Prognostic Role of Inflammasome Components in Human Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
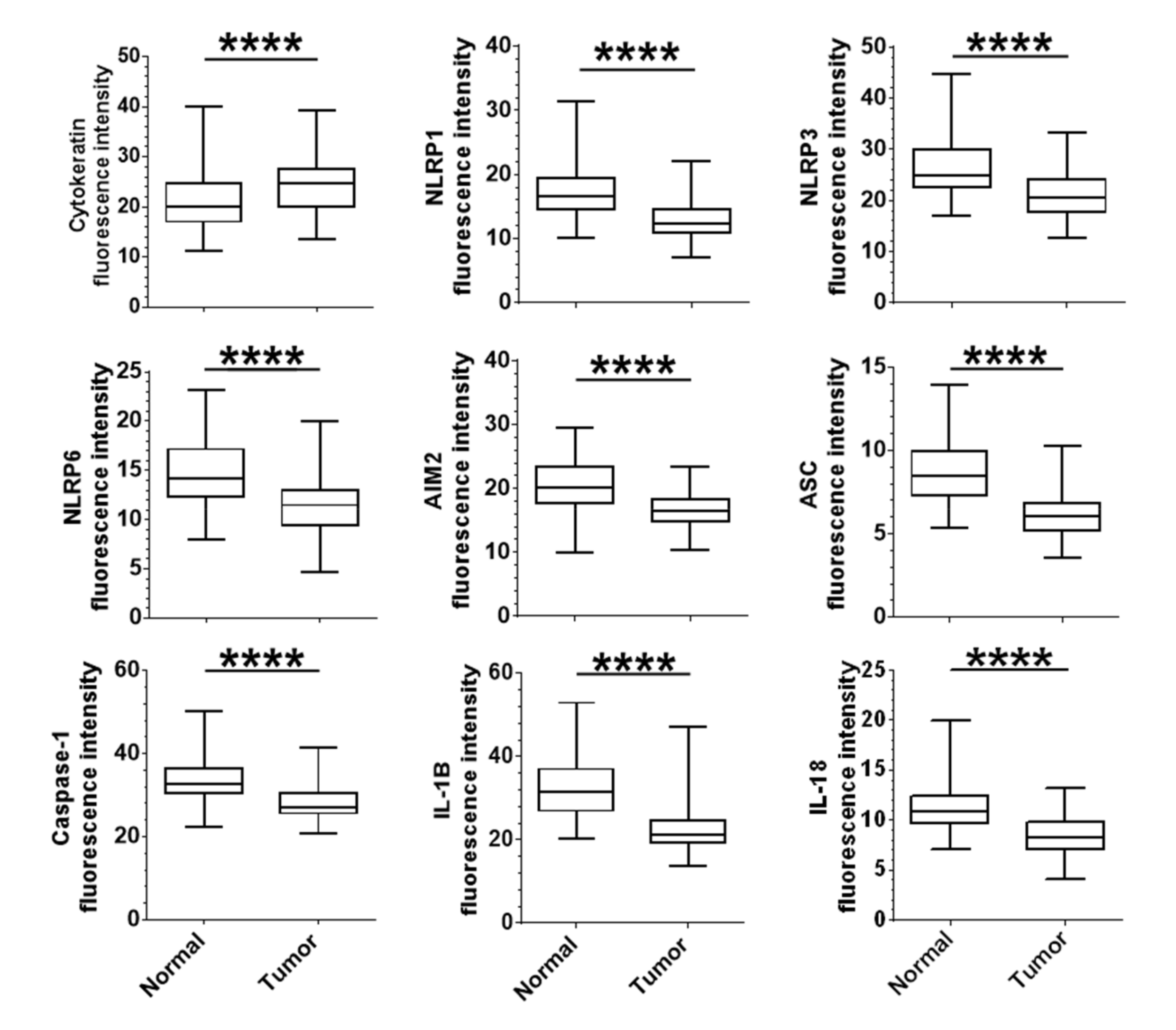
2.2. Correlation of the Expression of Epithelial Tumour Inflammasome Components with Clinical Outcomes
2.3. Correlation between Expression of Inflammasome Components and Clinical Parameters
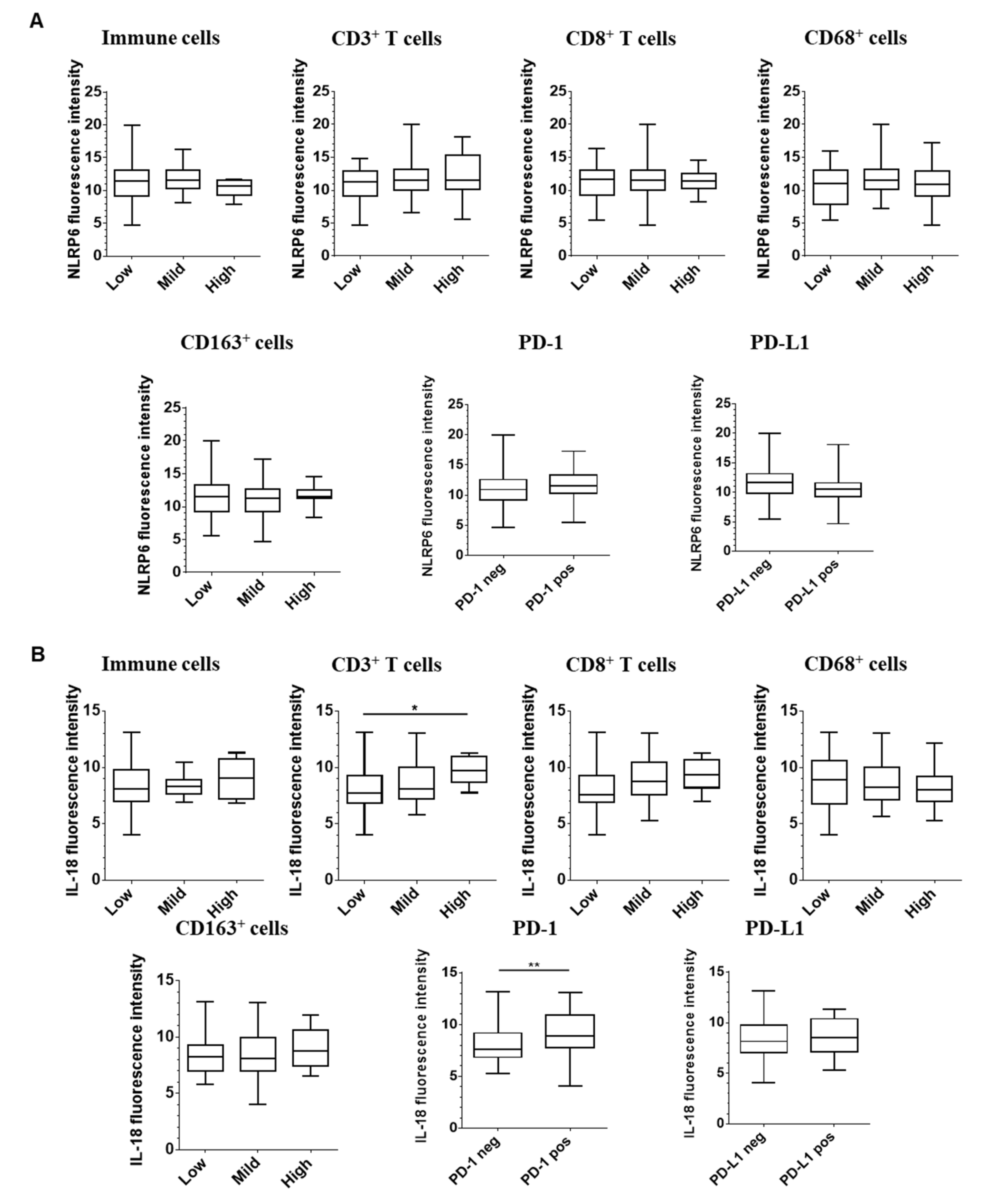
2.4. Correlation between Epithelial Expression of Inflammasome Components and Immune Infiltration
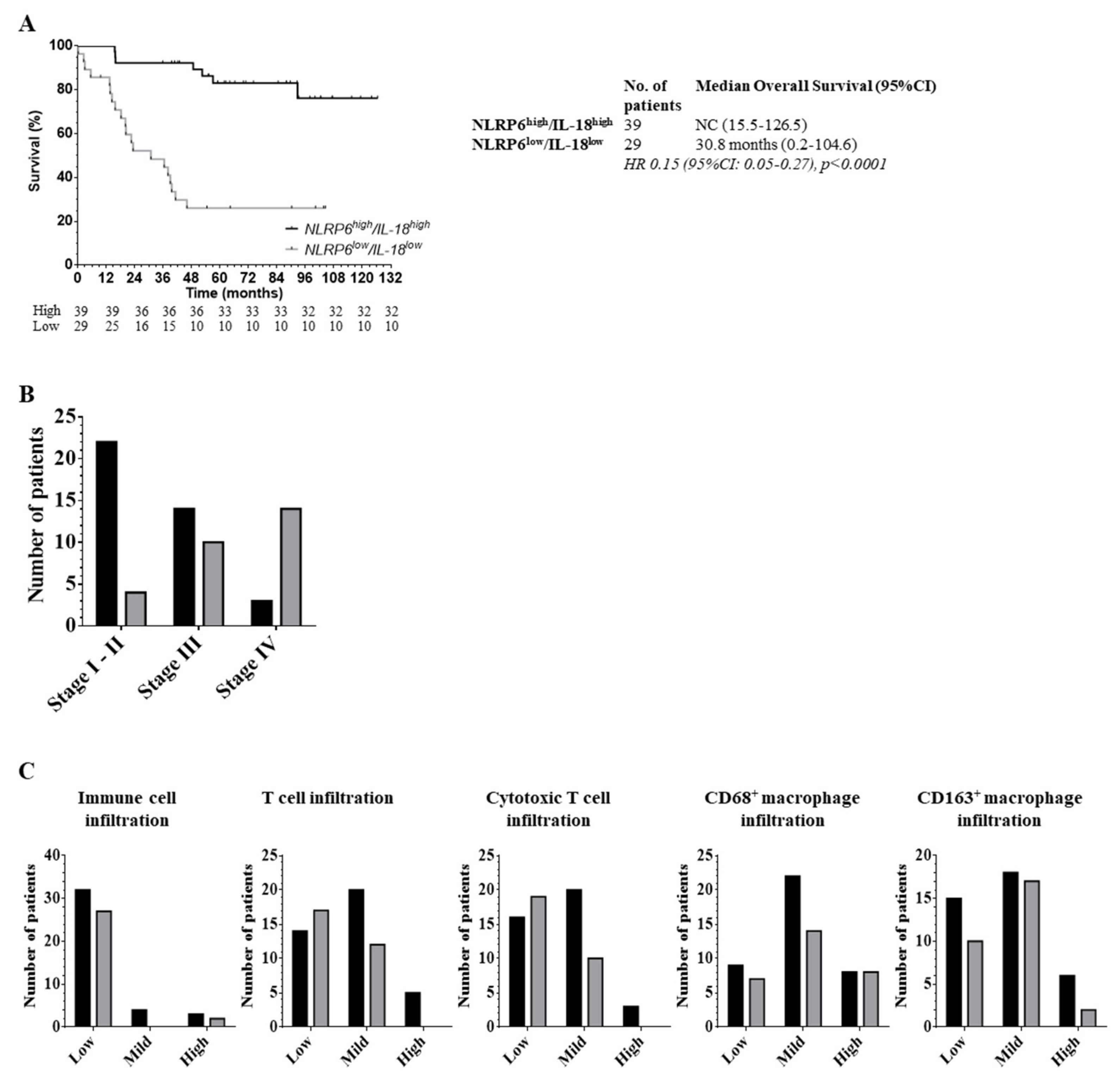
2.5. The Role of the Inflammasome Protein Profile in Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Tissue Micro-Array (TMA) Constitution
4.2. Fluorescent Tissue Staining
4.3. Slides Acquisition (Figure S1)
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | antimicrobial peptides |
| CRC | colorectal cancer |
| DAMP | danger-associated molecular patterns |
| DAPI | 4’,6-diamidino-2-phenylindole |
| FFPE | formalin-fixed, paraffin-embedded |
| IFN | interferon |
| MSI | microsatellite instability |
| NLR | nucleotide-binding domain and leucine-rich repeat proteins |
| OS | overall survival |
| PAMP | pathogen-associated molecular patterns |
| PRR | pattern-recognition receptors |
| ROC | receiver-operating characteristics |
| TMA | tissue micro-array |
| TNM | tumor-node-metastasis |
References
- Mok, T.S.-K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Agency for Research on Cancer. Estimated Number of Deaths in 2018, United Arab Emirates, All Cancers, Females, All Ages. 2018. Available online: http://gco.iarc.fr/today/home (accessed on 14 December 2019).
