhibitors: Multi-Targeted or Single-Targeted? World J. Clin. Oncol. 2011, 2, 80–93. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Honeywell, R.J.; Dekker, H.; Peters, G.J. Physicochemical Properties of Novel Protein Kinase Inhibitors in Relation to Their Substrate Specificity for Drug Transporters. Expert Opin Drug Metab Toxicol. 2015, 11, 703–717. [Google Scholar] [CrossRef]
- Nakagawa, K.; Tamura, T.; Negoro, S.; Kudoh, S.; Yamamoto, N.; Takeda, K.; Swaisland, H.; Nakatani, I.; Hirose, M.; Dong, R.P.; et al. Phase I Pharmacokinetic Trial of the Selective Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Gefitinib (’Iressa’, ZD1839) in Japanese Patients with Solid Malignant Tumors. Ann. Oncol. 2003, 14, 922–930. [Google Scholar] [CrossRef]
- Gross, M.E.; Leichman, L.; Lowe, E.S.; Swaisland, A.; Agus, D.B. Safety and Pharmacokinetics of High-Dose Gefitinib in Patients with Solid Tumors: Results of a Phase I Study. Cancer Chemother. Pharmacol. 2012, 69, 273–280. [Google Scholar] [CrossRef]
- Bergman, E.; Forsell, P.; Persson, E.M.; Knutson, L.; Dickinson, P.; Smith, R.; Swaisland, H.; Farmer, M.R.; Cantarini, M.V.; Lennernäs, H. Pharmacokinetics of Gefitinib in Humans: The Influence of Gastrointestinal Factors. Int. J. Pharm. 2007, 341, 134–142. [Google Scholar] [CrossRef]
- Peters, S.; Zimmermann, S.; Adjei, A.A. Anti-Tumour Treatment Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for the Treatment of Non-Small Cell Lung Cancer: Comparative Pharmacokinetics and Drug–drug Interactions. Cancer Treat. Rev. 2014, 40, 917–926. [Google Scholar] [CrossRef]
- Swaisland, H.C.; Smith, R.P.; Laight, A.; Kerr, D.J.; Ranson, M.; Wilder-Smith, C.H.; Duvauchelle, T. Single-Dose Clinical Pharmacokinetic Studies of Gefitinib. Clin. Pharmacokinet. 2005, 44, 1165–1177. [Google Scholar] [CrossRef]
- Scheffler, M.; Di Gion, P.; Doroshyenko, O.; Wolf, J.; Fuhr, U. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors: Focus on 4-Anilinoquinazolines. Clin. Pharmacokin. 2011, 50, 371–403. [Google Scholar] [CrossRef]
- Giaccone, G.; Gonzalez-Larriba, J.L.; van Oosterom, A.T.; Alfonso, R.; Smit, E.F.; Martens, M.; Peters, G.J.; van der Vijgh, W.J.F.; Smith, R.; Averbuch, S.; et al. Combination Therapy with Gefitinib, an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor, Gemcitabine and Cisplatin in Patients with Advanced Solid Tumors. Ann. Oncol. 2004, 15, 831–838. [Google Scholar] [CrossRef]
- Lind, J.S.W.; Dingemans, A.-M.C.M.; Groen, H.J.M.; Thunnissen, F.B.; Bekers, O.; Heideman, D.A.M.; Honeywell, R.J.; Giovannetti, E.; Peters, G.J.; Postmus, P.E.; et al. A Multicenter Phase II Study of Erlotinib and Sorafenib in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2010, 16, 3078–3087. [Google Scholar] [CrossRef]
