Trop2: Jack of All Trades, Master of None
Simple Summary
Abstract
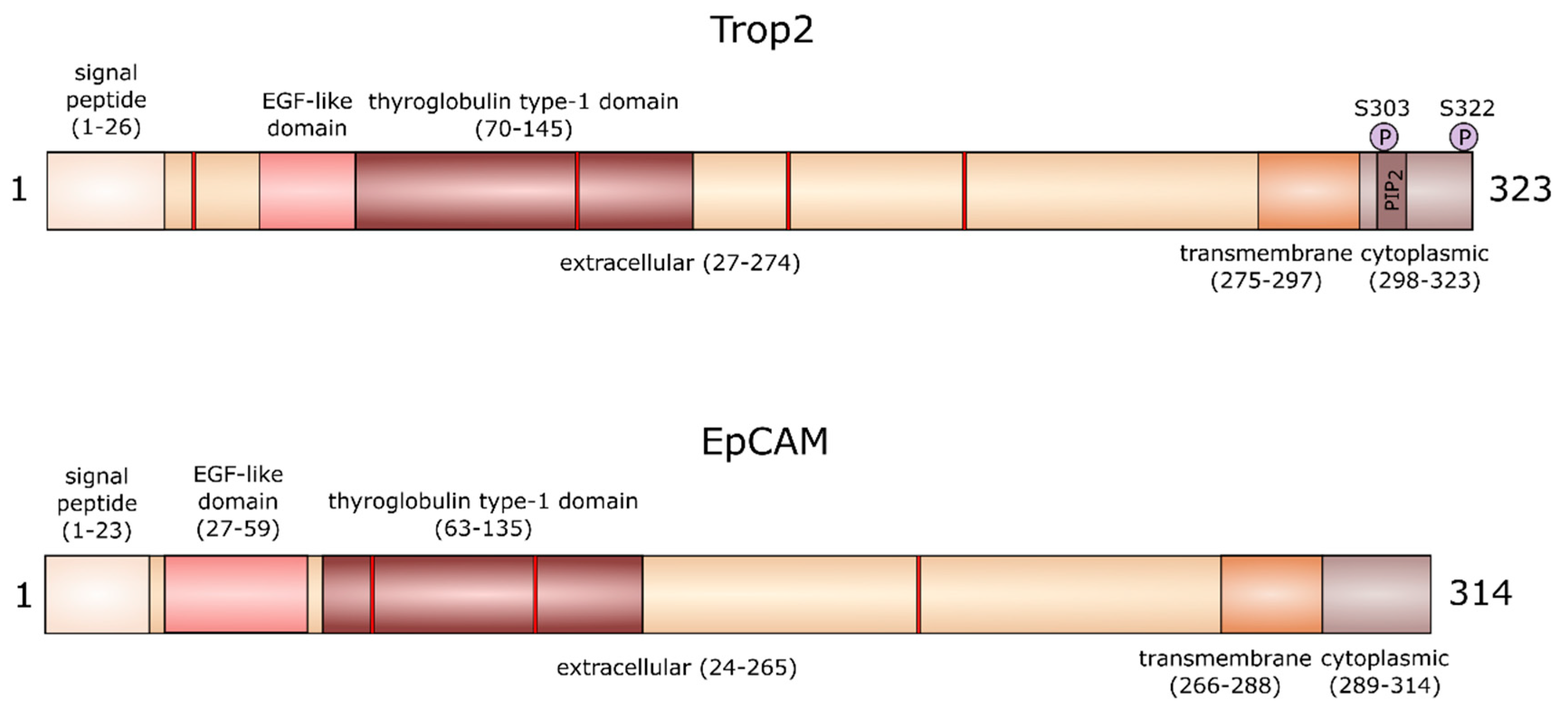
1. Gene and Protein
2. Trop2 in Healthy Tissue and Development
2.1. Germline Mutations in Trop2 (TACSTD2) Gene
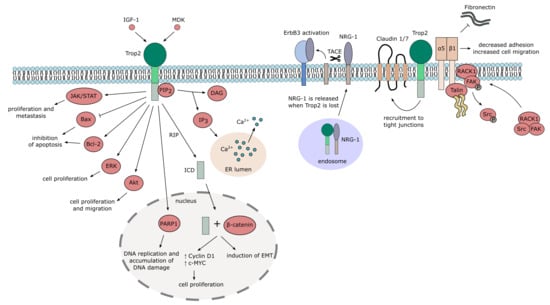
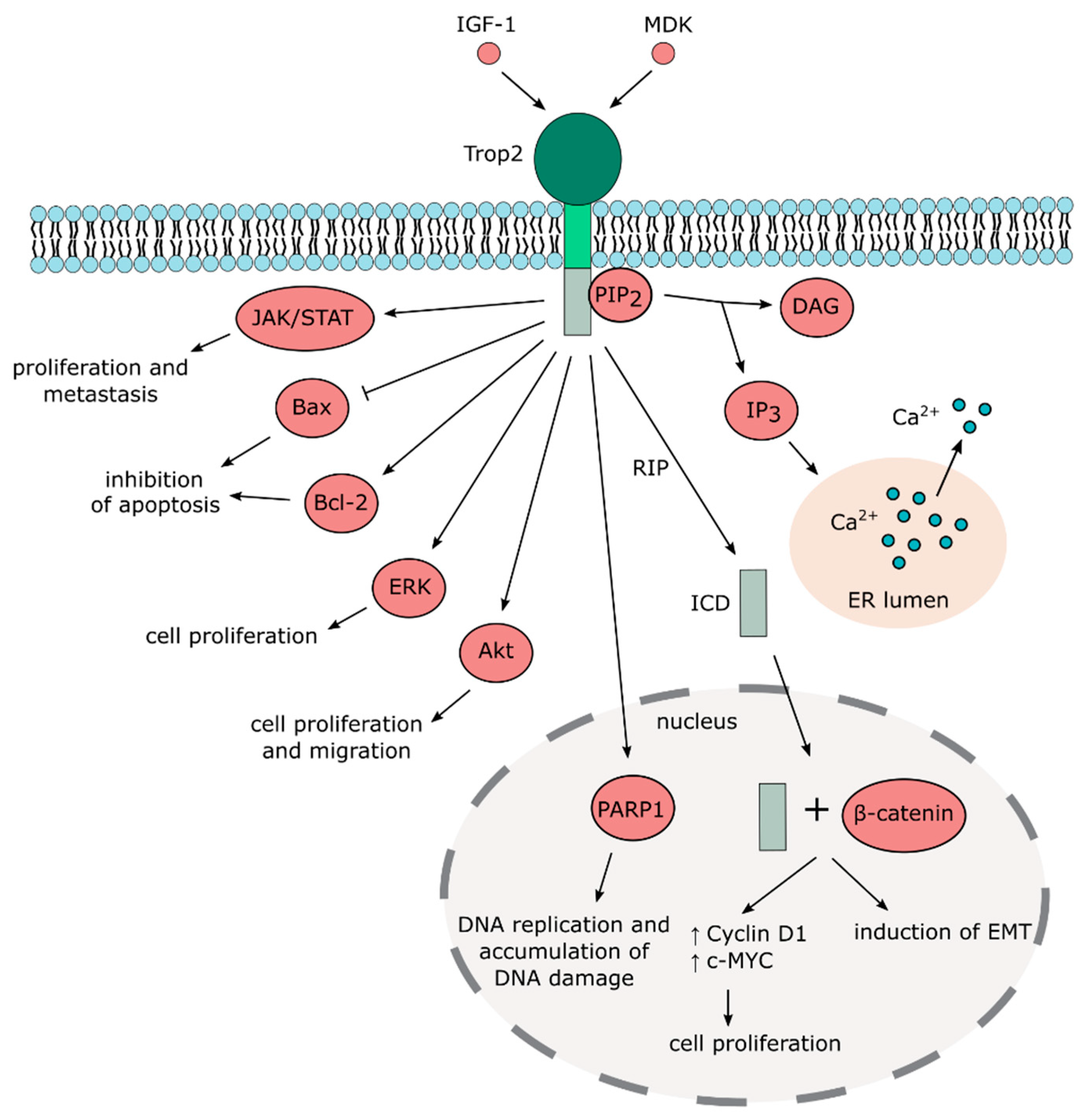
2.2. Trop2 Signaling and Interaction Network
3. Trop2 in Cancer
3.1. Regulation of Trop2 Expression in Cancer
3.2. Multiple Functions of Trop2 in Cancer
3.2.1. Trop2 and EMT
3.2.2. Trop2 and Cell Proliferation
3.2.3. Trop2 and Cell Adhesion and Migration
3.2.4. Trop2 and Drug Resistance
3.2.5. Other Functions in Cancer
3.2.6. Trop2 as Tumor Suppressor (Until It Is Not)
3.3. Trop2 in Cancer Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lipinski, M.; Parks, D.R.; Rouse, R.V.; Herzenberg, L.A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1981, 78, 5147–5150. [Google Scholar] [CrossRef]
- Cubas, R.; Li, M.; Chen, C.; Yao, Q. Trop2: A possible therapeutic target for late stage epithelial carcinomas. Biochim. Biophys. Acta 2009, 1796, 309–314. [Google Scholar] [CrossRef]
- Calabrese, G.; Crescenzi, C.; Morizio, E.; Palka, G.; Guerra, E.; Alberti, S. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet. Cell Genet. 2001, 92, 164–165. [Google Scholar] [CrossRef]
- Linnenbach, A.J.; Wojcierowski, J.; Wu, S.A.; Pyrc, J.J.; Ross, A.H.; Dietzschold, B.; Speicher, D.; Koprowski, H. Sequence investigation of the major gastrointestinal tumor-associated antigen gene family, GA733. Proc. Natl. Acad. Sci. USA. 1989, 86, 27–31. [Google Scholar] [CrossRef]
- McDougall, A.R.A.; Tolcos, M.; Hooper, S.B.; Cole, T.J.; Wallace, M.J. Trop2: From development to disease. Dev. Dyn. 2015, 244, 99–109. [Google Scholar] [CrossRef]
- Szala, S.; Froehlich, M.; Scollon, M.; Kasai, Y.; Steplewski, Z.; Koprowski, H.; Linnenbach, A.J. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc. Natl. Acad. Sci. USA 1990, 87, 3542–3546. [Google Scholar] [CrossRef]
- Linnenbach, A.J.; Seng, B.A.; Wu, S.; Robbins, S.; Scollon, M.; Pyrc, J.J.; Druck, T.; Huebner, K. Retroposition in a family of carcinoma-associated antigen genes. Mol. Cell. Biol. 1993, 13, 1507–1515. [Google Scholar] [CrossRef]
- El Sewedy, T.; Fornaro, M.; Alberti, S. Cloning of the murine TROP2 gene: Conservation of a PIP2-binding sequence in the cytoplasmic domain of TROP-2. Int. J. Cancer 1998, 75, 324–330. [Google Scholar] [CrossRef]
- UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [CrossRef]
- Basu, A.; Goldenberg, D.M.; Stein, R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int. J. Cancer 1995, 62, 472–479. [Google Scholar] [CrossRef]
- Pavšič, M.; Ilc, G.; Vidmar, T.; Plavec, J.; Lenarčič, B. The cytosolic tail of the tumor marker protein Trop2—A structural switch triggered by phosphorylation. Sci. Rep. 2015, 5, 10324. [Google Scholar] [CrossRef]
- Vidmar, T.; Pavšič, M.; Lenarčič, B. Biochemical and preliminary X-ray characterization of the tumor-associated calcium signal transducer 2 (Trop2) ectodomain. Protein Expr. Purif. 2013, 91, 69–76. [Google Scholar] [CrossRef]
- Mori, Y.; Akita, K.; Ojima, K.; Iwamoto, S.; Yamashita, T.; Morii, E.; Nakada, H. Trophoblast cell surface antigen 2 (Trop-2) phosphorylation by protein kinase C α/δ (PKCα/δ) enhances cell motility. J. Biol. Chem. 2019, 294, 11513–11524. [Google Scholar] [CrossRef]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, In Vivo, and Site-Specific Phosphorylation Dynamics in Signaling Networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef]
- Stepan, L.P.; Trueblood, E.S.; Hale, K.; Babcook, J.; Borges, L.; Sutherland, C.L. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: Potential implications as a cancer therapeutic target. J. Histochem. Cytochem. 2011, 59, 701–710. [Google Scholar] [CrossRef]
- Trerotola, M.; Cantanelli, P.; Guerra, E.; Tripaldi, R.; Aloisi, A.L.; Bonasera, V.; Lattanzio, R.; de Lange, R.; Weidle, U.H.; Piantelli, M.; et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene 2013, 32, 222–233. [Google Scholar] [CrossRef]
- McDougall, A.R.A.; Hooper, S.B.; Zahra, V.A.; Sozo, F.; Lo, C.Y.; Cole, T.J.; Doran, T.; Wallace, M.J. The oncogene Trop2 regulates fetal lung cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L478–L489. [Google Scholar] [CrossRef]
- Sozo, F.; Wallace, M.J.; Zahra, V.A.; Filby, C.E.; Hooper, S.B. Gene expression profiling during increased fetal lung expansion identifies genes likely to regulate development of the distal airways. Physiol. Genomics 2006, 24, 105–113. [Google Scholar] [CrossRef]
- Mustata, R.C.; Vasile, G.; Fernandez-Vallone, V.; Strollo, S.; Lefort, A.; Libert, F.; Monteyne, D.; Pérez-Morga, D.; Vassart, G.; Garcia, M.-I. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 2013, 5, 421–432. [Google Scholar] [CrossRef]
- Vallone, V.F.; Leprovots, M.; Strollo, S.; Vasile, G.; Lefort, A.; Libert, F.; Vassart, G.; Garcia, M.-I. Trop2 marks transient gastric fetal epithelium and adult regenerating cells after epithelial damage. Development 2016, 143, 1452–1463. [Google Scholar] [CrossRef]
- Sun, W.; Wilhelmina Aalders, T.; Oosterwijk, E. Identification of potential bladder progenitor cells in the trigone. Dev. Biol. 2014, 393, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, Y.; Tanaka, M.; Miyajima, A. TROP2 expressed in the trunk of the ureteric duct regulates branching morphogenesis during kidney development. PLoS ONE 2011, 6, e28607. [Google Scholar] [CrossRef] [PubMed]
- McDougall, A.R.A.; Wiradjaja, V.; Azhan, A.; Li, A.; Hale, N.; Wlodek, M.E.; Hooper, S.B.; Wallace, M.J.; Tolcos, M. Intrauterine Growth Restriction Alters the Postnatal Development of the Rat Cerebellum. Dev. Neurosci. 2017, 39, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, S.; Zong, Y.; Janzen, D.M.; Goldstein, A.S.; Cheng, D.; Kurita, T.; Schafenacker, A.M.; Huang, J.; Witte, O.N. Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc. Natl. Acad. Sci. USA 2010, 107, 17298–17303. [Google Scholar] [CrossRef]
- Li, T.; Su, Y.; Yu, X.; Mouniir, D.S.A.; Masau, J.F.; Wei, X.; Yang, J. Trop2 Guarantees Cardioprotective Effects of Cortical Bone-Derived Stem Cells on Myocardial Ischemia/Reperfusion Injury. Cell Transplant. 2018, 27, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Z.; Wang, H.; Li, F.; Du, X.; Ma, R.Z. Trop2 regulates the proliferation and differentiation of murine compact-bone derived MSCs. Int. J. Oncol. 2013, 43, 859–867. [Google Scholar] [CrossRef]