- Ullman, T.; Itzkowitz, S.H. Intestinal Inflammation and Cancer. Gastroenterology 2011, 140, 1807-e1. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: mechanism of action, role in disease and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Palazon-Riquelme, P.; Lopez-Castejon, G. The inflammasomes, immune guardians at defence barriers. Immunol. 2018, 155, 320–330. [Google Scholar] [CrossRef]
- Liu, R.; Truax, A.D.; Chen, L.; Hu, P.; Li, Z.; Chen, J.; Song, C.; Chen, L.; Ting, J.P.-Y. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget 2015, 6, 33456–33469. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Kanneganti, T.-D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 2019, 19, 197–214. [Google Scholar] [CrossRef]
- Wilson, J.E.; Petrucelli, A.S.; Chen, L.; Koblansky, A.A.; Truax, A.D.; Oyama, Y.; Rogers, A.B.; Brickey, W.J.; Wang, Y.; Schneider, M.; et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat. Med. 2015, 21, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Zaki, H.; Vogel, P.; Malireddi, R.K.S.; Body-Malapel, M.; Anand, P.K.; Bertin, J.; Green, D.R.; Lamkanfi, M.; Kanneganti, T.-D. The NOD-Like Receptor NLRP12 Attenuates Colon Inflammation and Tumorigenesis. Cancer Cell 2011, 20, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kauppinen, A.; Hiltunen, M.; Kaarniranta, K. Epigenetic regulation of ASC/TMS1 expression: potential role in apoptosis and inflammasome function. Cell. Mol. Life Sci. 2013, 71, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Janowski, A.M.; Colegio, O.R.; Hornick, E.E.; McNiff, J.M.; Martin, M.D.; Badovinac, V.P.; Norian, L.A.; Zhang, W.; Cassel, S.L.; Sutterwala, F.S. NLRC4 suppresses melanoma tumor progression independently of inflammasome activation. J. Clin. Investig. 2016, 126, 3917–3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.-M.; Wang, E.; Ma, W.; Haines, D.; O’Huigin, C.; et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 2010, 207, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Vogel, P.; Body-Malapel, M.; Lamkanfi, M.; Kanneganti, T.-D. IL-18 Production Downstream of the Nlrp3 Inflammasome Confers Protection against Colorectal Tumor Formation. J. Immunol. 2010, 185, 4912–4920. [Google Scholar] [CrossRef] [Green Version]
- Ni, L.; Lu, J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018, 7, 4509–4516. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef] [Green Version]
- Dupaul-Chicoine, J.; Arabzadeh, A.; Dagenais, M.; Douglas, T.; Champagne, C.; Morizot, A.; Rodrigue-Gervais, I.G.; Breton, V.; Colpitts, S.L.; Beauchemin, N.; et al. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunology 2015, 43, 751–763. [Google Scholar] [CrossRef] [Green Version]
- Dagenais, M.; Saleh, M. Linking cancer-induced Nlrp3 inflammasome activation to efficient NK cell-mediated immunosurveillance. OncoImmunology 2016, 5, e1129484. [Google Scholar] [CrossRef] [Green Version]
- Nishio, S.; Yamada, N.; Ohyama, H.; Yamanegi, K.; Nakasho, K.; Hata, M.; Nakamura, Y.; Fukunaga, S.; Futani, H.; Yoshiya, S.; et al. Enhanced suppression of pulmonary metastasis of malignant melanoma cells by combined administration of α-galactosylceramide and interleukin-18. Cancer Sci. 2007, 99, 113–120. [Google Scholar] [CrossRef]
