Teratoma Growth Retardation by HDACi Treatment of the Tumor Embryonal Source
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Histopathology of Teratomas
2.2. The Effect of HDAC Inhibition on Teratoma Growth
2.3. The Effect of HDACi on Histone Acetylation in Teratomas
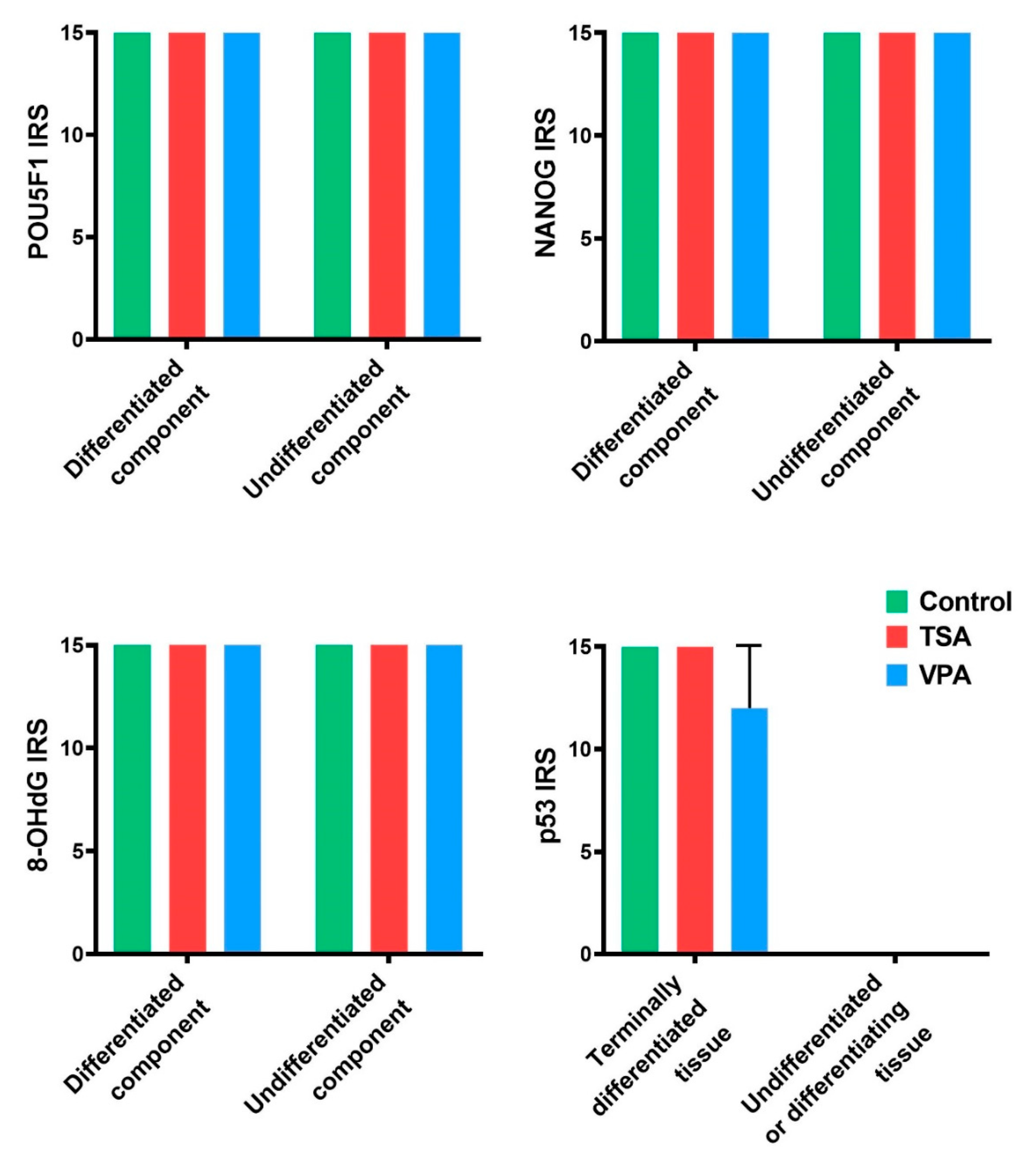
2.4. HDACi Effect on Teratoma Differentiation
2.5. Proliferation and Apoptosis
2.6. Oxidative Stress Levels
2.7. Gene Expression Analysis
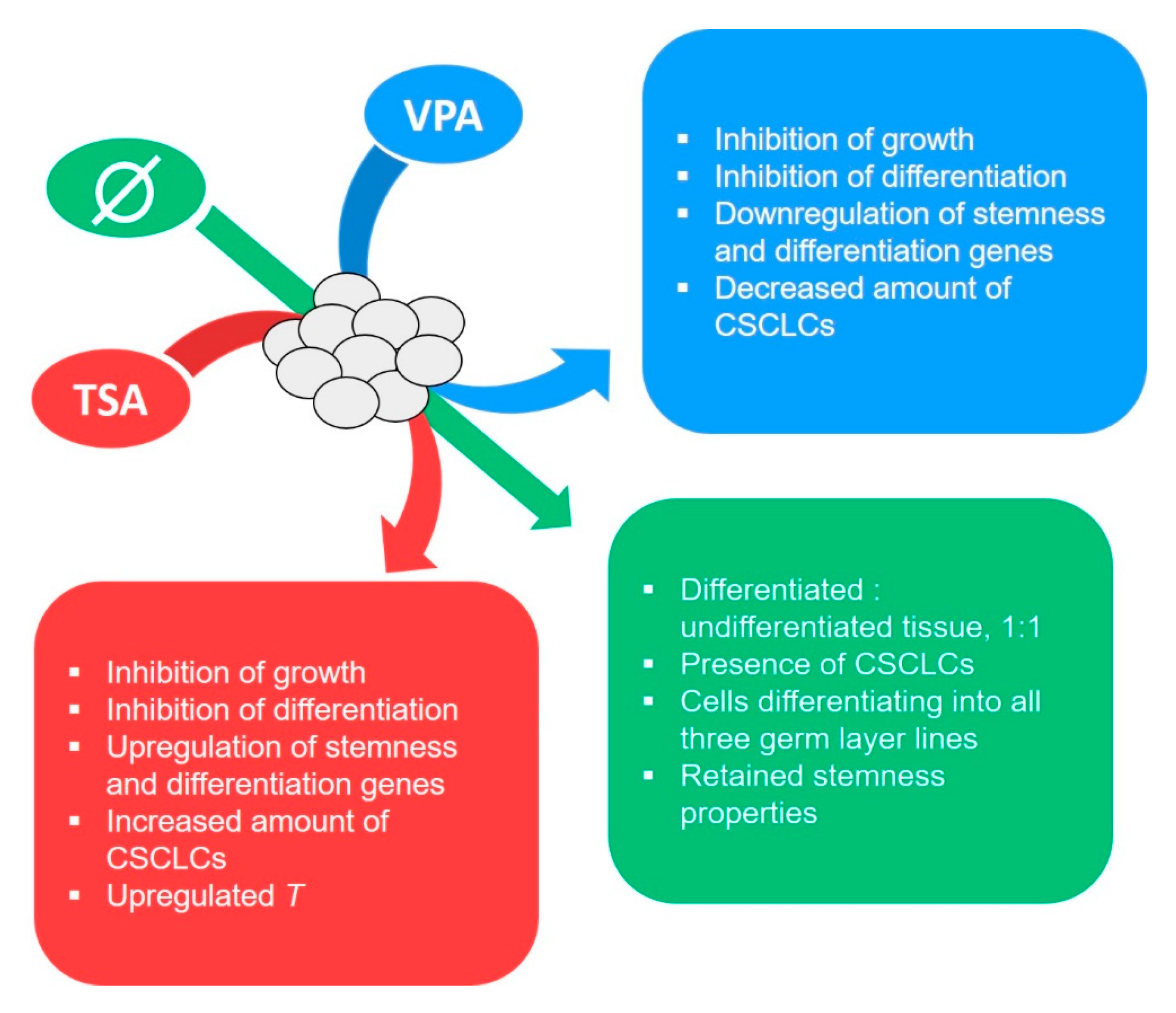
3. Discussion
3.1. Histopathological and Molecular Analysis of the Experimental Teratoma In Vitro System
3.2. Teratoma Growth In Vitro
3.3. Gene Expression
4. Materials and Methods
4.1. Ethical Statement
4.2. Animals
4.3. Isolation of the Embryo-Proper
4.4. HDACi Treatment
4.5. In Vitro Culture
4.6. Morphometric Analysis, Hematoxylin and Eosin (HE) and Immunohistochemistry (IHC)
4.7. SDS-PAGE and Western Blot
4.8. Functional Enrichment Analysis and RNA Coexpression Analysis
4.9. RNA Isolation and cDNA Reverse Transcription
4.10. Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batool, A.; Karimi, N.; Wu, X.N.; Chen, S.R.; Liu, Y.X. Testicular germ cell tumor: A comprehensive review. Cell. Mol. Life Sci. 2019, 76, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Parraga-Alava, J. Meta-analysis of gene expressions in testicular germ cell tumor histologies. Int. J. Mol. Sci. 2020, 21, 4487. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Skakkebæk, N.E.; Rajpert-De Meyts, E.; Laversanne, M.; Kuliš, T.; Gurney, J.; Sarfati, D.; McGlynn, K.A.; Bray, F. Testicular cancer incidence predictions in Europe 2010–2035: A rising burden despite population ageing. Int. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Costa, A.L.; Vilela-Salgueiro, B.; Rodrigues, Â.; Guimarães, R.; Cantante, M.; Lopes, P.; Antunes, L.; Jerónimo, C.; Henrique, R. Testicular germ cell tumors: Revisiting a series in light of the new WHO classification and AJCC staging systems, focusing on challenges for pathologists. Hum. Pathol. 2018, 82, 113–124. [Google Scholar] [CrossRef]
- Selfe, J.; Goddard, N.C.; McIntyre, A.; Taylor, K.R.; Renshaw, J.; Popov, S.D.; Thway, K.; Summersgill, B.; Huddart, R.A.; Gilbert, D.C.; et al. IGF1R signalling in testicular germ cell tumour cells impacts on cell survival and acquired cisplatin resistance. J. Pathol. 2018, 244, 242–253. [Google Scholar] [CrossRef]
- Bakardjieva-Mihaylova, V.; Kramarzova, K.S.; Slamova, M.; Svaton, M.; Rejlova, K.; Zaliova, M.; Dobiasova, A.; Fiser, K.; Stuchly, J.; Grega, M.; et al. Molecular basis of cisplatin resistance in testicular germ cell tumors. Cancers 2019, 11, 1316. [Google Scholar] [CrossRef] [Green Version]
- Lobo, J.; Henrique, R.; Jerónimo, C. The role of DNA/histone modifying enzymes and chromatin remodeling complexes in testicular germ cell tumors. Cancers 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Funt, S.A.; Patil, S.; Feldman, D.R.; Motzer, R.J.; Bajorin, D.F.; Sheinfeld, J.; Tickoo, S.K.; Reuter, V.E.; Bosl, G.J. Impact of teratoma on the cumulative incidence of disease-related death in patients with advanced germ cell tumors. J. Clin. Oncol. 2019, 37, 2329–2337. [Google Scholar] [CrossRef]