- Grünherz, L.; Prein, C.; Winkler, T.; Kirsch, M.; Hopfner, U.; Streichert, T.; Clausen-Schaumann, H.; Zustin, J.; Kirchhof, K.; Morlock, M.M.; et al. Osteoidosis leads to altered differentiation and function of osteoclasts. J. Cell. Mol. Med. 2020, 24, 5665–5674. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Lawson, D.A.; Cheng, D.; Sun, W.; Garraway, I.P.; Witte, O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 20882–20887. [Google Scholar] [CrossRef]
- Kahounová, Z.; Remšík, J.; Fedr, R.; Bouchal, J.; Mičková, A.; Slabáková, E.; Binó, L.; Hampl, A.; Souček, K. Slug-expressing mouse prostate epithelial cells have increased stem cell potential. Stem Cell Res. 2020, 46, 101844. [Google Scholar] [CrossRef]
- Crowell, P.D.; Fox, J.J.; Hashimoto, T.; Diaz, J.A.; Navarro, H.I.; Henry, G.H.; Feldmar, B.A.; Lowe, M.G.; Garcia, A.J.; Wu, Y.E.; et al. Expansion of Luminal Progenitor Cells in the Aging Mouse and Human Prostate. Cell Rep. 2019, 28, 1499–1510. [Google Scholar] [CrossRef]
- Okabe, M.; Tsukahara, Y.; Tanaka, M.; Suzuki, K.; Saito, S.; Kamiya, Y.; Tsujimura, T.; Nakamura, K.; Miyajima, A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Dev. Camb. Engl. 2009, 136, 1951–1960. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Wang, Q.; Zhang, Y.; Wang, W.; Dou, S.; Xiao, W. Increased expression of TROP2 in airway basal cells potentially contributes to airway remodeling in chronic obstructive pulmonary disease. Respir. Res. 2016, 17, 159. [Google Scholar] [CrossRef]
- McDougall, A.R.A.; Hooper, S.B.; Zahra, V.A.; Cole, T.J.; Lo, C.Y.; Doran, T.; Wallace, M.J. Trop2 regulates motility and lamellipodia formation in cultured fetal lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L508–L521. [Google Scholar] [CrossRef] [PubMed]
- Aizarani, N.; Saviano, A.; Sagar; Mailly, L.; Durand, S.; Herman, J.S.; Pessaux, P.; Baumert, T.F.; Grün, D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 2019, 572, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Grabowska, D.; Li, A.; Dong, Y.; Day, R.; Humphrey, P.; Lewis, J.; Kladney, R.D.; Arbeit, J.M.; et al. Loss of Trop2 promotes carcinogenesis and features of epithelial to mesenchymal transition in squamous cell carcinoma. Mol. Cancer Res. MCR 2011, 9, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Maeda, T.; Tamura, A.; Nakamura, T.; Yamazaki, Y.; Shiratori, H.; Yashiro, K.; Tsukita, S.; Hamada, H. EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins. Dev. Biol. 2012, 371, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Lattanzio, R.; La Sorda, R.; Dini, F.; Tiboni, G.M.; Piantelli, M.; Alberti, S. mTrop1/Epcam Knockout Mice Develop Congenital Tufting Enteropathy through Dysregulation of Intestinal E-cadherin/β-catenin. PLoS ONE 2012, 7, e49302. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.L.; McGeough, M.D.; Peña, C.A.; Sivagnanam, M. Functional consequences of EpCam mutation in mice and men. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G278–G288. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, M.; Mueller, J.L.; Lee, H.; Chen, Z.; Nelson, S.F.; Turner, D.; Zlotkin, S.H.; Pencharz, P.B.; Ngan, B.-Y.; Libiger, O.; et al. Identification of EpCAM as the Gene for Congenital Tufting Enteropathy. Gastroenterology 2008, 135, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Balzar, M.; Winter, M.J.; de Boer, C.J.; Litvinov, S.V. The biology of the 17–1A antigen (Ep-CAM). J. Mol. Med. 1999, 77, 699–712. [Google Scholar] [CrossRef]
- Nakato, G.; Morimura, S.; Lu, M.; Feng, X.; Wu, C.; Udey, M.C. Amelioration of Congenital Tufting Enteropathy in EpCAM (TROP1)-Deficient Mice via Heterotopic Expression of TROP2 in Intestinal Epithelial Cells. Cells 2020, 9, 1847. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, M.; Kurahashi, H.; Tanaka, T.; Nishida, K.; Shimomura, Y.; Tano, Y.; Nakamura, Y. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat. Genet. 1999, 21, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, Y.; Tsujikawa, M.; Takigawa, T.; Xu, P.; Kai, C.; Kawasaki, S.; Nakatsukasa, M.; Inatomi, T.; Kinoshita, S.; Nishida, K. A novel mutation in gelatinous drop-like corneal dystrophy and functional analysis. Hum. Genome Var. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Nishida, K.; Maeda, N.; Tsujikawa, M.; Yamamoto, S.; Watanabe, H.; Tano, Y. A spectrum of clinical manifestations of gelatinous drop-like corneal dystrophy in japan. Am. J. Ophthalmol. 2004, 137, 1081–1084. [Google Scholar] [CrossRef]
- Takaoka, M.; Nakamura, T.; Ban, Y.; Kinoshita, S. Phenotypic investigation of cell junction-related proteins in gelatinous drop-like corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1095–1101. [Google Scholar] [CrossRef]
- Nakatsukasa, M.; Kawasaki, S.; Yamasaki, K.; Fukuoka, H.; Matsuda, A.; Tsujikawa, M.; Tanioka, H.; Nagata-Takaoka, M.; Hamuro, J.; Kinoshita, S. Tumor-associated calcium signal transducer 2 is required for the proper subcellular localization of claudin 1 and 7: Implications in the pathogenesis of gelatinous drop-like corneal dystrophy. Am. J. Pathol. 2010, 177, 1344–1355. [Google Scholar] [CrossRef]
- Xu, P.; Kai, C.; Kawasaki, S.; Kobayashi, Y.; Yamamoto, K.; Tsujikawa, M.; Hayashi, R.; Nishida, K. A New in Vitro Model of GDLD by Knocking Out TACSTD2 and Its Paralogous Gene EpCAM in Human Corneal Epithelial Cells. Transl. Vis. Sci. Technol. 2018, 7, 30. [Google Scholar] [CrossRef]
- Nübel, T.; Preobraschenski, J.; Tuncay, H.; Weiss, T.; Kuhn, S.; Ladwein, M.; Langbein, L.; Zöller, M. Claudin-7 regulates EpCAM-mediated functions in tumor progression. Mol. Cancer Res. MCR 2009, 7, 285–299. [Google Scholar] [CrossRef]
- Sekhar, V.; Pollicino, T.; Diaz, G.; Engle, R.E.; Alayli, F.; Melis, M.; Kabat, J.; Tice, A.; Pomerenke, A.; Altan-Bonnet, N.; et al. Infection with hepatitis C virus depends on TACSTD2, a regulator of claudin-1 and occludin highly downregulated in hepatocellular carcinoma. PLoS Pathog. 2018, 14, e1006916. [Google Scholar] [CrossRef]
- Wu, C.-J.; Lu, M.; Feng, X.; Nakato, G.; Udey, M.C. Matriptase Cleaves EpCAM and TROP2 in Keratinocytes, Destabilizing Both Proteins and Associated Claudins. Cells 2020, 9, 1027. [Google Scholar] [CrossRef]