- Thomas, F.; Rochaix, P.; White-Koning, M.; Hennebelle, I.; Sarini, J.; Benlyazid, A.; Malard, L.; Lefebvre, J.-L.; Chatelut, E.; Delord, J.P. Population Pharmacokinetics of Erlotinib and Its Pharmacokinetic/pharmacodynamic Relationships in Head and Neck Squamous Cell Carcinoma. Eur. J. Cancer 2009, 45, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Jakacki, R.I.; Hamilton, M.; Gilbertson, R.J.; Blaney, S.M.; Tersak, J.; Krailo, M.D.; Ingle, A.M.; Voss, S.D.; Dancey, J.E.; Adamson, P.C. Pediatric Phase I and Pharmacokinetic Study of Erlotinib Followed by the Combination of Erlotinib and Temozolomide: A Children’s Oncology Group Phase I Consortium Study. J. Clin. Oncol. 2008, 26, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Masago, K.; Togashi, Y.; Fukudo, M.; Terada, T.; Irisa, K.; Sakamori, Y.; Kim, Y.H.; Mio, T.; Inui, K.; Mishima, M. Plasma and Pleural Fluid Pharmacokinetics of Erlotinib and Its Active Metabolite OSI-420 in Patients with Non-Small-Cell Lung Cancer with Pleural Effusion. Clin. Lung Cancer 2011, 12, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Fettner, S.; Lum, B.L.; Riek, M.; Rakhit, A. Effect of Food on the Pharmacokinetics of Erlotinib, an Orally Active Epidermal Growth Factor Receptor Tyrosine-Kinase Inhibitor, in Healthy Individuals. Anticancer Drugs 2008, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ranson, M.; Shaw, H.; Wolf, J.; Hamilton, M.; McCarthy, S.; Dean, E.; Reid, A.; Judson, I. A Phase I Dose-Escalation and Bioavailability Study of Oral and Intravenous Formulations of Erlotinib (Tarceva®, OSI-774) in Patients with Advanced Solid Tumors of Epithelial Origin. Cancer Chemother. Pharmacol. 2010, 66, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wind, S.; Schmid, M.; Erhardt, J.; Goeldner, R.G.; Stopfer, P. Pharmacokinetics of Afatinib, a Selective Irreversible ErbB Family Blocker, in Patients with Advanced Solid Tumours. Clin. Pharmacokinet. 2013, 52, 1101–1109. [Google Scholar] [CrossRef]
- Bello, C.L.; Garrett, M.; Sherman, L.; Smeraglia, J.; Ryan, B.; Toh, M. Pharmacokinetics of Sunitinib Malate in Subjects with Hepatic Impairment. Cancer Chemother. Pharmacol. 2010, 699–707. [Google Scholar] [CrossRef]
- Bello, C.L.; Sherman, L.; Zhou, J.; Verkh, L.; Smeraglia, J.; Mount, J.; Klamerus, K.J. Effect of Food on the Pharmacokinetics of Sunitinib Malate (SU11248), a Multi-Targeted Receptor Tyrosine Kinase Inhibitor: Results from a Phase I Study in Healthy Subjects. Anticancer Drugs 2006, 17, 353–358. [Google Scholar] [CrossRef]
- Di Gion, P.; Kanefendt, F.; Lindauer, A.; Scheffler, M.; Doroshyenko, O.; Fuhr, U.; Wolf, J.; Jaehde, U. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors: Focus on Pyrimidines, Pyridines and Pyrroles. Clin. Pharmacokinet. 2011, 50, 551–603. [Google Scholar] [CrossRef]
