Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
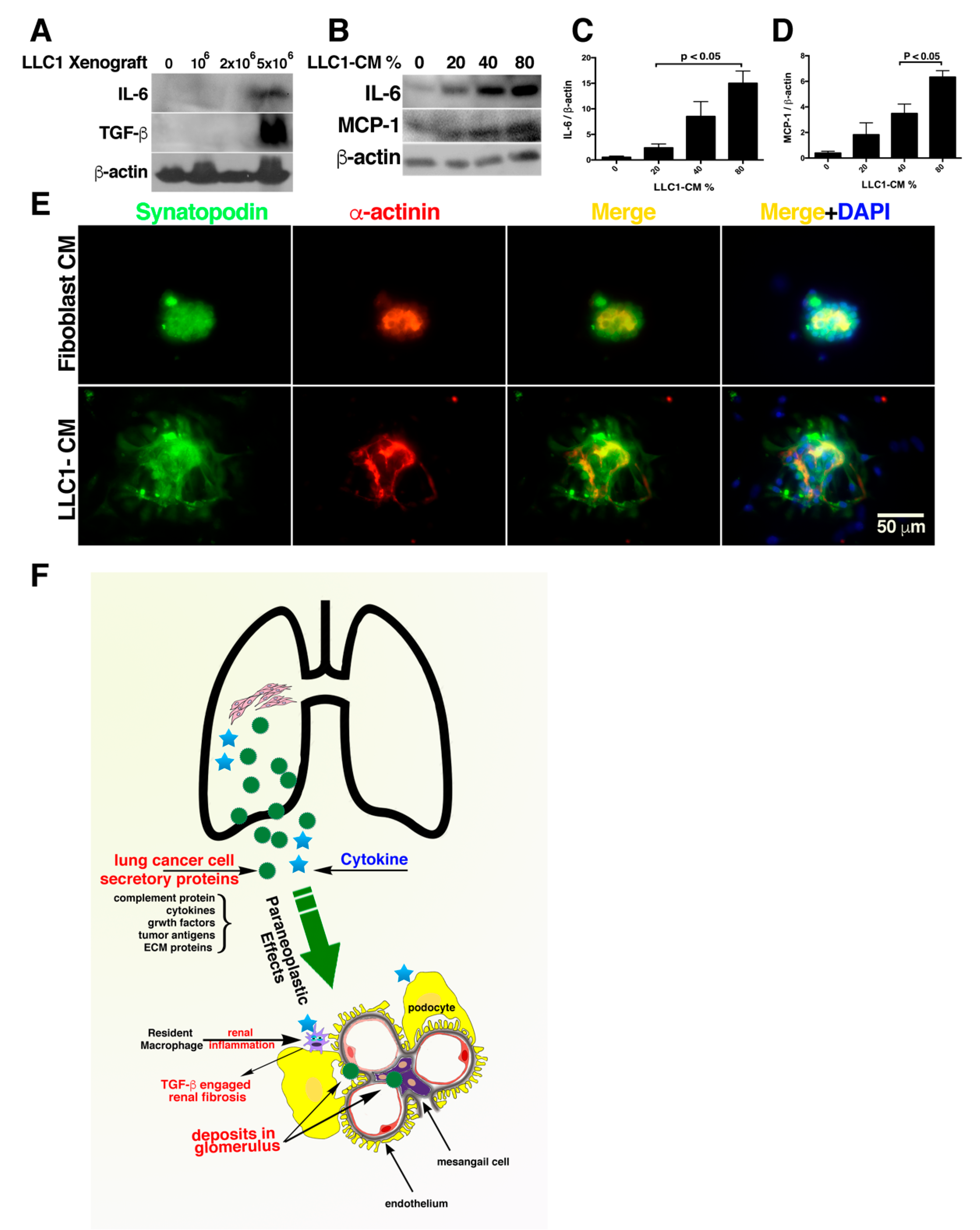
2. Results
2.1. The LLC1 Cells Grown in Mice Lung Caused Albuminuria
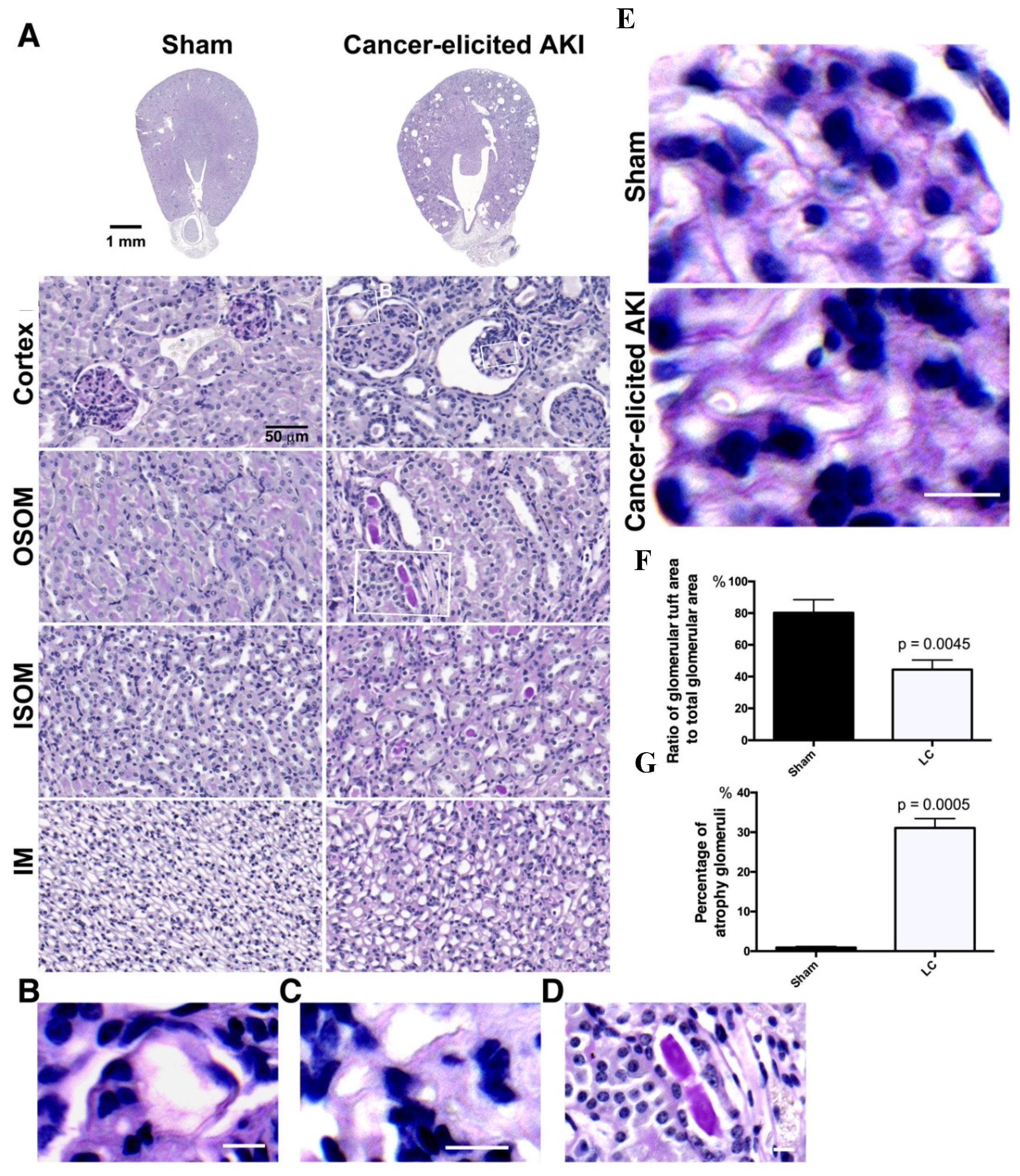
2.2. The Collapse of Glomerular Tufts and Structural Lesions in Tubules Were Present in the Kidneys of the Lung Cancer Mice
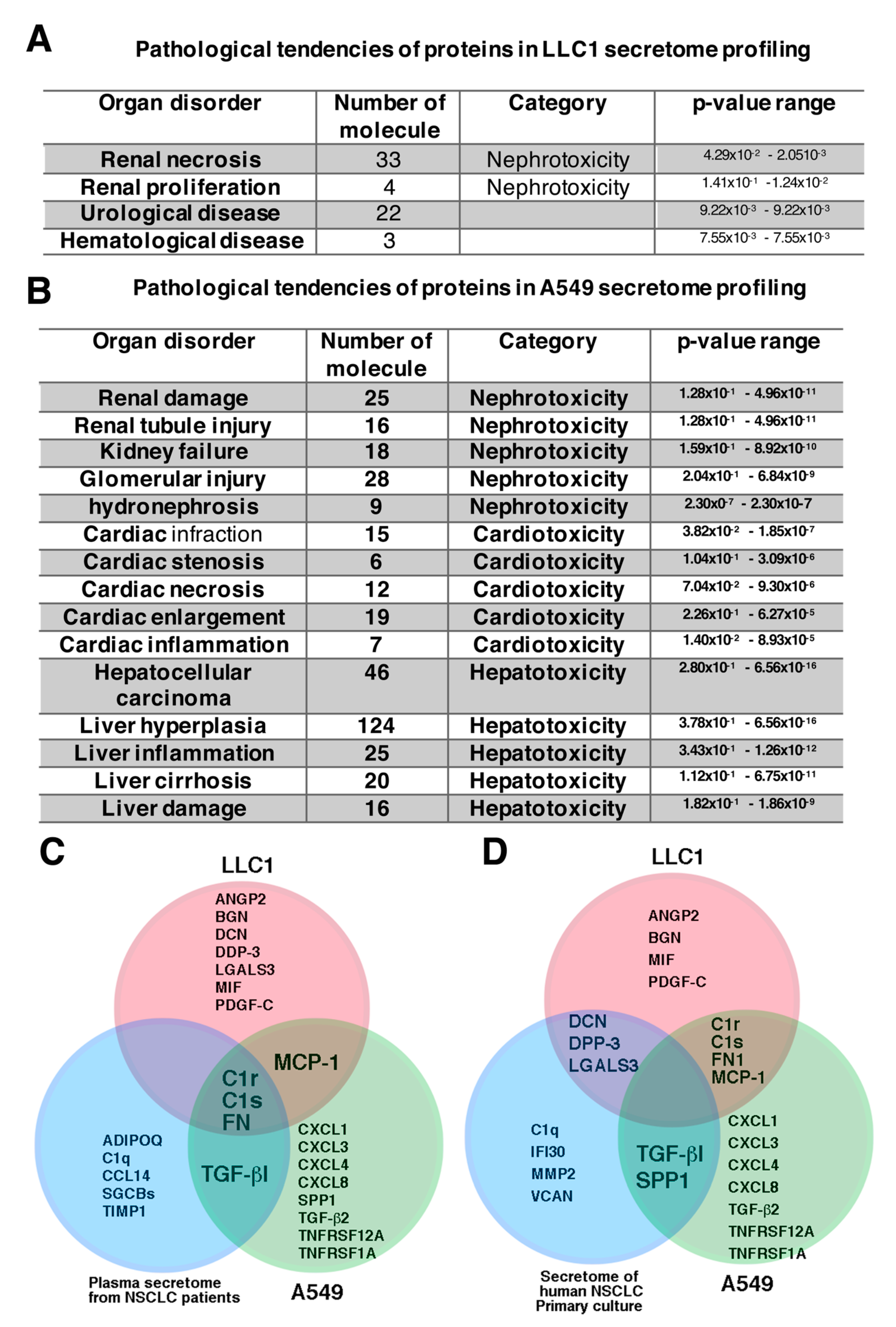
2.3. Proteins Identified in Lung Cancer Cell Secretome Potentiated Nephrotoxicity
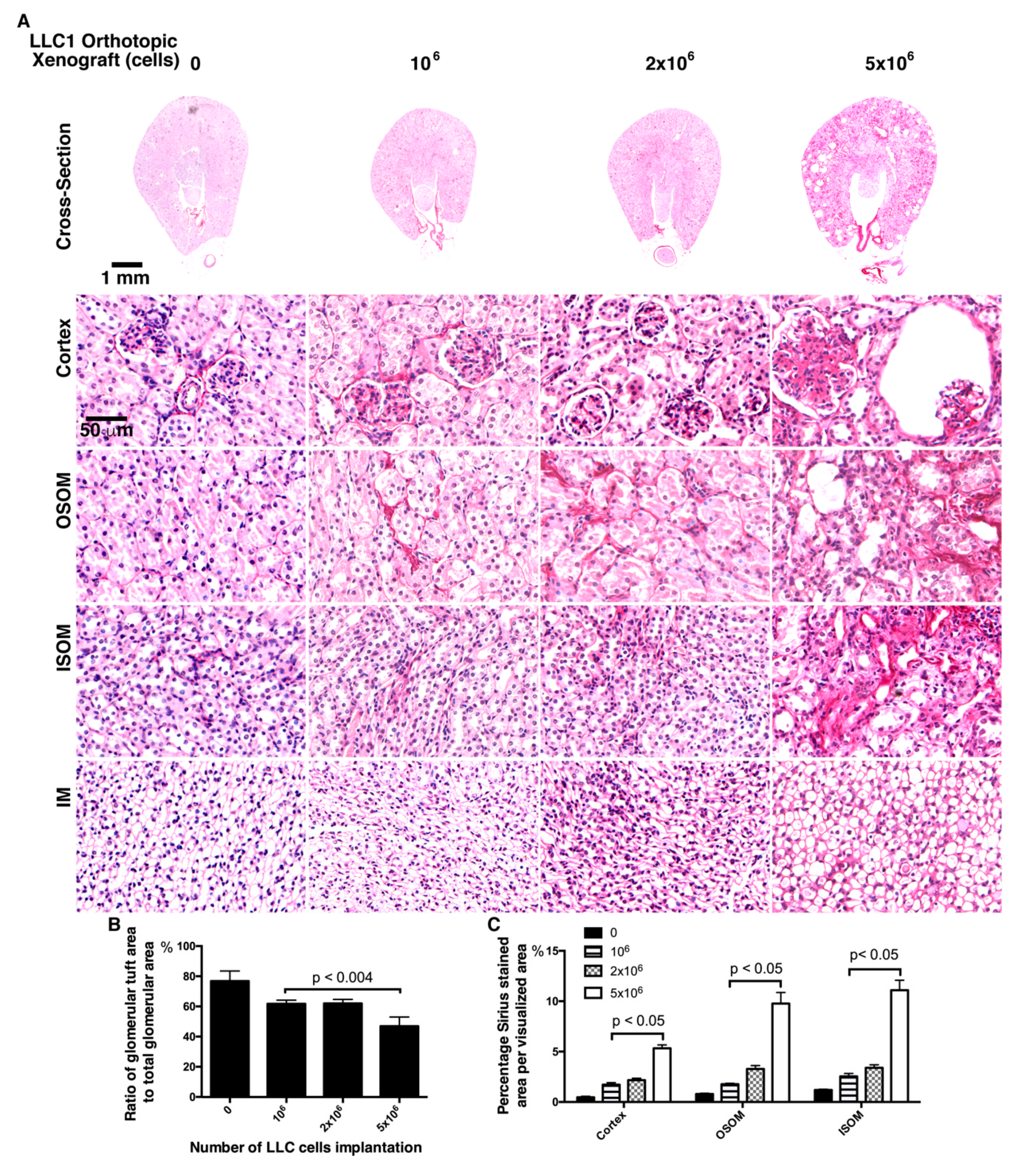
2.4. Fewer LLC1 Cells Implanted in Mice Develop Relatively Mitigaged Renal Insult and Inflammation
2.5. Mice Transplanted with Fewer LLC1 Cells Had Milder Renal Fibrosis
2.6. Activation of TGF-β Signaling in the Kidneys of the Lung Cancer Mice
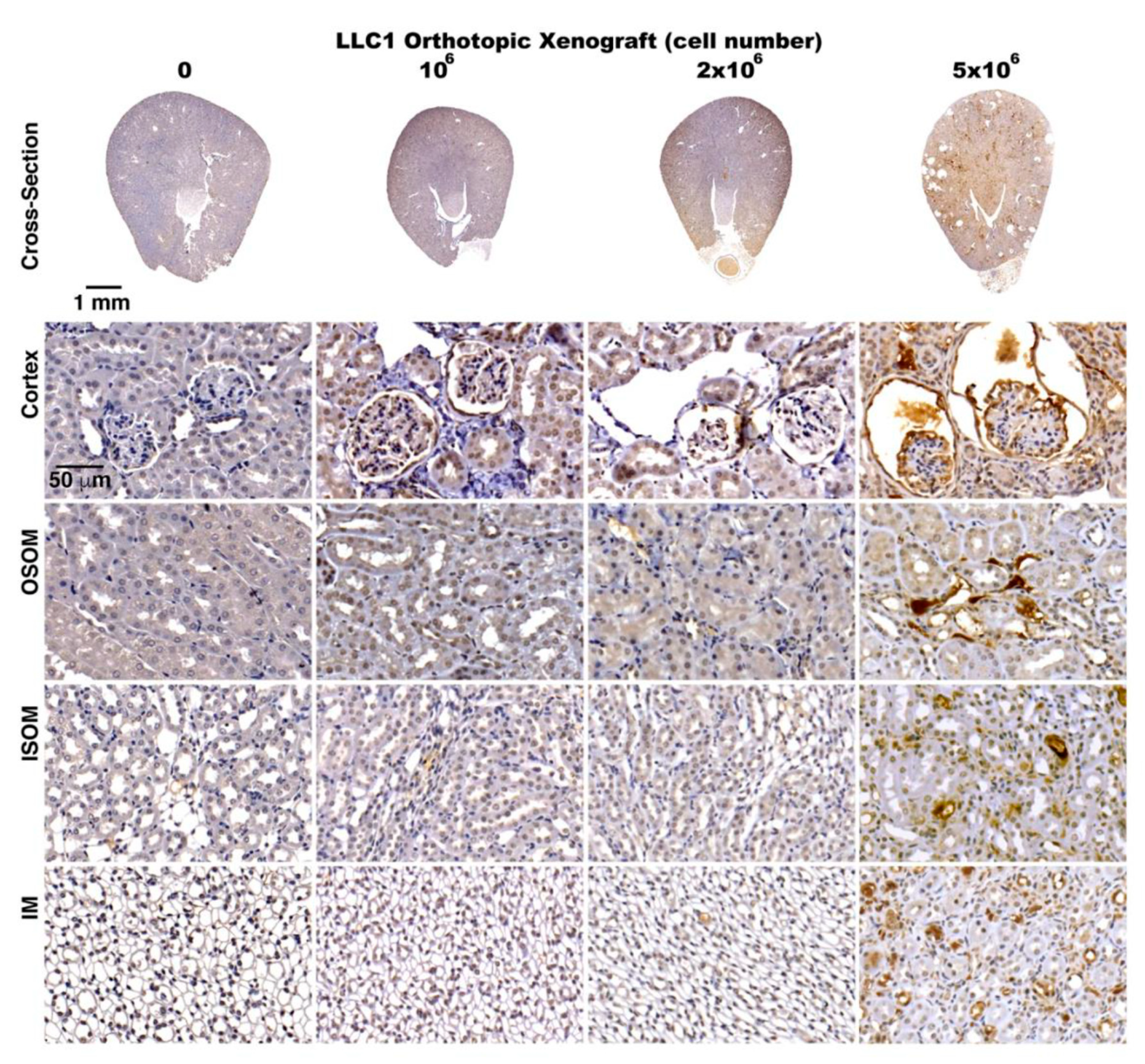
2.7. Cancer Secretome-Activated Renal Inflammation
2.8. Exposure of Glomeruli to LLC1 Cell-Conditioned Medium Leads to Loss of Glomerular Integrity
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Animal Care and Lung Cancer Cell Orthotropic Xenograft Model