- Cheng, L.; Lyu, B.; Roth, L.M. Perspectives on testicular germ cell neoplasms. Hum. Pathol. 2017, 59, 10–25. [Google Scholar] [CrossRef]
- David, S.; András, F.; Endre, K.; Balint, K.; Árpad, K.; Csaba, P.; Karoly, S.; Tamás, T. More cases of benign testicular teratomas are detected in adults than in children. A clinicopathological study of 543 testicular germ cell tumor cases. Pathol. Oncol. Res. 2017, 23, 513–517. [Google Scholar] [CrossRef]
- Calaminus, G.; Schneider, D.T.; von Schweinitz, D.; Jürgens, H.; Infed, N.; Schönberger, S.; Olson, T.A.; Albers, P.; Vokuhl, C.; Stein, R.; et al. Age-dependent presentation and clinical course of 1465 patients aged 0 to less than 18 years with ovarian or testicular germ cell tumors; data of the MAKEI 96 protocol revisited in the light of prenatal germ cell biology. Cancers 2020, 12, 611. [Google Scholar] [CrossRef] [Green Version]
- Almstrup, K.; Lobo, J.; Mørup, N.; Belge, G.; Rajpert-De Meyts, E.; Looijenga, L.H.J.; Dieckmann, K.P. Application of miRNAs in the diagnosis and monitoring of testicular germ cell tumours. Nat. Rev. Urol. 2020, 17, 201–213. [Google Scholar] [CrossRef]
- Stevens, L.C. The biology of teratomas. Adv. Morphog. 1967, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Damjanov, I.; Solter, D.; Belicza, M.; Skreb, N. Teratomas obtained through extrauterine growth of seven-day mouse embryos1. J. Natl. Cancer Inst. 1971, 46, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Plazibat, M.; Bojanac, A.K.; Himerleich Perić, M.; Gamulin, O.; Rašić, M.; Radonić, V.; Škrabić, M.; Krajačić, M.; Krasić, J.; Sinčić, N.; et al. Embryo-derived teratoma in vitro biological system reveals antitumor and embryotoxic activity of valproate. FEBS J. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulic-Jakus, F.; Katusic Bojanac, A.; Juric-Lekic, G.; Vlahovic, M.; Sincic, N. Teratoma: From spontaneous tumors to the pluripotency/malignancy assay. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 186–209. [Google Scholar] [CrossRef]
- Bustamante-Marín, X.; Garness, J.A.; Capel, B. Testicular teratomas: An intersection of pluripotency, differentiation and cancer biology. Int. J. Dev. Biol. 2013, 57, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Solter, D. From teratocarcinomas to embryonic stem cells and beyond: A history of embryonic stem cell research. Nat. Rev. Genet. 2006, 7, 319–327. [Google Scholar] [CrossRef]
- Kremenskoy, M.; Kremenska, Y.; Ohgane, J.; Hattori, N.; Tanaka, S.; Hashizume, K.; Shiota, K. Genome-wide analysis of DNA methylation status of CpG islands in embryoid bodies, teratomas, and fetuses. Biochem. Biophys. Res. Commun. 2003, 311, 884–890. [Google Scholar] [CrossRef]
- Saraiva, N.Z. Histone acetylation and its role in embryonic stem cell differentiation. World J. Stem Cells 2010, 2, 121. [Google Scholar] [CrossRef]