- Wulkersdorfer, B.; Zeitlinger, M.; Schmid, M. Pharmacokinetic Aspects of Vascular Endothelial Growth Factor Tyrosine Kinase Inhibitors. Clin. Pharmacokin. 2016, 55, 47–77. [Google Scholar] [CrossRef]
- Jain, L.; Woo, S.; Gardner, E.R.; Dahut, W.L.; Kohn, E.C.; Kummar, S.; Mould, D.R.; Giaccone, G.; Yarchoan, R.; Venitz, J.; et al. Population Pharmacokinetic Analysis of Sorafenib in Patients with Solid Tumours. Br. J. Clin. Pharmacol. 2011, 72, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Strumberg, D.; Clark, J.W.; Awada, A.; Moore, M.J.; Richly, H.; Hendlisz, A.; Hirte, H.W.; Eder, J.P.; Lenz, H.-J.; Schwartz, B. Safety, Pharmacokinetics, and Preliminary Antitumor Activity of Sorafenib: A Review of Four Phase I Trials in Patients with Advanced Refractory Solid Tumors. Oncologist 2007, 12, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I. Sorafenib. Expert Opin. Pharmacother. 2006, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Rath, B.; Burghuber, O. Pharmacokinetics of Crizotinib in NSCLC Patients. Expert Opin. Drug Metab. Toxicol. 2015, 11, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Vicini, P.; Shen, Z.; Zou, H.Y.; Lee, J.; Li, Q.; Christensen, J.G.; Smith, B.J.; Shetty, B. Pharmacokinetic/pharmacodynamic Modeling of Crizotinib for Anaplastic Lymphoma Kinase Inhibition and Antitumor Efficacy in Human Tumor Xenograft Mouse Models. J. Pharmacol. Exp. Ther. 2012, 340, 549–557. [Google Scholar] [CrossRef]
- Xu, H.; O’Gorman, M.; Boutros, T.; Brega, N.; Kantaridis, C.; Tan, W.; Bello, A. Evaluation of Crizotinib Absolute Bioavailability, the Bioequivalence of Three Oral Formulations, and the Effect of Food on Crizotinib Pharmacokinetics in Healthy Subjects. J. Clin. Pharmacol. 2015, 55, 104–113. [Google Scholar] [CrossRef]
- Herbrink, M.; Nuijen, B.; Schellens, J.H.M.; Beijnen, J.H. Variability in Bioavailability of Small Molecular Tyrosine Kinase Inhibitors. Cancer Treat. Rev. 2015, 41, 412–422. [Google Scholar] [CrossRef]
- Takahashi, N.; Miura, M.; Scott, S.A.; Niioka, T.; Sawada, K. Pharmacokinetics of Dasatinib for Philadelphia-Positive Acute Lymphocytic Leukemia with Acquired T315I Mutation. J. Hematol. Oncol. 2012, 5, 23. [Google Scholar] [CrossRef]
- Stuurman, F.E.; Nuijen, B.; Beijnen, J.H.; Schellens, J.H.M. Oral Anticancer Drugs: Mechanisms of Low Bioavailability and Strategies for Improvement. Clin. Pharmacokinet. 2013, 52, 399–414. [Google Scholar] [CrossRef]
- van Erp, N.P.; Gelderblom, H.; Guchelaar, H.-J. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors. Cancer Treat. Rev. 2009, 35, 692–706. [Google Scholar] [CrossRef]
- Eley, T.; Luo, F.R.; Agrawal, S.; Sanil, A.; Manning, J.; Li, T.; Blackwood-Chirchir, A.; Bertz, R. Phase I Study of the Effect of Gastric Acid pH Modulators on the Bioavailability of Oral Dasatinib in Healthy Subjects. J. Clin. Pharmacol. 2009, 49, 700–709. [Google Scholar] [CrossRef]