- Wu, C.-J.; Feng, X.; Lu, M.; Morimura, S.; Udey, M.C. Matriptase-mediated cleavage of EpCAM destabilizes claudins and dysregulates intestinal epithelial homeostasis. J. Clin. Investig. 2017, 127, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.R.; Rane, S.; Breed, A.A.; Joseph, S.; Mahale, S.D.; Pathak, B.R. Proteolytic cleavage of Trop2 at Arg87 is mediated by matriptase and regulated by Val194. FEBS Lett. 2020, 594, 3156–3169. [Google Scholar] [CrossRef] [PubMed]
- Ripani, E.; Sacchetti, A.; Corda, D.; Alberti, S. Human Trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer 1998, 76, 671–676. [Google Scholar] [CrossRef]
- Cheng, N.; Li, H.; Luo, J. Trop2 promotes proliferation, invasion and EMT of nasopharyngeal carcinoma cells through the NF-κB pathway. RSC Adv. 2017, 7, 53087–53096. [Google Scholar] [CrossRef]
- Gu, Q.-Z.; Nijiati, A.; Gao, X.; Tao, K.-L.; Li, C.-D.; Fan, X.-P.; Tian, Z. TROP2 promotes cell proliferation and migration in osteosarcoma through PI3K/AKT signaling. Mol. Med. Rep. 2018, 18, 1782–1788. [Google Scholar] [CrossRef]
- Li, X.; Teng, S.; Zhang, Y.; Zhang, W.; Zhang, X.; Xu, K.; Yao, H.; Yao, J.; Wang, H.; Liang, X.; et al. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget 2017, 8, 47052–47063. [Google Scholar] [CrossRef]
- Sun, X.; Xing, G.; Zhang, C.; Lu, K.; Wang, Y.; He, X. Knockdown of Trop2 inhibits proliferation and migration and induces apoptosis of endometrial cancer cells via AKT/β-catenin pathway. Cell Biochem. Funct. 2020. [Google Scholar] [CrossRef]
- Tang, G.; Tang, Q.; Jia, L.; Chen, Y.; Lin, L.; Kuai, X.; Gong, A.; Feng, Z. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway. Int. J. Mol. Med. 2019, 44, 2161–2170. [Google Scholar] [CrossRef]
- Guerra, E.; Trerotola, M.; Tripaldi, R.; Aloisi, A.L.; Simeone, P.; Sacchetti, A.; Relli, V.; D’Amore, A.; La Sorda, R.; Lattanzio, R.; et al. Trop-2 Induces Tumor Growth Through AKT and Determines Sensitivity to AKT Inhibitors. Clin. Cancer Res. 2016, 22, 4197–4205. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer 2010, 9, 253. [Google Scholar] [CrossRef]
- Guan, H.; Guo, Z.; Liang, W.; Li, H.; Wei, G.; Xu, L.; Xiao, H.; Li, Y. Trop2 enhances invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways. BMC Cancer 2017, 17, 486. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Bao, X.; Tian, J.; Liu, Y.; Yang, X. Overexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathway. PLoS ONE 2013, 8, e75864. [Google Scholar] [CrossRef] [PubMed]
- Redlich, N.; Robinson, A.M.; Nickel, K.P.; Stein, A.P.; Wheeler, D.L.; Adkins, D.R.; Uppaluri, R.; Kimple, R.J.; Van Tine, B.A.; Michel, L.S. Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma. Cell Death Dis. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.; Yang, P.; Guo, L.; Liu, C.; Li, H.; Long, S.; Shen, Y.; Wan, H. Loss of TACSTD2 contributed to squamous cell carcinoma progression through attenuating TAp63-dependent apoptosis. Cell Death Dis. 2014, 5, e1133. [Google Scholar] [CrossRef]
- Zhang, K.; Jones, L.; Lim, S.; Maher, C.A.; Adkins, D.; Lewis, J.; Kimple, R.J.; Fertig, E.J.; Chung, C.H.; Van Tine, B.A.; et al. Loss of Trop2 causes ErbB3 activation through a neuregulin-1-dependent mechanism in the mesenchymal subtype of HNSCC. Oncotarget 2014, 5, 9281–9294. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, L.; Kuai, X.; Tang, Q.; Huang, X.; Yang, T.; Qiu, Z.; Zhu, J.; Huang, J.; Huang, W.; et al. The role and molecular mechanism of Trop2 induced epithelial-mesenchymal transition through mediated β-catenin in gastric cancer. Cancer Med. 2019, 8, 1135–1147. [Google Scholar] [CrossRef]
- Stoyanova, T.; Goldstein, A.S.; Cai, H.; Drake, J.M.; Huang, J.; Witte, O.N. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via β-catenin signaling. Genes Dev. 2012, 26, 2271–2285. [Google Scholar] [CrossRef]
- Hou, J.; Lv, A.; Deng, Q.; Zhang, G.; Hu, X.; Cui, H. TROP2 promotes the proliferation and metastasis of glioblastoma cells by activating the JAK2/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 753–764. [Google Scholar] [CrossRef]
- Trerotola, M.; Ganguly, K.K.; Fazli, L.; Fedele, C.; Lu, H.; Dutta, A.; Liu, Q.; De Angelis, T.; Riddell, L.W.; Riobo, N.A.; et al. Trop-2 is up-regulated in invasive prostate cancer and displaces FAK from focal contacts. Oncotarget 2015, 6, 14318–14328. [Google Scholar] [CrossRef]
- Trerotola, M.; Jernigan, D.L.; Liu, Q.; Siddiqui, J.; Fatatis, A.; Languino, L.R. Trop-2 promotes prostate cancer metastasis by modulating β(1) integrin functions. Cancer Res. 2013, 73, 3155–3167. [Google Scholar] [CrossRef]
- Trerotola, M.; Li, J.; Alberti, S.; Languino, L.R. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the β1 integrin-RACK1 axis. J. Cell. Physiol. 2012, 227, 3670–3677. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Wu, Y.-Y.; Wu, J.-Y.; Lin, T.-C.; Wu, C.-T.; Chang, Y.-L.; Jou, Y.-S.; Hong, T.-M.; Yang, P.-C. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol. Med. 2012, 4, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.G.; Shin, D.H.; Lee, C.H.; Lee, M.K. Significance of EpCAM and TROP2 expression in non-small cell lung cancer. World J. Surg. Oncol. 2012, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Sawanyawisuth, K.; Tantapotinan, N.; Wongkham, C.; Riggins, G.J.; Kraiklang, R.; Wongkham, S.; Puapairoj, A. Suppression of trophoblast cell surface antigen 2 enhances proliferation and migration in liver fluke-associated cholangiocarcinoma. Ann. Hepatol. 2016, 15, 71–81. [Google Scholar] [CrossRef]
- Kobayashi, H.; Minami, Y.; Anami, Y.; Kondou, Y.; Iijima, T.; Kano, J.; Morishita, Y.; Tsuta, K.; Hayashi, S.; Noguchi, M. Expression of the GA733 gene family and its relationship to prognosis in pulmonary adenocarcinoma. Virchows Arch. Int. J. Pathol. 2010, 457, 69–76. [Google Scholar] [CrossRef]
- Sin, S.T.K.; Li, Y.; Liu, M.; Ma, S.; Guan, X.-Y. TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol. Oncol. 2018, 152, 185–193. [Google Scholar] [CrossRef]
- Eyvazi, S.; Farajnia, S.; Dastmalchi, S.; Kanipour, F.; Zarredar, H.; Bandehpour, M. Antibody Based EpCAM Targeted Therapy of Cancer, Review and Update. Curr. Cancer Drug Targets 2018, 18, 857–868. [Google Scholar] [CrossRef]