- Jostes, S.; Nettersheim, D.; Schorle, H. Epigenetic drugs and their molecular targets in testicular germ cell tumours. Nat. Rev. Urol. 2019, 16, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Tung, E.W.Y.; Winn, L.M. Epigenetic modifications in valproic acid-induced teratogenesis. Toxicol. Appl. Pharmacol. 2010, 248, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, S.; Wu, N.; Cho, K.S. Acetylation and deacetylation in cancer stem-like cells. Oncotarget 2017, 8, 89315–89325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, L.; Yang, X.B. Harnessing the HDAC-histone deacetylase enzymes, inhibitors and how these can be utilised in tissue engineering. Int. J. Oral Sci. 2019, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Zalewska, T.; Jaworska, J.; Sypecka, J.; Ziemka-Nalecz, M. Impact of a histone deacetylase inhibitor- trichostatin a on neurogenesis after hypoxia- ischemia in immature rats. Int. J. Mol. Sci. 2020, 21, 3808. [Google Scholar] [CrossRef]
- Nervi, C.; Borello, U.; Fazi, F.; Buffa, V.; Pelicci, P.G.; Cossu, G. Inhibition of histone deacetylase activity by trichostatin a modulates gene expression during mouse embryogenesis without apparent toxicity. Cancer Res. 2001, 61, 1247–1249. [Google Scholar]
- Lagger, S.; Meunier, D.; Mikula, M.; Brunmeir, R.; Schlederer, M.; Artaker, M.; Pusch, O.; Egger, G.; Hagelkruys, A.; Mikulits, W.; et al. Crucial function of histone deacetylase 1 for differentiation of teratomas in mice and humans. EMBO J. 2010, 29, 3992–4007. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; He, G.; Zhang, K.; Guan, X.; Wang, Y.; Zhang, B. Trichostatin A induces p53-dependent endoplasmic reticulum stress in human colon cancer cells. Oncol. Lett. 2019, 17, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.C.; Wang, S.T.; Liu, H.T.; Wang, X.Y.; Wu, S.C.; Chen, L.C.; Liu, Y.W. Trichostatin A induces bladder cancer cell death via intrinsic apoptosis at the early phase and Sp1-survivin downregulation at the late phase of treatment. Oncol. Rep. 2017, 38, 1587–1596. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, X.; Zhu, S.; Hu, X.; Niu, H.; Zhang, X.; Zhu, D.; Nesa, E.U.; Tian, K.; Yuan, H. Hyper-acetylation contributes to the sensitivity of chemo-resistant prostate cancer cells to histone deacetylase inhibitor Trichostatin A. J. Cell. Mol. Med. 2018, 22, 1909–1922. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhao, X.; Liu, H.; Jin, H.; Ji, Y. Trichostatin a inhibits proliferation of PC3 prostate cancer cells by disrupting the EGFR pathway. Oncol. Lett. 2019, 18, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, S.; Shen, T.; Lu, H.; Xiao, D.; Zhao, M.; Yao, Y.; Li, X.; Zhang, G.; Zhou, X.; et al. Trichostatin A reverses epithelial-mesenchymal transition and attenuates invasion and migration in MCF-7 breast cancer cells. Exp. Ther. Med. 2020, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’amato, L.; Pastorino, O.; Teresa Gentile, M.; Mancini, A.; Del Gaudio, N.; Di Costanzo, A.; Bajetto, A.; Franco, P.; Altucci, L.; Florio, T.; et al. Histone deacetylase inhibitors impair vasculogenic mimicry from glioblastoma