- Kamath, A.V.; Wang, J.; Lee, F.Y.; Punit, A.; Marathe, H.; Kamath, A.V.; Wang, J.; Lee, F.Y.; Marathe, P.H. Preclinical Pharmacokinetics and in Vitro Metabolism of Dasatinib (BMS-354825): A Potent Oral Multi-Targeted Kinase Inhibitor against SRC and BCR-ABL. Cancer Chemother. Pharmacol. 2008, 61, 365–376. [Google Scholar] [CrossRef]
- Tsuji, A.; Tamai, I. Carrier-Mediated Intestinal Transport of Drugs. Pharm. Res. 1996, 13, 963–977. [Google Scholar] [CrossRef]
- Fromm, M.F. Importance of P-Glycoprotein at Blood-Tissue Barriers. Trends Pharmacol. Sci. 2004, 25, 423–429. [Google Scholar] [CrossRef]
- Takano, M.; Yumoto, R.; Murakami, T. Expression and Function of Efflux Drug Transporters in the Intestine. Pharmacol. Ther. 2006, 109, 137–161. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugiyama, Y. Role of Metabolic Enzymes and Efflux Transporters in the Absorption of Drugs from the Small Intestine. Eur. J. Pharm. Sci. 2000, 12, 3–12. [Google Scholar] [CrossRef]
- Chan, L.M.S.; Lowes, S.; Hirst, B.H. The ABCs of Drug Transport in Intestine and Liver: Efflux Proteins Limiting Drug Absorption and Bioavailability. Eur. J. Pharm. Sci. 2004, 21, 25–51. [Google Scholar] [CrossRef]
- Honeywell, R.J.; Hitzerd, S.; Kathmann, I.; Peters, G.J. Transport of Six Tyrosine Kinase Inhibitors: Active or Passive? ADMET DMPK 2016, 4, 23–34. [Google Scholar] [CrossRef]
- Honeywell, R.; Fatmawati, C.; Buddha, M.; Hitzerd, S.; Kathman, I.; Peters, G.J. Adaptation of a Human Gut Epithelial Model in Relation to the Assessment of Clinical Pharmacokinetic Parameters for Selected Tyrosine Kinase Inhibitors. ADMET DMPK 2015, 3, 51–67. [Google Scholar] [CrossRef]
- Keating, G.M. Afatinib: A Review of Its Use in the Treatment of Advanced Non-Small Cell Lung Cancer. Drugs 2014, 74, 207–221. [Google Scholar] [CrossRef]
- Keating, G.M.; Santoro, A. Sorafenib: A Review of Its Use in Advanced Hepatocellular Carcinoma. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D.; Keating, G.M. Sunitinib. Drugs 2006, 66, 2255–2266. [Google Scholar] [CrossRef]
- Shaw, A.T.; Yasothan, U.; Kirkpatrick, P. Crizotinib. Nat. Rev. Drug Discov. 2011, 10, 897–898. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Dasatinib: A Review in Chronic Myeloid Leukaemia and Ph+ Acute Lymphoblastic Leukaemia. Drugs 2017, 77, 85–96. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, D.J.; Honeywell, R.J.; Jansen, G.; Peters, G.J. Transporter and Lysosomal Mediated (Multi)drug Resistance to Tyrosine Kinase Inhibitors and Potential Strategies to Overcome Resistance. Cancers 2018, 10, 503. [Google Scholar] [CrossRef]