4.3. Biopsy Collection, Histochemical Staining, and Immunohistochemical Staining
4.4. Evaluation of Serum Physiological Parameters
4.5. Cell Culture and Orthotopic Xenograft
4.6. Cell Culture and Proteomic Profiling of Secretome
4.7. Cell Culture and Secretome-Induced Inflammation
4.8. Renal Cells Primary Culture
4.9. Glomerular Integrity Assay
4.10. Immunofluorescent Staining and Immunofluorescent Microscopic Imagination
4.11. Immunoblot
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, C.F.; Xie, Q.; Su, Y.L.; Koeman, J.; Khoo, S.K.; Gustafson, M.; Knudsen, B.S.; Hay, R.; Shinomiya, N.; Vande Woude, G.F. Proliferation and invasion: Plasticity in tumor cells. Proc. Natl. Acad. Sci. USA 2005, 102, 10528–10533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, G.; Moser, D.; Hochmair, M. Metastasis: Circulating Tumor Cells in Small Cell Lung Cancer. Trends Cancer 2016, 2, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaji, N.; Watanabe, N.; Kita, N.; Bandoh, S.; Tadokoro, A.; Ishii, T.; Dobashi, H.; Matsunaga, T. Paraneoplastic syndromes associated with lung cancer. World. J. Clin. Oncol. 2014, 5, 197–223. [Google Scholar] [CrossRef]