- Keller, L.; Werner, S.; Pantel, K. Biology and clinical relevance of EpCAM. Cell Stress 2019, 3, 165–180. [Google Scholar] [CrossRef]
- Martowicz, A.; Seeber, A.; Untergasser, G. The role of EpCAM in physiology and pathology of the epithelium. Histol. Histopathol. 2016, 31, 349–355. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Aguilar-Gallardo, C.; Calabuig-Fariñas, S.; Sirera, R.; Jantus-Lewintre, E.; Camps, C. EpCAM duality becomes this molecule in a new Dr. Jekyll and Mr. Hyde tale. Crit. Rev. Oncol. Hematol. 2018, 126, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Moser, P.; Krammel, C.; Gostner, J.M.; Margreiter, R.; Mitterer, M.; Gastl, G.; Spizzo, G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br. J. Cancer 2008, 99, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Spizzo, G.; Gostner, J.M.; Gastl, G.; Moser, P.; Krammel, C.; Gerhard, S.; Rasse, M.; Laimer, K. TROP2: A novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod. Pathol. 2008, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.-C.; Rice, M.A.; Bermudez, A.; Marques, F.J.G.; Aslan, M.; Liu, S.; Ghoochani, A.; Zhang, C.A.; Chen, Y.-S.; Zlitni, A.; et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc. Natl. Acad. Sci. USA 2020, 117, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Mühlmann, G.; Spizzo, G.; Gostner, J.; Zitt, M.; Maier, H.; Moser, P.; Gastl, G.; Zitt, M.; Müller, H.M.; Margreiter, R.; et al. TROP2 expression as prognostic marker for gastric carcinoma. J. Clin. Pathol. 2009, 62, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Guo, S.; Xie, J.; Xu, Y.; Lu, X.; Chen, Y. TROP2 correlates with microvessel density and poor prognosis in hilar cholangiocarcinoma. J. Gastrointest. Surg. 2013, 17, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kuai, X.; Zhou, X.; Jia, L.; Wang, J.; Yang, X.; Tian, Z.; Wang, X.; Lv, Q.; Wang, B.; et al. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol. Rep. 2018, 40, 759–766. [Google Scholar] [CrossRef]
- Sin, S.T.K.; Li, Y.; Liu, M.; Yuan, Y.-F.; Ma, S.; Guan, X.-Y. Down-regulation of TROP-2 Predicts Poor Prognosis of Hepatocellular Carcinoma Patients. Hepatol. Commun. 2018, 2, 1408–1414. [Google Scholar] [CrossRef]
- Ambrogi, F.; Fornili, M.; Boracchi, P.; Trerotola, M.; Relli, V.; Simeone, P.; La Sorda, R.; Lattanzio, R.; Querzoli, P.; Pedriali, M.; et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE 2014, 9, e96993. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, H.; Zhang, S.; Yong, H.; Wang, W.; Zhou, Y.; Wang, B.; Wen, J.; Qiu, Z.; Ding, G.; et al. Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget 2016, 7, 6136–6145. [Google Scholar] [CrossRef]
- Riera, K.M.; Jang, B.; Min, J.; Roland, J.T.; Yang, Q.; Fesmire, W.T.; Camilleri-Broet, S.; Ferri, L.; Kim, W.H.; Choi, E.; et al. Trop2 is upregulated in the transition to dysplasia in the metaplastic gastric mucosa. J. Pathol. 2020, 251, 336–347. [Google Scholar] [CrossRef]
- Addati, T.; Achille, G.; Centrone, M.; Petroni, S.; Popescu, O.; Russo, S.; Grammatica, L.; Simone, G. TROP-2 expression in papillary thyroid cancer: A preliminary cyto-histological study. Cytopathology 2015, 26, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.; Sampatanukul, P.; Shuangshoti, S.; Keelawat, S. TROP-2 immunohistochemistry: A highly accurate method in the differential diagnosis of papillary thyroid carcinoma. Pathology 2016, 48, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Simms, A.; Jacob, R.P.; Cohen, C.; Siddiqui, M.T. TROP-2 expression in papillary thyroid carcinoma: Potential Diagnostic Utility. Diagn. Cytopathol. 2016, 44, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Lu, Z.H.; Wang, G.Q.; Pan, Z.Z.; Zhou, Z.W.; Yun, J.P.; Zhang, M.F.; Wan, D.S. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int. J. Colorectal Dis. 2009, 24, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Ohmachi, T.; Tanaka, F.; Mimori, K.; Inoue, H.; Yanaga, K.; Mori, M. Clinical Significance of TROP2 Expression in Colorectal Cancer. Clin. Cancer Res. 2006, 12, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Gao, X.; Zhang, D.; Zhang, L.; Lu, H. Expression and clinical significance of the Trop-2 gene in advanced non-small cell lung carcinoma. Oncol. Lett. 2013, 6, 375–380. [Google Scholar] [CrossRef]
- Mito, R.; Matsubara, E.; Komohara, Y.; Shinchi, Y.; Sato, K.; Yoshii, D.; Ohnishi, K.; Fujiwara, Y.; Tomita, Y.; Ikeda, K.; et al. Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol. Int. 2020, 70, 187–294. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Zhang, W. TROP2 overexpression promotes proliferation and invasion of lung adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2016, 470, 197–204. [Google Scholar] [CrossRef]
- Lin, H.; Huang, J.-F.; Qiu, J.-R.; Zhang, H.-L.; Tang, X.-J.; Li, H.; Wang, C.-J.; Wang, Z.-C.; Feng, Z.-Q.; Zhu, J. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp. Mol. Pathol. 2013, 94, 73–78. [Google Scholar] [CrossRef]
- Bignotti, E.; Todeschini, P.; Calza, S.; Falchetti, M.; Ravanini, M.; Tassi, R.A.; Ravaggi, A.; Bandiera, E.; Romani, C.; Zanotti, L.; et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur. J. Cancer 2010, 46, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Varughese, J.; Cocco, E.; Bellone, S.; Bellone, M.; Todeschini, P.; Carrara, L.; Schwartz, P.E.; Rutherford, T.J.; Pecorelli, S.; Santin, A.D. High-grade, chemotherapy-resistant primary ovarian carcinoma cell lines overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Gynecol. Oncol. 2011, 122, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Perrone, E.; Lopez, S.; Zeybek, B.; Bellone, S.; Bonazzoli, E.; Pelligra, S.; Zammataro, L.; Manzano, A.; Manara, P.; Bianchi, A.; et al. Preclinical Activity of Sacituzumab Govitecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2 (Trop-2) Linked to the Active Metabolite of Irinotecan (SN-38), in Ovarian Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Avellini, C.; Licini, C.; Lazzarini, R.; Procopio, A.D.; Muzzonigro, G.; Tossetta, G.; Mazzucchelli, R.; Gesuita, R.; Castellucci, M.; Olivieri, F.; et al. Expression of Trop2 in bladder cancer is modulated by miR125b: In vivo and in vitro analyses. Ital. J. Anat. Embryol. 2015, 120, 46. [Google Scholar] [CrossRef]