- Hinderling, P.H. Red Blood Cells: A Neglected Compartment in Pharmacokinetics and Pharmacodynamics. Pharmacol. Rev. 1997, 49, 279–295. [Google Scholar]
- Labots, M.; Pham, T.V.; Honeywell, R.J.; Knol, J.C.; Beekhof, R.; De Goeij-de Haas, R.; Dekker, H.; Neerincx, M.; Piersma, S.R.; Van der Mijn, J.C.; et al. Kinase Inhibitor Treatment of Patients with Advanced Cancer Results in High Tumor Drug Concentrations and in Specific Alterations of the Tumor Phosphoproteome. Cancers 2020, 12, 330. [Google Scholar] [CrossRef]
- Lagas, J.S.; Van Waterschoot, R.A.B.; Van Tilburg, V.A.C.J.; Hillebrand, M.J.; Lankheet, N.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Brain Accumulation of Dasatinib Is Restricted by P-Glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) and Can Be Enhanced by Elacridar Treatment. Clin. Cancer Res. 2009, 15, 2344–2351. [Google Scholar] [CrossRef]
- Van Der Steen, N.; Keller, K.; Dekker, H.; Porcelli, L.; Honeywell, R.J.; Van Meerloo, J.; Musters, R.J.P.; Kathmann, I.; Frampton, A.E.; Lui, D.S.K.; et al. Crizotinib sensitizes the erlotinib resistant HCC827GR5 cell line by influencing lysosomal function. J. Cell. Physiol. 2020, 235, 8085–8097. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, A.E.; Lubberman, F.J.; Tol, J.; Gerritsen, W.R.; van Herpen, C.M.; van Erp, N.P. Effect of food and acid-reducing agents on the absorption of oral targeted therapies in solid tumors. Drug Discov. Today 2016, 21, 962–976. [Google Scholar] [CrossRef]
- Halley, A.; Leonetti, A.; Gregori, A.; Tiseo, M.; Deng, D.M.; Giovannetti, E.; Peters, G.J. The Role of the Microbiome in Cancer and Therapy Efficacy: Focus on Lung Cancer. Anticancer Res. 2020, 40, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.M.; Reddy, N.J.; Cohen, R.B.; Lewis, N.L.; Whitehead, B.; Mackay, K.; Stead, A.; Beelen, A.P.; Lewis, L.D. Effects of Food on the Relative Bioavailability of Lapatinib in Cancer Patients. J. Clin. Oncol. 2009, 27, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Rovithi, M.; Gerritse, S.L.; Honeywell, R.J.; Ten Tije, A.J.; Ruijter, R.; Peters, G.J.; Voortman, J.; Labots, M.; Verheul, H.M.W. Phase 1 dose-escalation study of once weekly or once every two weeks administration of high dose sunitinib in patients with refractory solid tumors. J. Clin. Oncol. 2019, 37, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mammatas, L.H.; Zandvliet, A.S.; Rovithi, M.; Honeywell, R.J.; Swart, E.L.; Peters, G.J.; Menke-van der Houven van Oordt, C.W.; Verheul, H.M.W. Sorafenib administered using a high-dose, pulsatile regimen in patients with advanced solid malignancies: A phase I exposure escalation study. Cancer Chemother. Pharmacol. 