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nat. Cell Biol. 2019, 571, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Pantschenko, A.G.; Pushkar, I.; Anderson, K.H.; Wang, Y.; Miller, L.J.; Kurtzman, S.H.; Barrows, G.; Kreutzer, D.L. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int. J. Oncol. 2003, 23, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. 2003, 100, 2645–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. IL-1β mediated up-regulation of HIF-lα via an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003, 17, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Krelin, Y.; Dvorkin, T.; Bjorkdahl, O.; Segal, S.; Dinarello, C.A.; Voronov, E.; Apte, R.N. CD11b+/Gr-1+ Immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J. Immunol. 2005, 175, 8200–8208. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Liu, M.; Wang, F.; Bertin, J.; Núñez, G. A Functional Role for Nlrp6 in Intestinal Inflammation and Tumorigenesis. J. Immunol. 2011, 186, 7187–7194. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Nyström, E.E.L.; Johansson, M.E.V.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Wlodarska, M.; Thaiss, C.A.; Nowarski, R.; Henao-Mejia, J.; Zhang, J.-P.; Brown, E.M.; Frankel, G.; Levy, M.; Katz, M.N.; Philbrick, W.M.; et al. NLRP6 Inflammasome Orchestrates the Colonic Host-Microbial Interface by Regulating Goblet Cell Mucus Secretion. Cell 2014, 156, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Elinav, E.; Huber, S.; Strowig, T.; Hao, L.; Hafemann, A.; Jin, C.; Wunderlich, C.; Eisenbarth, S.C.; Flavell, R.A. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl. Acad. Sci. 2013, 110, 9862–9867. [Google Scholar] [CrossRef] [Green Version]
- Normand, S.; Delanoye-Crespin, A.; Bressenot, A.; Huot, L.; Grandjean, T.; Peyrin-Biroulet, L.; Lemoine, Y.; Hot, D.; Chamaillard, M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. 2011, 108, 9601–9606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zhu, S.; Yang, L.; Cui, S.; Pan, W.; Jackson, R.; Zheng, Y.; Rongvaux, A.; Sun, Q.; Yang, G.; et al. Nlrp6 regulates intestinal antiviral innate immunity. Science 2015, 350, 826–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, T.M.; Leeth, R.A.; Rothschild, D.E.; Coutermarsh-Ott, S.L.; McDaniel, D.K.; Simmons, A.E.; Heid, B.; Cecere, T.E.; Allen, I.C. The NLRP1 Inflammasome Attenuates Colitis and Colitis-Associated Tumorigenesis. J. Immunol. 2015, 194, 3369–3380. [Google Scholar] [CrossRef] [Green Version]
- Nowarski, R.; Jackson, R.; Gagliani, N.; De Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef]


| Characteristic | N | % | |
|---|---|---|---|
| Gender | Male | 60 | 57.7 |
| Female | 44 | 42.3 | |
| Median age (years [range]) | 71.7 [38.6–88.4] | ||
| Stage | I | 8 | 7.7 |
| II | 36 | 34.6 | |
| III | 37 | 35.6 | |
| IV | 23 | 22.1 | |
| Grade | 1 | 8 | 7.7 |
| 1-2 | 9 | 8.7 | |
| 2 | 76 | 73.1 | |
| 3 | 8 | 7.7 | |
| Mutational status | KRAS | 19 | 18.3 |
| BRAF | 4 | 3.8 | |
| MMR status | MSS | 88 | 84.6 |
| MSI | 16 | 15.4 | |
| 5 years overall survival | 64.4% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domblides, C.; Soubeyran, I.; Lartigue, L.; Mahouche, I.; Lefort, F.; Velasco, V.; Barnetche, T.; Blanco, P.; Déchanet-Merville, J.; Faustin, B. Prognostic Role of Inflammasome Components in Human Colorectal Cancer. Cancers 2020, 12, 3500. https://doi.org/10.3390/cancers12123500
Domblides C, Soubeyran I, Lartigue L, Mahouche I, Lefort F, Velasco V, Barnetche T, Blanco P, Déchanet-Merville J, Faustin B. Prognostic Role of Inflammasome Components in Human Colorectal Cancer. Cancers. 2020; 12(12):3500. https://doi.org/10.3390/cancers12123500
Chicago/Turabian StyleDomblides, Charlotte, Isabelle Soubeyran, Lydia Lartigue, Isabelle Mahouche, Félix Lefort, Valérie Velasco, Thomas Barnetche, Patrick Blanco, Julie Déchanet-Merville, and Benjamin Faustin. 2020. "Prognostic Role of Inflammasome Components in Human Colorectal Cancer" Cancers 12, no. 12: 3500. https://doi.org/10.3390/cancers12123500