cells. Cancers 2019, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- Boudadi, E.; Stower, H.; Halsall, J.A.; Rutledge, C.E.; Leeb, M.; Wutz, A.; O’Neill, L.P.; Nightingale, K.P.; Turner, B.M. The histone deacetylase inhibitor sodium valproate causes limited transcriptional change in mouse embryonic stem cells but selectively overrides Polycomb-mediated Hoxb silencing. Epigenet. Chromatin 2013, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sukhorum, W.; Iamsaard, S. Changes in testicular function proteins and sperm acrosome status in rats treated with valproic acid. Reprod. Fertil. Dev. 2017, 29, 1585–1592. [Google Scholar] [CrossRef]
- Miziak, B.; Konarzewska, A.; Ułamek-Kozioł, M.; Dudra-Jastrzębska, M.; Pluta, R.; Czuczwar, S.J. Anti-epileptogenic effects of antiepileptic drugs. Int. J. Mol. Sci. 2020, 21, 2340. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Chen, X.; Yu, D.; Li, T.; Cui, J.; Wang, G.; Hu, J.F.; Li, W. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming-induced senescence stress. Exp. Cell Res. 2015, 337, 61–67. [Google Scholar] [CrossRef]
- Takai, N.; Ueda, T.; Nishida, M.; Nasu, K.; Narahara, H. Histone deacetylase inhibitors induce growth inhibition, cell cycle arrest and apoptosis in human choriocarcinoma cells. Int. J. Mol. Med. 2008, 21, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Iamsaard, S.; Sukhorum, W.; Arun, S.; Phunchago, N.; Uabundit, N.; Boonruangsri, P.; Namking, M. Valproic acid induces histologic changes and decreases androgen receptor levels of testis and epididymis in rats. Int. J. Reprod. Biomed. 2017, 15, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Kretsovali, A.; Hadjimichael, C.; Charmpilas, N. Histone deacetylase inhibitors in cell pluripotency, differentiation, and reprogramming. Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef]
- Moschidou, D.; Mukherjee, S.; Blundell, M.P.; Drews, K.; Jones, G.N.; Abdulrazzak, H.; Nowakowska, B.; Phoolchund, A.; Lay, K.; Ramasamy, T.S.; et al. Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol. Ther. 2012, 20, 1953–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hezroni, H.; Tzchori, I.; Davidi, A.; Mattout, A.; Biran, A.; Nissim-Rafinia, M.; Westphal, H.; Meshorer, E. H3K9 histone acetylation predicts pluripotency and reprogramming capacity of ES cells. Nucleus 2011, 2, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurvich, N.; Berman, M.G.; Wittner, B.S.; Gentleman, R.C.; Klein, P.S.; Green, J.B.A. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 2005, 19, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Faiella, A.; Wernig, M.; Consalez, G.G.; Hostick, U.; Hofmann, C.; Hustert, E.; Boncinelli, E.; Balling, R.; Nadeau, J.H. A mouse model for valproate teratogenicity: Parental effects, homeotic transformations, and altered HOX expression. Hum. Mol. Genet. 2000, 9, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.-i.; Sugimura, S.; Wakai, T.; Kawahara, M.; Sato, E. Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J. Reprod. Dev. 2009, 55, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, M.C.; Pope, C.E.; Ricks, D.M.; Lyons, J.; Dumas, C.; Dresser, B.L. Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer. Reprod. Fertil. Dev. 2009, 21, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hasibeder, A.; Venkataramani, V.; Thelen, P.; Radzun, H.J.; Schweyer, S. Phytoestrogens regulate the proliferation and expression of stem cell factors in cell lines of malignant testicular germ cell tumors. Int. J. Oncol. 2013, 43, 1385–1394. [Google Scholar] [CrossRef]
- Venkataramani, V.; Thiele, K.; Behnes, C.L.; Wulf, G.G.; Thelen, P.; Opitz, L.; Salinas-Riester, G.; Wirths, O.; Bayer, T.A.; Schweyer, S. Amyloid precursor protein is a biomarker for transformed human pluripotent stem cells. Am. J. Pathol. 2012, 180, 1636–1652. [Google Scholar] [CrossRef]
- Aldahmash, A.; Atteya, M.; Elsafadi, M.; Al-Nbaheen, M.; Al-Mubarak, H.A.; Vishnubalaji, R.; Al-Roalle, A.; Al-Harbi, S.; Manikandan, M.; Matthaei, K.I.; et al. Teratoma formation in immunocompetent mice after syngeneic and allogeneic implantation of germline capable mouse embryonic stem cells. Asian Pac. J. Cancer Prev. 2013, 14, 5705–5711. [Google Scholar] [CrossRef] [Green Version]
- Howitt, B.E.; Berney, D.M. Tumors of the Testis: Morphologic Features and Molecular Alterations. Surg. Pathol. Clin. 2015, 8, 687–716. [Google Scholar] [CrossRef]
- Buljubašić, R.; Buljubašić, M.; Bojanac, A.K.; Ulamec, M.; Vlahović, M.; Ježek, D.; Bulić-Jakuš, F.; Sinčić, N. Epigenetics and testicular germ cell tumors. Gene 2018, 661, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, M.P.; Adhikarla, S.; Mukherjee, O.; Panicker, M.M. Genotoxic effects of culture media on human pluripotent stem cells. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saykali, B.; Mathiah, N.; Nahaboo, W.; Racu, M.L.; Hammou, L.; Defrance, M.; Migeotte, I. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. Elife 2019, 8, 1–27. [Google Scholar] [CrossRef]
- Tsukada, M.; Ota, Y.; Wilkinson, A.C.; Becker, H.J.; Osato, M.; Nakauchi, H.; Yamazaki, S. In Vivo Generation of Engraftable Murine Hematopoietic Stem Cells by Gfi1b, c-Fos, and Gata2 Overexpression within Teratoma. Stem Cell Rep. 2017, 9, 1024–1033. [Google Scholar] [CrossRef] [Green Version]
- Siska, P.J.; Johnpulle, R.A.N.; Zhou, A.; Bordeaux, J.; Kim, J.Y.; Dabbas, B.; Dakappagari, N.; Rathmell, J.C.; Rathmell, W.K.; Morgans, A.K.; et al. Deep exploration of the immune infiltrate and outcome prediction in testicular cancer by quantitative multiplexed immunohistochemistry and gene expression profiling. Oncoimmunology 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Lobo, J.; Rodrigues, Â.; Guimarães, R.; Cantante, M.; Lopes, P.; Maurício, J.; Oliveira, J.; Jerónimo, C.; Henrique, R. Detailed characterization of immune cell infiltrate and expression of immune checkpoint molecules PD-L1/CTLA-4 and MMR proteins in testicular germ cell tumors disclose novel disease biomarkers. Cancers 2019, 11, 1535. [Google Scholar] [CrossRef] [Green Version]