2020, 85, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Dumez, H.; Guetens, G.; De Boeck, G.; Highley, M.S.; Maes, R.A.; van Oosterom, A.T.; de Bruijn, E.A. The relevance of therapeutic drug monitoring in plasma and erythrocytes in anti-cancer drug treatment. Clin. Chem. Lab. Med. 2004, 42, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A.; Tam, K.Y. How well can the CaCo2/Madin-Darby Canine kidney models predict effective human jejunal permeability? J. Med. Chem. 2010, 53, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Honeywell, R.; Yarzadah, K.; Giovannetti, E.; Losekoot, N.; Smit, E.F.; Walraven, M.; Lind, J.S.W.; Tibaldi, C.; Verheul, H.M.; Peters, G.J. Simple and Selective Method for the Determination of Various Tyrosine Kinase Inhibitors Used in the Clinical Setting by Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. B 2010, 878, 1059–1068. [Google Scholar] [CrossRef]
- Lemos, C.; Peters, G.J.; Jansen, G.; Martel, F.; Calhau, C. Modulation of folate uptake in cultured human colon adenocarcinoma Caco-2 cells by diet compounds. Eur. J. Nutr. 2007, 46, 429–436. [Google Scholar] [CrossRef]
- Lemos, C.; Giovannetti, E.; Zucali, P.A.; Assaraf, Y.G.; Scheffer, G.L.; van der Straaten, T.; D’Incecco, A.; Falcone, A.; Guchelaar, H.-J.; Danesi, R.; et al. Impact of ABCG2 Polymorphisms on the Clinical Outcome and Toxicity of Gefitinib in Non-Small-Cell Lung Cancer Patients. Pharmacogenomics 2011, 12, 159–170. [Google Scholar] [CrossRef]
- Westgeest, H.M.; van Erp, N.P.; Honeywell, R.J.; Hoekstra, R.; Peters, G.J.; Verheul, H.M. Successful Treatment of Renal Cell Carcinoma with Sorafenib after Effective but Hepatotoxic Sunitinib Exposure. J Clin. Oncol. 2013, 31, e83–e86. [Google Scholar] [CrossRef]
- Driessen, O.; Highley, M.S.; Harper, P.G.; Maes, R.A.A.; De Bruijn, E.A. Description of an Instrument for Separation of Red Cells from Plasma and Measurement of Red Cell Volume. Clin. Biochem. 1994, 27, 195–196. [Google Scholar] [CrossRef]

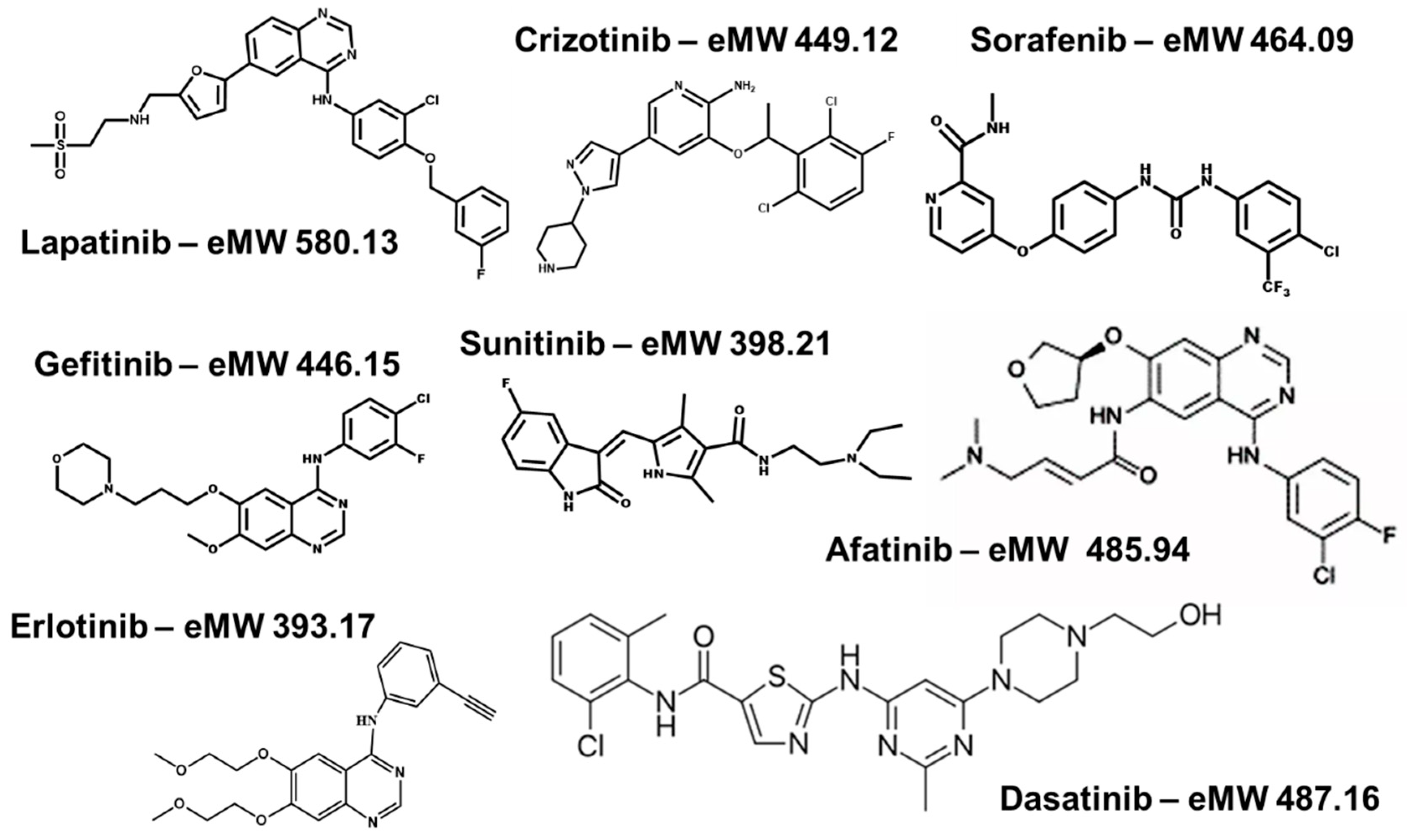

| Drug | Targets | Disease | Dose | Effect of Food (%) | Protein (%) | Steady State Concentration (µM) | Vd (l) | t½ (h) | Bioavailability (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gefitinib [3,4,5,6] | EGFR + mutation | NSCLC | 250 mg daily | ~ 37% increase [7] | ~90 | 0.11–0.33 | 1700 | 32 | 35–60 [8,9] |
| Erlotinib [8,10,11,12,13] | EGFR + mutation | NSCLC, pancreatic cancer | 150 mg daily | ~ 40% increase [14] | ~95 | 2.54–5.08 | 232 | 15–30 | 76 [15] |
| Afatinib [16] | EGFR + mutation | NSCLC | 40 mg daily | ~ 50% decrease | ~95 | 0.062–0.16 | 2870 | 36.6 | No data available |
| Sunitinib [17] | VEGFR, KDR, FLT3 | GIST, RCC, HCC, pNET | 50 mg daily | ~12% increase [18] | ~98 | 0.15–0.25 | 1900 | 25–60 | 39 |
| Sorafenib [19,20,21] 1 | KDR, Ras/Raf pathway | HCC, RCC, DTC | 100–600 mg BID | Inconsistent ~ 29% decrease ~14% increase [22,23] | ~95 | 6.28–23.45 | 212 | 22–28 | Estimated 38–49% |