- Anwar, A.; Jafri, F.; Ashraf, S.; Jafri, M.A.S.; Fanucchi, M. Paraneoplastic syndromes in lung cancer and their management. Ann. Transl. Med. 2019, 7, 359. [Google Scholar] [CrossRef]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [Green Version]
- Wilson, F.P.; Berns, J.S. Tumor lysis syndrome: New challenges and recent advances. Adv. Chronic Kidney Dis. 2014, 21, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Wilson, F.P.; Berns, J.S. Onco-nephrology: Tumor lysis syndrome. Clin. J. Am. Soc. Nephrol. 2012, 7, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Johnson, B.E. Paraneoplastic syndromes associated with small cell lung cancer. J. Natl. Compr. Canc. Netw. 2006, 4, 631–638. [Google Scholar] [CrossRef]
- Rosner, M.H.; Perazella, M.A. Acute Kidney Injury in Patients with Cancer. N. Engl. J. Med. 2017, 377, 500–501. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Hutchins, G.M.; Moore, G.W. Paraneoplastic syndromes and constitutional symptoms in prediction of metastatic behavior of small cell carcinoma of the lung. Am. J. Med. 1984, 77, 851–857. [Google Scholar] [CrossRef]
- Henry, K. Paraneoplastic syndromes: Definitions, classification, pathophysiology and principles of treatment. Semin. Diagn. Pathol. 2019, 36, 204–210. [Google Scholar] [CrossRef]
- Graus, F.; Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Hsu, C.W.; Chen, C.D.; Yu, C.J.; Chang, K.P.; Tai, D.I.; Liu, H.P.; Su, W.H.; Chang, Y.S.; Yu, J.S. Candidate serological biomarkers for cancer identified from the secretomes of 23 cancer cell lines and the human protein atlas. Mol. Cell. Proteom. 2010, 9, 1100–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, T.; Alsina, M.A.; Chavez, J.B.; Bonewald, L.; Nishimura, R.; Mundy, G.R. Evidence that tumor necrosis factor plays a pathogenetic role in the paraneoplastic syndromes of cachexia, hypercalcemia, and leukocytosis in a human tumor in nude mice. J. Clin. Investig. 