- Aponte, P.M.; Caicedo, A. Stemness in cancer: Stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise review: Cancer cells, cancer stem cells, and mesenchymal stem cells: Influence in cancer development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.R.; Seo, M.S.; Roh, K.H.; Park, S.B.; Hwang, J.W.; Sun, B.; Seo, K.; Lee, Y.S.; Kang, S.K.; et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009, 42, 711–720. [Google Scholar] [CrossRef]
- Sang, Z.; Sun, Y.; Ruan, H.; Cheng, Y.; Ding, X.; Yu, Y. Anticancer effects of valproic acid on oral squamous cell carcinoma via SUMOylation in vivo and in vitro. Exp. Ther. Med. 2016, 12, 3979–3987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, T.X.; Ma, Y.; Zhang, Y.; Li, D.Y.; Xu, H.R. Bursopentin (BP5) induces G1 phase cell cycle arrest and endoplasmic reticulum stress/mitochondria-mediated caspase-dependent apoptosis in human colon cancer HCT116 cells. Cancer Cell Int. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El Gaafary, M.; Hafner, S.; Lang, S.J.; Jin, L.; Sabry, O.M.; Vogel, C.V.; Vanderwal, C.D.; Syrovets, T.; Simmet, T. A novel polyhalogenated monoterpene induces cell cycle arrest and apoptosis in breast cancer cells. Mar. Drugs 2019, 17, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa-González, G.; García-Castillo, V.; Coronel-Hernández, J.; López-Urrutia, E.; León-Cabrera, S.; Arias-Romero, L.E.; Terrazas, L.; Rodríguez-Sosa, M.; Campos-Parra, A.D.; Zúñiga-Calzada, E.; et al. Anti-inflammatory and antitumor activity of a triple therapy for a colitis-related colorectal cancer. J. Cancer 2016, 7, 1632–1644. [Google Scholar] [CrossRef] [Green Version]
- Rithanya, P.; Ezhilarasan, D. Sodium valproate, a histone deacetylase inhibitor, provokes reactive oxygen species–mediated cytotoxicity in human hepatocellular carcinoma cells. J. Gastrointest. Cancer 2020. [Google Scholar] [CrossRef]
- Comel, A.; Sorrentino, G.; Capaci, V.; Del Sal, G. The cytoplasmic side of p53’s oncosuppressive activities. FEBS Lett. 2014, 588, 2600–2609. [Google Scholar] [CrossRef] [Green Version]
- Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Aranda, I.; Ramos-Mejia, V.; Bueno, C.; Munoz-Lopez, M.; Real, P.J.; Mácia, A.; Sanchez, L.; Ligero, G.; Garcia-Parez, J.L.; Menendez, P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010, 28, 1568–1570. [Google Scholar] [CrossRef] [Green Version]
- Petkov, S.; Glage, S.; Nowak-Imialek, M.; Niemann, H. Long-Term culture of porcine induced pluripotent stem-like cells under feeder-free conditions in the presence of histone deacetylase inhibitors. Stem Cells Dev. 2016, 25, 386–394. [Google Scholar] [CrossRef]
- Kelly, G.M.; Gatie, M.I. Mechanisms regulating stemness and differentiation in embryonal carcinoma cells. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Jergil, M.; Forsberg, M.; Salter, H.; Stockling, K.; Gustafson, A.L.; Dencker, L.; Stigson, M. Short-time gene expression response to valproic acid and valproic acid analogs in mouse embryonic stem cells. Toxicol. Sci. 2011, 121, 328–342. [Google Scholar] [CrossRef]