- Chen, M.-B.; Wu, H.-F.; Zhan, Y.; Fu, X.-L.; Wang, A.-K.; Wang, L.-S.; Lei, H.-M. Prognostic value of TROP2 expression in patients with gallbladder cancer. Tumor Biol. 2014, 35, 11565–11569. [Google Scholar] [CrossRef]
- Varughese, J.; Cocco, E.; Bellone, S.; Ratner, E.; Silasi, D.-A.; Azodi, M.; Schwartz, P.E.; Rutherford, T.J.; Buza, N.; Pecorelli, S.; et al. Cervical carcinomas overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Am. J. Obstet. Gynecol. 2011, 205, 567.e1–567.e7. [Google Scholar] [CrossRef]
- Zeybek, B.; Manzano, A.; Bianchi, A.; Bonazzoli, E.; Bellone, S.; Buza, N.; Hui, P.; Lopez, S.; Perrone, E.; Manara, P.; et al. Cervical carcinomas that overexpress human trophoblast cell-surface marker (Trop-2) are highly sensitive to the antibody-drug conjugate sacituzumab govitecan. Sci. Rep. 2020, 10, 973. [Google Scholar] [CrossRef]
- Varughese, J.; Cocco, E.; Bellone, S.; de Leon, M.; Bellone, M.; Todeschini, P.; Schwartz, P.E.; Rutherford, T.J.; Pecorelli, S.; Santin, A.D. Uterine serous papillary carcinomas overexpress human trophoblast-cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized anti-Trop-2 monoclonal antibody. Cancer 2011, 117, 3163–3172. [Google Scholar] [CrossRef]
- Han, C.; Perrone, E.; Zeybek, B.; Bellone, S.; Tymon-Rosario, J.; Altwerger, G.; Menderes, G.; Feinberg, J.; Haines, K.; Muller Karger, M.E.; et al. In vitro and in vivo activity of sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2) in uterine serous carcinoma. Gynecol. Oncol. 2020, 156, 430–438. [Google Scholar] [CrossRef]
- Lopez, S.; Perrone, E.; Bellone, S.; Bonazzoli, E.; Zeybek, B.; Han, C.; Tymon-Rosario, J.; Altwerger, G.; Menderes, G.; Bianchi, A.; et al. Preclinical activity of sacituzumab govitecan (IMMU-132) in uterine and ovarian carcinosarcomas. Oncotarget 2020, 11, 560–570. [Google Scholar] [CrossRef]
- Bignotti, E.; Ravaggi, A.; Romani, C.; Falchetti, M.; Lonardi, S.; Facchetti, F.; Pecorelli, S.; Varughese, J.; Cocco, E.; Bellone, S.; et al. Trop-2 overexpression in poorly differentiated endometrial endometrioid carcinoma: Implications for immunotherapy with hRS7, a humanized anti-trop-2 monoclonal antibody. Int. J. Gynecol. Cancer 2011, 21, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Bignotti, E.; Zanotti, L.; Calza, S.; Falchetti, M.; Lonardi, S.; Ravaggi, A.; Romani, C.; Todeschini, P.; Bandiera, E.; Tassi, R.A.; et al. Trop-2 protein overexpression is an independent marker for predicting disease recurrence in endometrioid endometrial carcinoma. BMC Clin. Pathol. 2012, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.-F.; Zhang, D.-J.; Wen, L.-J.; Yu, D.-J.; Zhao, Y.; Zhu, L.; Guo, Y.-Y.; Zheng, Y. Prognostic value of TROP2 in human nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 10995–11004. [Google Scholar] [PubMed]
- Tang, G.; Tang, Q.; Jia, L.; Xia, S.; Li, J.; Chen, Y.; Li, H.; Ding, X.; Wang, F.; Hou, D.; et al. High expression of TROP2 is correlated with poor prognosis of oral squamous cell carcinoma. Pathol. Res. Pract. 2018, 214, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, T.; Ding, G.; Kuai, X.; Wang, X.; Wang, B.; Zhao, W.; Zhao, Y. Trop2 inhibition of P16 expression and the cell cycle promotes intracellular calcium release in OSCC. Int. J. Biol. Macromol. 2020, 164, 2409–2417. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, S.; Li, R.; Li, Y.; Cao, R.; Cheng, J.; Guo, Y.; Wang, E.; Huang, Y.; Zhang, K. Tissue mechanics and expression of TROP2 in oral squamous cell carcinoma with varying differentiation. BMC Cancer 2020, 20, 815. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Shimada, H.; Ochiai, T.; Kuboshima, M.; Kuroiwa, N.; Okazumi, S.; Matsubara, H.; Nomura, F.; Takiguchi, M.; Hiwasa, T. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int. J. Cancer 2004, 112, 1029–1035. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, D.; Guo, Y.; Fu, Z.; Yu, D.; Guan, G. miR-488-3p sponged by circ-0000495 and mediated upregulation of TROP2 in head and neck squamous cell carcinoma development. J. Cancer 2020, 11, 3375–3386. [Google Scholar] [CrossRef]
- Wu, H.; Xu, H.; Zhang, S.; Wang, X.; Zhu, H.; Zhang, H.; Zhu, J.; Huang, J. Potential therapeutic target and independent prognostic marker of TROP2 in laryngeal squamous cell carcinoma. Head Neck 2013, 35, 1373–1378. [Google Scholar] [CrossRef]
- Salerno, E.P.; Bedognetti, D.; Mauldin, I.S.; Deacon, D.H.; Shea, S.M.; Pinczewski, J.; Obeid, J.M.; Coukos, G.; Wang, E.; Gajewski, T.F.; et al. Human melanomas and ovarian cancers overexpressing mechanical barrier molecule genes lack immune signatures and have increased patient mortality risk. Oncoimmunology 2016, 5, e1240857. [Google Scholar] [CrossRef]
- Chen, R.; Lu, M.; Wang, J.; Zhang, D.; Lin, H.; Zhu, H.; Zhang, W.; Xiong, L.; Ma, J.; Mao, Y.; et al. Increased expression of Trop2 correlates with poor survival in extranodal NK/T cell lymphoma, nasal type. Virchows Arch. Int. J. Pathol. 2013, 463, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Liang, N.; Liu, B.; Chen, X.; Pang, Q.; Xin, T. TROP2 expression and its correlation with tumor proliferation and angiogenesis in human gliomas. Neurol. Sci. 2013, 34, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pang, B.; Liang, Y.; Xu, S.-C.; Xin, T.; Fan, H.-T.; Yu, Y.-B.; Pang, Q. Overexpression of EpCAM and Trop2 in pituitary adenomas. Int. J. Clin. Exp. Pathol. 2014, 7, 7907–7914. [Google Scholar] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Guerra, E.; Trerotola, M.; Aloisi, A.L.; Tripaldi, R.; Vacca, G.; La Sorda, R.; Lattanzio, R.; Piantelli, M.; Alberti, S. The Trop-2 signalling network in cancer growth. Oncogene 2013, 32, 1594–1600. [Google Scholar] [CrossRef]
- Wu, M.; Liu, L.; Hijazi, H.; Chan, C. A multi-layer inference approach to reconstruct condition-specific genes and their regulation. Bioinformatics 2013, 29, 1541–1552. [Google Scholar] [CrossRef]
- Hidalgo-Estévez, A.M.; Stamatakis, K.; Jiménez-Martínez, M.; López-Pérez, R.; Fresno, M. Cyclooxygenase 2-Regulated Genes an Alternative Avenue to the Development of New Therapeutic Drugs for Colorectal Cancer. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827. [Google Scholar] [CrossRef]