| Crizotinib [24,25,26] | ALK, c-MET | NSCLC | 250 mg daily | ~ 14% decrease | ~ 90% | 0.22–0.44 | 4200–4900 | 43–51 | 43 [27] |
| Dasatinib [19,28,29,30] | BCR-Abl | CML | 100–140 mg daily | ~ 14% increase [31] | ~ 94% | 0.19–0.27 | 2500 | 3–4 | 14–27 [32] (animal) |
| Drug | µM | Papp (×10−6) cm/s (Apical Basolateral) | Papp (×10−6) cm/s (Basolateral Apical) | Efflux Ratio | Log P | Log D (pH 5.5) | Log D (pH 7.5) |
|---|---|---|---|---|---|---|---|
| Gefitinib | 20 | 0.38 ± 0.05 | 0.39 ± 0.038 | 1.0 | 4.11 | 1.96 | 3.55 |
| 10 | 0.57 ± 0.43 | 0.19 ± 0.12 | 0.3 | ||||
| Erlotinib | 20 | 0.13 ± 0.001 | 0.70 ± 0.30 | 5.4 | 2.39 | 2.74 | 3.04 |
| 10 | 0.48 ± 0.26 | 0.79 ± 0.65 | 1.6 | ||||
| with sorafenib | 10 | 0.35 ± 0.17 | 0.48 ± 0.30 | 1.4 | |||
| Afatinib | 10 | 1.5 ± 2.2 | 10.9 ± 5.0 | 7.3 | 3.59 | 1.00 | 2.87 |
| Sunitinib | 20 | 7.7 ± 4.6 | 12.6 ± 12.3 | 1.6 | 3.15 | −0.09 | 1.09 |
| Sorafenib | 20 | 14.1 ± 11.5 | 11.4 ± 5.6 | 0.81 | 5.16 | 4.26 | 4.26 |
| 10 | 10.1 ± 9.5 | 9.3 ± 5.8 | 0.92 | ||||
| with Erlotinib | 10 | 18.6 ± 13.2 | 13.3 ± 10.5 | 0.71 | |||
| Crizotinib | 10 | Unmeasurable | 7.7 ± 5.5 | >7 * | 4.73 | 0.30 | 1.80 |
| Dasatinib | 20 | 3.4 ± 3.4 | 20.4 ± 13.5 | 6.0 | 2.24 | −0.44 | 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honeywell, R.J.; Kathmann, I.; Giovannetti, E.; Tibaldi, C.; Smit, E.F.; Rovithi, M.N.; Verheul, H.M.W.; Peters, G.J. Epithelial Transfer of the Tyrosine Kinase Inhibitors Erlotinib, Gefitinib, Afatinib, Crizotinib, Sorafenib, Sunitinib, and Dasatinib: Implications for Clinical Resistance. Cancers 2020, 12, 3322. https://doi.org/10.3390/cancers12113322
Honeywell RJ, Kathmann I, Giovannetti E, Tibaldi C, Smit EF, Rovithi MN, Verheul HMW, Peters GJ. Epithelial Transfer of the Tyrosine Kinase Inhibitors Erlotinib, Gefitinib, Afatinib, Crizotinib, Sorafenib, Sunitinib, and Dasatinib: Implications for Clinical Resistance. Cancers. 2020; 12(11):3322. https://doi.org/10.3390/cancers12113322
Chicago/Turabian StyleHoneywell, Richard J., Ietje Kathmann, Elisa Giovannetti, Carmelo Tibaldi, Egbert F. Smit, Maria N. Rovithi, Henk M.W. Verheul, and Godefridus J. Peters. 2020. "Epithelial Transfer of the Tyrosine Kinase Inhibitors Erlotinib, Gefitinib, Afatinib, Crizotinib, Sorafenib, Sunitinib, and Dasatinib: Implications for Clinical Resistance" Cancers 12, no. 11: 3322. https://doi.org/10.3390/cancers12113322
APA StyleHoneywell, R. J., Kathmann, I., Giovannetti, E., Tibaldi, C., Smit, E. F., Rovithi, M. N., Verheul, H. M. W., & Peters, G. J. (2020). Epithelial Transfer of the Tyrosine Kinase Inhibitors Erlotinib, Gefitinib, Afatinib, Crizotinib, Sorafenib, Sunitinib, and Dasatinib: Implications for Clinical Resistance. Cancers, 12(11), 3322. https://doi.org/10.3390/cancers12113322