- Batista, M.R.; Diniz, P.; Torres, A.; Murta, D.; Murta, D.; Lopes-Da-Costa, L.; Silva, E. Notch signaling in mouse blastocyst development and hatching. BMC Dev. Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Israel, S.; Ernst, M.; Psathaki, O.E.; Drexler, H.C.A.; Casser, E.; Suzuki, Y.; Makalowski, W.; Boiani, M.; Fuellen, G.; Taher, L. An integrated genome-wide multi-omics analysis of gene expression dynamics in the preimplantation mouse embryo. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, M.; Deng, Y.; Su, G.; Guo, C.; Zhang, D.; Kim, D.; Bai, Z.; Xiao, Y.; Fan, R. High-Spatial-Resolution Multi-Omics Atlas Sequencing of Mouse Embryos via Deterministic Barcoding in Tissue. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Ghassemi, S.; Vejdovszky, K.; Sahin, E.; Ratzinger, L.; Schelch, K.; Mohr, T.; Peter-Vörösmarty, B.; Brankovic, J.; Lackner, A.; Leopoldi, A.; et al. FGF5 is expressed in melanoma and enhances malignancy in vitro and in vivo. Oncotarget 2017, 8, 87750–87762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jostes, S.V.; Fellermeyer, M.; Arévalo, L.; Merges, G.E.; Kristiansen, G.; Nettersheim, D.; Schorle, H. Unique and redundant roles of SOX2 and SOX17 in regulating the germ cell tumor fate. Int. J. Cancer 2020, 146, 1592–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, F.; Cárcano, F.M.; da Silva, E.C.A.; Vidal, D.O.; Scapulatempo-Neto, C.; Lopes, L.F.; Reis, R.M. Brachyury oncogene is a prognostic factor in high-risk testicular germ cell tumors. Andrology 2018, 6, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Jasek, E.; Lis, G.; Jasińska, M.; Jurkowska, H.; Litwin, J. Effect of histone deacetylase inhibitors trichostatin a and valproic acid on etoposide-induced apoptosis in leukemia cells EWA. Anticancer Res. 2012, 32, 2791–2800. [Google Scholar] [CrossRef] [Green Version]
- Crump, N.T.; Hazzalin, C.A.; Bowers, E.M.; Alani, R.M.; Cole, P.A.; Mahadevan, L.C. Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc. Natl. Acad. Sci. USA 2011, 108, 7814–7819. [Google Scholar] [CrossRef] [Green Version]
- Katusic Bojanac, A.; Rogosic, S.; Sincic, N.; Juric-Lekic, G.; Vlahovic, M.; Serman, L.; Jezek, D.; Bulic-Jakus, F. Influence of hyperthermal regimes on experimental teratoma development in vitro. Int. J. Exp. Pathol. 2018, 99, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Belicza, M. Evaluacija morfološki utvrđenog apoptotičkog indeksa. Acta Med. Croat. 2009, 63, 3–12. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models. Genome Res. 2003, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, H.-J.; Jee, S.; Jin, S.; Koo, S.K.; Paik, S.S.; Jung, S.C.; Hwang, S.-Y.; Lee, K.S.; Oh, B. In Vitro Differentiation of Mouse Embryonic Stem Cells: Enrichment of Endodermal Cells in the Embryoid Body. Stem Cells 2005, 23, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Kim, S.M.; Jeon, Y.; Sim, J.; Jang, J.Y.; Son, J.; Hong, W.; Park, M.K.; Lee, H. Loss of Mob1a/b impairs the differentiation of mouse embryonic stem cells into the three germ layer lineages. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, T.; Bober, E.; Rudnicki, M.A.; Jaenisch, R.; Arnold, H.H. MyoD expression marks the onset of skeletal myogenesis in Myf-5 mutant mice. Development 1994, 120, 3083–3092. [Google Scholar]