- Lokody, I.B.; Francis, J.C.; Gardiner, J.R.; Erler, J.T.; Swain, A. Pten Regulates Epithelial Cytodifferentiation during Prostate Development. PLoS ONE 2015, 10, e0129470. [Google Scholar] [CrossRef]
- Suraneni, M.V.; Schneider-Broussard, R.; Moore, J.R.; Davis, T.C.; Maldonado, C.J.; Li, H.; Newman, R.A.; Kusewitt, D.; Hu, J.; Yang, P.; et al. Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence. Oncogene 2010, 29, 4261–4275. [Google Scholar] [CrossRef]
- Eisenwort, G.; Jurkin, J.; Yasmin, N.; Bauer, T.; Gesslbauer, B.; Strobl, H. Identification of TROP2 (TACSTD2), an EpCAM-Like Molecule, as a Specific Marker for TGF-β1-Dependent Human Epidermal Langerhans Cells. J. Investig. Dermatol. 2011, 131, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Izvolsky, K.I.; Qian, J.; Cardoso, W.V. Identification of FGF10 Targets in the Embryonic Lung Epithelium during Bud Morphogenesis. J. Biol. Chem. 2005, 280, 4834–4841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, Q.; Ju, Y.; Song, G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol. Med. 2020, 17, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Taccioli, C.; Palatini, J.; Fernandez-Cymering, C.; Cui, R.; Kim, T.; Volinia, S.; Croce, C. Loss of miR-125b-1 contributes to head and neck cancer development by dysregulating TACSTD2 and MAPK pathway. Oncogene 2014, 33, 702–712. [Google Scholar] [CrossRef]
- Avellini, C.; Licini, C.; Lazzarini, R.; Gesuita, R.; Guerra, E.; Tossetta, G.; Castellucci, C.; Giannubilo, S.R.; Procopio, A.; Alberti, S.; et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017, 8, 58642–58653. [Google Scholar] [CrossRef]
- Ibragimova, I.; de Cáceres, I.I.; Hoffman, A.M.; Potapova, A.; Dulaimi, E.; Al-Saleem, T.; Hudes, G.R.; Ochs, M.F.; Cairns, P. Global Reactivation of Epigenetically Silenced Genes in Prostate Cancer. Cancer Prev. Res. 2010, 3, 1084–1092. [Google Scholar] [CrossRef]
- Remšík, J.; Binó, L.; Kahounová, Z.; Kharaishvili, G.; Šimecková, Š.; Fedr, R.; Kucírková, T.; Lenárt, S.; Muresan, X.M.; Slabáková, E.; et al. Trop-2 plasticity is controlled by epithelial-to-mesenchymal transition. Carcinogenesis 2018, 39, 1411–1418. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Zhang, R.; Dong, L.; Chen, H.; Bo, J.; Xue, W.; Huang, Y. Curcumin inhibits cell proliferation and motility via suppression of TROP2 in bladder cancer cells. Int. J. Oncol. 2018, 53, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Day, R.; Dong, Y.; Weintraub, S.J.; Michel, L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol. Cancer Ther. 2008, 7, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Zimmers, S.M.; Browne, E.P.; Williams, K.E.; Jawale, R.M.; Otis, C.N.; Schneider, S.S.; Arcaro, K.F. TROP2 methylation and expression in tamoxifen-resistant breast cancer. Cancer Cell Int. 2018, 18, 94. [Google Scholar] [CrossRef]
- Wu, B.; Yu, C.; Zhou, B.; Huang, T.; Gao, L.; Liu, T.; Yang, X. Overexpression of TROP2 promotes proliferation and invasion of ovarian cancer cells. Exp. Ther. Med. 2017, 14, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Mølck, C.; Paquet-Fifield, S.; Butler, L.; Australian Prostate Cancer Bioresource, E.S.; Ventura, S.; Hollande, F. High expression of TROP2 characterizes different cell subpopulations in androgen-sensitive and androgen-independent prostate cancer cells. Oncotarget 2016, 7, 44492–44504. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.; Jia, L.; Yang, T.; Huang, X.; Zhao, W.; Zhang, M.; Chen, Y.; Zhu, J.; Feng, Z.; Tang, Q. Trop2 Promotes Multidrug Resistance by Regulating Notch1 Signaling Pathway in Gastric Cancer Cells. Med. Sci. Monit. 2020, 26, e919566-1–e919566-9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Long, M.; Dong, K.; Lin, F.; Weng, Y.; Ouyang, Y.; Liu, L.; Wei, J.; Chen, X.; He, T.; et al. Chemotherapy agents-induced immunoresistance in lung cancer cells could be reversed by trop-2 inhibition in vitro and in vivo by interaction with MAPK signaling pathway. Cancer Biol. Ther. 2013, 14, 1123–1132. [Google Scholar] [CrossRef]
- Frederick, B.A.; Helfrich, B.A.; Coldren, C.D.; Zheng, D.; Chan, D.; Bunn, P.A.; Raben, D. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non–small cell lung carcinoma. Mol. Cancer Ther. 2007, 6, 1683–1691. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Stein, R.; Chen, S.; Sharkey, R.M.; Goldenberg, D.M. Murine monoclonal antibodies raised against human non-small cell carcinoma of the lung: Specificity and tumor targeting. Cancer Res. 1990, 50, 1330–1336. [Google Scholar]
- Stein, R.; Basu, A.; Chen, S.; Shih, L.B.; Goldenberg, D.M. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int. J. Cancer 1993, 55, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Govindan, S.V.; Chen, S.; Reed, L.; Spiegelman, H.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. Successful therapy of a human lung cancer xenograft using MAb RS7 labeled with residualizing radioiodine. Crit. Rev. Oncol. Hematol. 2001, 39, 173–180. [Google Scholar] [CrossRef]
- Stein, R.; Chen, S.; Haim, S.; Goldenberg, D.M. Advantage of yttrium-90-labeled over iodine-131-labeled monoclonal antibodies in the treatment of a human lung carcinoma xenograft. Cancer 1997, 80, 2636–2641. [Google Scholar] [CrossRef]
- Stein, R.; Goldenberg, D.M.; Thorpe, S.R.; Mattes, M.J. Advantage of a residualizing iodine radiolabel for radioimmunotherapy of xenografts of human non-small-cell carcinoma of the lung. J. Nucl. Med. 1997, 38, 391–395. [Google Scholar]
- Shih, L.B.; Xuan, H.; Aninipot, R.; Stein, R.; Goldenberg, D.M. In Vitro and in Vivo Reactivity of an Internalizing Antibody, RS7, with Human Breast Cancer. Cancer Res. 1995, 55, 5857s–5863s. [Google Scholar] [PubMed]
- Chang, C.-H.; Gupta, P.; Michel, R.; Loo, M.; Wang, Y.; Cardillo, T.M.; Goldenberg, D.M. Ranpirnase (Frog RNase) Targeted with a Humanized, Internalizing, Anti–Trop-2 Antibody Has Potent Cytotoxicity against Diverse Epithelial Cancer Cells. Mol. Cancer Ther. 2010, 9, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cardillo, T.M.; Wang, Y.; Rossi, E.A.; Goldenberg, D.M.; Chang, C.-H. Trop-2-targeting tetrakis-ranpirnase has potent antitumor activity against triple-negative breast cancer. Mol. Cancer 2014, 13, 53. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin. Biol. Ther. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J., Jr.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A.; et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef]