1991, 87, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, T.; Aufdemorte, T.B.; Nishimura, R.; Nishikawa, N.; Sakuda, M.; Alsina, M.M.; Chavez, J.B.; Mundy, G.R. Occurrence of hypercalcemia and leukocytosis with cachexia in a human squamous cell carcinoma of the maxilla in athymic nude mice: A novel experimental model of three concomitant paraneoplastic syndromes. J. Clin. Oncol. 1991, 9, 468–477. [Google Scholar] [CrossRef]
- Ohara, G.; Satoh, H.; Kurishima, K.; Ohtsuka, M.; Hizawa, N. Paraneoplastic nephrotic syndrome in patients with lung cancer. Intern. Med. 2009, 48, 1817–1820. [Google Scholar] [CrossRef] [Green Version]
- Boon, E.S.; Vrij, A.A.; Nieuwhof, C.; van Noord, J.A.; Zeppenfeldt, E. Small cell lung cancer with paraneoplastic nephrotic syndrome. Eur. Respir. J. 1994, 7, 1192–1193. [Google Scholar]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Lien, Y.H.; Lai, L.W. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat. Rev. Nephrol. 2011, 7, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Ronco, P.M. Paraneoplastic glomerulopathies: New insights into an old entity. Kidney Int. 1999, 56, 