- Iwashita, H.; Shiraki, N.; Sakano, D.; Ikegami, T.; Shiga, M.; Kume, K.; Kume, S. Secreted Cerberus1 as a Marker for Quantification of Definitive Endoderm Differentiation of the Pluripotent Stem Cells. PLoS ONE 2013, 8, e64291. [Google Scholar] [CrossRef] [Green Version]
- Anchan, R.M.; Lachke, S.A.; Gerami-Naini, B.; Lindsey, J.; Ng, N.; Naber, C.; Nickerson, M.; Cavallesco, R.; Rowan, S.; Eaton, J.L.; et al. Pax6- And Six3-mediated induction of lens cell fate in mouse and human ES cells. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Loebel, D.A.F.; Watson, C.M.; De Young, R.A.; Tam, P.P.L. Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev. Biol. 2003, 264, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pfendler, K.C.; Catuar, C.S.; Meneses, J.J.; Pedersen, R.A. Overexpression of Nodal promotes differentiation of mouse embryonic stem cells into mesoderm and endoderm at the expense of neuroectoderm formation. Stem Cells Dev. 2005, 14, 162–172. [Google Scholar] [CrossRef]
- Russ, A.P.; Wattler, S.; Colledge, W.H.; Aparicio, S.A.J.R.; Carlton, M.B.L.; Pearce, J.J.; Barton, S.C.; Azim Surani, M.; Ryan, K.; Nehls, M.C.; et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 2000, 404, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.T.; Demarest, B.L.; Hill, J.T.; Palmer, J.D.; Brothman, A.R.; Yost, H.J.; Condic, M.L. Genome-Wide Analysis Reveals the Unique Stem Cell Identity of Human Amniocytes. PLoS ONE 2013, 8, e53372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsankov, A.M.; Akopian, V.; Pop, R.; Chetty, S.; Gifford, C.A.; Daheron, L.; Tsankova, N.M.; Meissner, A. A qPCR ScoreCard quantifies the differentiation potential of human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2008, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasic, J.; Skara, L.; Ulamec, M.; Katusic Bojanac, A.; Dabelic, S.; Bulic-Jakus, F.; Jezek, D.; Sincic, N. Teratoma Growth Retardation by HDACi Treatment of the Tumor Embryonal Source. Cancers 2020, 12, 3416. https://doi.org/10.3390/cancers12113416
Krasic J, Skara L, Ulamec M, Katusic Bojanac A, Dabelic S, Bulic-Jakus F, Jezek D, Sincic N. Teratoma Growth Retardation by HDACi Treatment of the Tumor Embryonal Source. Cancers. 2020; 12(11):3416. https://doi.org/10.3390/cancers12113416
Chicago/Turabian StyleKrasic, Jure, Lucija Skara, Monika Ulamec, Ana Katusic Bojanac, Sanja Dabelic, Floriana Bulic-Jakus, Davor Jezek, and Nino Sincic. 2020. "Teratoma Growth Retardation by HDACi Treatment of the Tumor Embryonal Source" Cancers 12, no. 11: 3416. https://doi.org/10.3390/cancers12113416
APA StyleKrasic, J., Skara, L., Ulamec, M., Katusic Bojanac, A., Dabelic, S., Bulic-Jakus, F., Jezek, D., & Sincic, N. (2020). Teratoma Growth Retardation by HDACi Treatment of the Tumor Embryonal Source. Cancers, 12(11), 3416. https://doi.org/10.3390/cancers12113416