- Ocean, A.J.; Starodub, A.N.; Bardia, A.; Vahdat, L.T.; Isakoff, S.J.; Guarino, M.; Messersmith, W.A.; Picozzi, V.J.; Mayer, I.A.; Wegener, W.A.; et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer 2017, 123, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Faltas, B.; Goldenberg, D.M.; Ocean, A.J.; Govindan, S.V.; Wilhelm, F.; Sharkey, R.M.; Hajdenberg, J.; Hodes, G.; Nanus, D.M.; Tagawa, S.T. Sacituzumab Govitecan, a Novel Antibody--Drug Conjugate, in Patients With Metastatic Platinum-Resistant Urothelial Carcinoma. Clin. Genitourin. Cancer 2016, 14, e75–e79. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Petrylak, D.P.; Grivas, P.; Agarwal, N.; Sternberg, C.N.; Hernandez, C.; Siemon-Hryczyk, P.; Goswami, T.; Loriot, Y. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan (IMMU-132) in patients with advanced urothelial cancer after progression on platinum-based chemotherapy and/or anti-PD-1/PD-L1 checkpoint inhibitor therapy. J. Clin. Oncol. 2019, 37, TPS3153. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Faltas, B.; Lam, E.; Saylor, P.; Bardia, A.; Hajdenberg, J.; Morgans, A.K.; Lim, E.; Kalinsky, K.; Petrylak, D.P.; et al. Sacituzumab govitecan (IMMU-132) for patients with pretreated metastatic urothelial uancer (UC): Interim results. Ann. Oncol. 2017, 28, v301–v302. [Google Scholar] [CrossRef]
- Gray, J.E.; Heist, R.S.; Starodub, A.N.; Camidge, D.R.; Kio, E.A.; Masters, G.A.; Purcell, W.T.; Guarino, M.J.; Misleh, J.; Schneider, C.J.; et al. Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I-inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5711–5719. [Google Scholar] [CrossRef] [PubMed]
- Heist, R.S.; Guarino, M.J.; Masters, G.; Purcell, W.T.; Starodub, A.N.; Horn, L.; Scheff, R.J.; Bardia, A.; Messersmith, W.A.; Berlin, J.; et al. Therapy of Advanced Non–Small-Cell Lung Cancer With an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan. J. Clin. Oncol. 2017, 35, 2790–2797. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Bardia, A.; Diamond, J.R.; Vahdat, L.T.; Tolaney, S.M.; O’Shaughnessy, J.; Moroose, R.L.; Mayer, I.A.; Abramson, V.G.; Juric, D.; Sharkey, R.M.; et al. Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC). J. Clin. Oncol. 2018, 36, 1004. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- King, G.T.; Eaton, K.D.; Beagle, B.R.; Zopf, C.J.; Wong, G.Y.; Krupka, H.I.; Hua, S.Y.; Messersmith, W.A.; El-Khoueiry, A.B. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Investig. New Drugs 2018, 36, 836–847. [Google Scholar] [CrossRef]
- Strop, P.; Tran, T.-T.; Dorywalska, M.; Delaria, K.; Dushin, R.; Wong, O.K.; Ho, W.-H.; Zhou, D.; Wu, A.; Kraynov, E.; et al. RN927C, a Site-Specific Trop-2 Antibody–Drug Conjugate (ADC) with Enhanced Stability, Is Highly Efficacious in Preclinical Solid Tumor Models. Mol. Cancer Ther. 2016, 15, 2698–2708. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, H.; Wang, J.; Lu, M.; Zheng, F.; Wang, C.; Tang, X.; Xu, N.; Chen, R.; Zhang, D.; et al. A novel human Fab antibody for Trop2 inhibits breast cancer growth in vitro and in vivo. Int. J. Cancer 2014, 134, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, X.; Zheng, F.; Wang, C.; Tang, Q.; Tang, X.; Xu, N.; Zhang, H.; Zhang, D.; Xiong, L.; et al. The tumor-inhibitory effectiveness of a novel anti-Trop2 Fab conjugate in pancreatic cancer. Oncotarget 2016, 7, 24810–24823. [Google Scholar] [CrossRef]
- Son, S.; Shin, S.; Rao, N.V.; Um, W.; Jeon, J.; Ko, H.; Deepagan, V.G.; Kwon, S.; Lee, J.Y.; Park, J.H. Anti-Trop2 antibody-conjugated bioreducible nanoparticles for targeted triple negative breast cancer therapy. Int. J. Biol. Macromol. 2018, 110, 406–415. [Google Scholar] [CrossRef]
- Chang, C.-H.; Goldenberg, D.M. Enhancing the antitumor potency of T cells redirected by bispecific antibodies. Oncoscience 2017, 4, 120–121. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Chimeric Trop2 Virus-like Particles: A Potential Immunotherapeutic Approach Against Pancreatic Cancer. J. Immunother. 2011, 34, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Ke, D.; Min, L.; Lin, W.; Jiahui, Z.; Fang, L.; Zhaowei, G.; Zhe, Z.; Xi, C.; Huizhong, Z. Incorporation of CD40 ligand enhances the immunogenicity of tumor-associated calcium signal transducer 2 virus-like particles against lung cancer. Int. J. Mol. Med. 2018, 41, 3671–3679. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tian, J.; Chen, Z.; Liang, Y.; Liu, J.; Liu, S.; Li, H.; Zhan, J.; Yang, X. Anti-TROP2 conjugated hollow gold nanospheres as a novel nanostructure for targeted photothermal destruction of cervical cancer cells. Nanotechnology 2014, 25, 345103. [Google Scholar] [CrossRef] [PubMed]
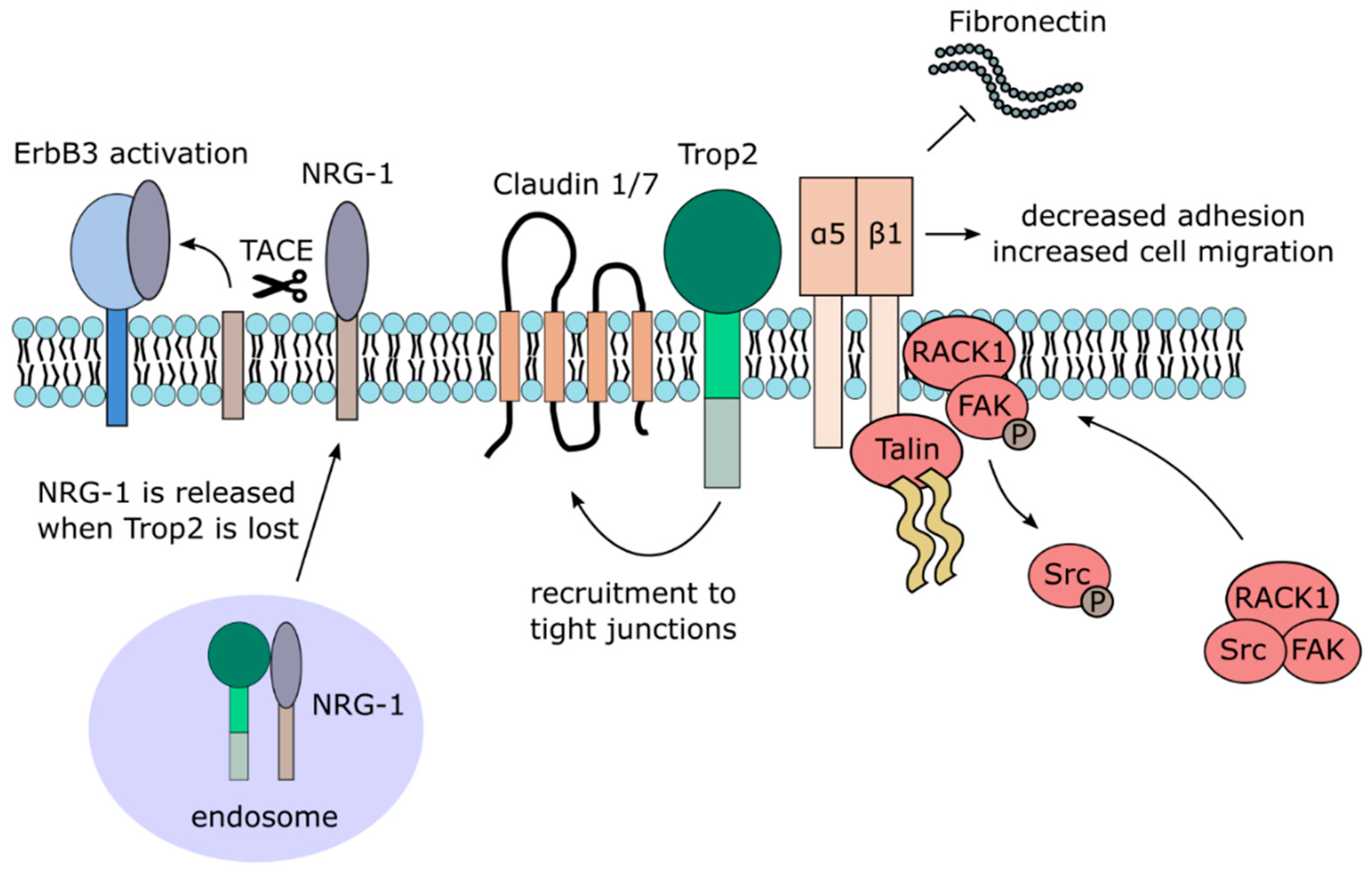
| Trop2-Interacting Partner | Function | Reference |
|---|---|---|
| β-catenin | activation of β-catenin signaling | [66,67] |
| Claudin 1 and 7 | facilitation of proper localization | [46] |
| Occludin | facilitation of proper localization | [49] |
| α5β1 integrin/Talin complex | relocalization from focal adhesions to leading edges, rearrangement of focal adhesions sites through displacement of FAK | [69,70] |
| IGF-1 | inhibition of IGF-1R signaling | [73,77] |
| MDK | inhibition of ALK signaling | [77] |
| NRG-1 | inhibition of ErbB3 activation | [65] |
| Trop2 Overexpression | |||||
| Type of Carcinoma | Detection Method | Number of Subjects | Protein Location | Prognosis | Reference |
| gastric | IHC | 104 | membrane | decreased overall and disease-free survival (in patients with intestinal-type carcinoma and lymph-node-positive patients) | [85] |
| IHC | 830 | membrane, cytoplasm | decreased overall and disease-free survival | [90] | |
| qRT-PCR | 41 pairs | - | - | ||
| IHC | 330 | membrane, cytoplasm | decreased overall survival (in patients with Trop2+/vimentin+ expression) | [66] | |
| IHC | 844 | membrane | N/A | [91] | |
| ICC | 192 | - | - | ||
| thyroid | IHC | 124 | membrane, cytoplasm | - | [61] |
| qRT-PCR | 18 pairs | - | - | ||
| RNA seq | 59 pairs | - | - | ||
| papillary thyroid | ICC | 60 | - | - | [92] |
| IHC | 94 | membrane, cytoplasm | - | ||
| IHC | 433 | membrane | - | [93] | |
| IHC | 468 | membrane | - | [94] | |
| colorectal | IHC | 620 | - | decreased overall survival and increased disease recurrence | [95] |
| IHC | 34 | - | - | [96] | |
| qRT-PCR | 74 pairs | - | decreased overall survival | ||
| non-small cell lung | IHC | 104 | membrane, nucleus, cytoplasm | decreased overall survival (in patients with adenocarcinoma) | [97] |
| IHC | 334 | membrane | decreased overall survival (in patients with adenocarcinoma) | [98] | |
| IHC | 164 | membrane | better overall and disease-free survival (in patients with adenocarcinoma) | [74] | |
| lung adenocarcinoma | IHC | 130 | membrane, cytoplasm | decreased overall survival (in patients with non-lepidic type tumors) | [76] |
| IHC | 68 | membrane, cytoplasm | decreased overall survival | [99] | |
| qRT-PCR | 20 pairs | - | - | ||
| breast | IHC | 702 | membrane, cytoplasm | decreased overall survival associated with membrane Trop2, intracellular Trop2 associated with better overall survival and disease recurrence | [89] |
| IHC | 354 | decreased overall survival (in patients with Trop2+/E-cadherin- expression) | [87] | ||
| qRT-PCR | 20 pairs | - | - | ||
| ductal breast | IHC | 152 | membrane | decreased overall survival | [100] |
| qRTPCR | 15 pairs | - | - | ||
| pancreatic | IHC | 207 | membrane | decreased overall survival and shortened progression-free survival in patients who underwent surgery with curative intent | [82] |
| ovarian | IHC | 117 | membrane, cytoplasm | decreased overall and progression-free survival | [101] |
| qRT-PCR | 128 | - | N/A | ||
| IHC | 55 | membrane | - | [102] | |
| IHC | 90 | membrane, cytoplasm | - | [103] | |
| prostate | IHC | 339 | membrane, cytoplasm | - | [84] |
| IHC | 148 | membrane | - | [69] | |
| bladder | IHC | 40 | membrane, cytoplasm | - | [104] |
| gallbladder | IHC | 93 | membrane, cytoplasm | decreased overall survival | [105] |
| IHC | 105 | membrane, cytoplasm | decreased overall survival | [56] | |
| qRT-PCR | 56 | - | - | ||
| WB | 10 | - | - | ||
| cervical | IHC | 160 | membrane | decreased overall and progression-free survival | [62] |
| IHC | 13 | membrane | - | [106] | |
| IHC | 147 | membrane, cytoplasm | - | [107] | |
| uterine serous papillary | IHC | 28 | membrane | - | [108] |
| IHC | 104 | membrane, cytoplasm | - | [109] | |
| uterine and ovarian carcinosarcomas | IHC | 10 | membrane, cytoplasm | - | [110] |
| endometrial | IHC | 163 | membrane | - | [111] |
| IHC | 118 | membrane | decreased disease-free survival | [112] | |
| nasopharyngeal | qRT-PCR | 37 | - | - | [54] |
| IHC | 58 | membrane, cytoplasm | decreased overall and disease-free survival | [113] | |
| hilar cholangiocarcinoma | IHC | 70 | membrane | decreased overall survival | [86] |
| qRT-PCR | 56 pairs | - | - | ||
| oral squamous cell | IHC | 90 | membrane | decreased overall survival | [83] |
| IHC | 443 | membrane, cytoplasm | decreased overall and disease-free survival | [114] | |
| qRT-PCR | 93 | - | - | ||
| IHC | 320 | membrane, cytoplasm | decreased overall survival (in patients with Trop2+/P16- expression) | [115] | |
| IHC | 108 | cytoplasm | decreased overall survival | [116] | |
| esophageal squamous cell | IHC | 55 | membrane, cytoplasm | - | [117] |
| head-and-neck squamous cell | IHC | 42 | membrane | decreased overall and disease-free survival | [118] |
| laryngeal squamous cell | IHC | 137 | membrane, cytoplasm | decreased overall and disease-free survival | [119] |
| qRT-PCR | 15 pairs | - | - | ||
| Trop2 Downregulation | |||||
| Type of Carcinoma | Detection Method | Number of Subjects | Protein Location | Prognosis | Reference |
| liver fluke-associated cholangiocarcinoma | IHC | 85 | membrane, cytoplasm | N/A | [75] |
| lung adenocarcinoma | IHC | 55 pairs | membrane | - | [73] |
| LOH analysis | 119 | - | - | ||
| hepatocellular | qRT-PCR | 205 pairs | - | decreased overall survival | [88] |
| RNA seq | 3 pairs | - | - | ||
| squamous cell carcinoma of cervix | IHC | 79 | membrane, cytoplasm | - | [64] |
| squamous cell carcinoma of head and neck | IHC | 5 | membrane, cytoplasm | - | |
| squamous cell carcinoma of esophagus | IHC | 6 | membrane, cytoplasm | - | |
| ClinicalTrials.gov Identifier | Disease | Combined Therapy | Phase |
|---|---|---|---|
| NCT03964727 | Metastatic NSCLC, head-and-neck squamous cell carcinoma, endometrial cancer | - | 2 |
| NCT03725761 | Metastatic castration-resistant prostate cancer | - | 2 |
| NCT04230109 | Invasive localized TNBC | - | 2 |
| NCT01631552 | Advanced epithelial cancers | - | 1/2 |
| NCT03547973 | Metastatic urothelial carcinoma | Pembrolizumab | 2 |
| NCT03901339 | HR+/HER2- metastatic breast cancer | - | 3 |
| NCT02574455 | Refractory/relapsed metastatic TNBC | - | 3 |
| NCT04039230 | Metastatic breast cancer | Talazoparib | 1/2 |
| NCT03995706 | Glioblastoma, metastatic brain tumors | - | early 1 |
| NCT03992131 | Metastatic solid tumors—ovarian cancer, TNBC, urothelial carcinoma | Rucaparib | 1/2 |
| NCT04251416 | Persistent of recurrent endometrial carcinoma | - | 2 |
| NCT04319198 | Metastatic solid tumor | - | 3 |
| NCT03424005 | Metastatic or inoperable locally advanced TNBC | Atezolizumab | 1/2 |
| NCT04454437 | Metastatic TNBC | - | 2 |
| NCT04468061 | Metastatic TNBC | Pembrolizumab | 2 |
| NCT04448886 | Metastatic HR+/HER2- breast cancer | Pembrolizumab | 2 |
| NCT03337698 | Metastatic NSCLC | Atezolizumab | 1/2 |
| NCT04559230 | Glioblastoma | - | 2 |
| NCT04527991 | Metastatic or locally advanced unresectable urothelial cancer | - | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenárt, S.; Lenárt, P.; Šmarda, J.; Remšík, J.; Souček, K.; Beneš, P. Trop2: Jack of All Trades, Master of None. Cancers 2020, 12, 3328. https://doi.org/10.3390/cancers12113328
Lenárt S, Lenárt P, Šmarda J, Remšík J, Souček K, Beneš P. Trop2: Jack of All Trades, Master of None. Cancers. 2020; 12(11):3328. https://doi.org/10.3390/cancers12113328
Chicago/Turabian StyleLenárt, Sára, Peter Lenárt, Jan Šmarda, Ján Remšík, Karel Souček, and Petr Beneš. 2020. "Trop2: Jack of All Trades, Master of None" Cancers 12, no. 11: 3328. https://doi.org/10.3390/cancers12113328
APA StyleLenárt, S., Lenárt, P., Šmarda, J., Remšík, J., Souček, K., & Beneš, P. (2020). Trop2: Jack of All Trades, Master of None. Cancers, 12(11), 3328. https://doi.org/10.3390/cancers12113328