355–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascal, R.R.; Slovin, S.F. Tumor directed antibody and carcinoembryonic antigen in the glomeruli of a patient with gastric carcinoma. Hum. Pathol. 1980, 11, 679–682. [Google Scholar] [CrossRef]
- Sardhara, J.; Shukla, M.; Jamdar, J.; Jaiswal, A.K.; Jaiswal, S.; Kaul, A.; Bhaisora, K.S.; Das, K.K.; Mehrotra, A.; Behari, S. Paraneoplastic Nephrotic Syndrome in a Patient with Planum Sphenoidale Meningioma. Asian J. Neurosurg. 2018, 13, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A.; Hu, D.; Okusa, M.D. Acute kidney injury in the cancer patient. Adv. Chronic Kidney Dis. 2014, 21, 64–71. [Google Scholar] [CrossRef]
- Guabello, G.; Brunetti, L.; Palladini, G.; Musumeci, S.; Lovati, E.; Perfetti, V. Paraneoplastic Cushing’s syndrome and nephrotic syndrome in a patient with disseminated small cell lung cancer. Am. J. Clin. Oncol. 2008, 31, 102–103. [Google Scholar] [CrossRef]
- Antonov, A.S.; Antonova, G.N.; Munn, D.H.; Mivechi, N.; Lucas, R.; Catravas, J.D.; Verin, A.D. alphaVbeta3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-kappaB activation. J. Cell. Physiol. 2011, 226, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Kassiotis, G.; Kollias, G. TNF and receptors in organ-specific autoimmune disease: Multi-layered functioning mirrored in animal models. J. Clin. Investig. 2001, 107, 1507–1508. [Google Scholar] [CrossRef] [Green Version]
- Aytekin, A.; Ozet, A.; Bilgetekin, I.; Ogut, B.; Ciltas, A.; Benekli, M. A case of membranous glomerulopathy associated with lung cancer and review of the literature. Mol. Clin. Oncol. 2017, 7, 241–243. [Google Scholar] [CrossRef] [Green Version]
- Plaisier, E.; Ronco, P. Screening for Cancer in Patients with Glomerular Diseases. Clin. J. Am. Soc. Nephrol. 2020. [Google Scholar] [CrossRef]
- Lin, F.C.; Chen, J.Y.; Yang, A.H.; Chang, S.C. The association of non-small-cell lung cancer, focal segmental glomerulosclerosis, and platelet dysfunction. Am. J. Med. Sci. 2002, 324, 161–165. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Juillard, L.; Cochat, P.; Droz, J.P. Paraneoplastic glomerular diseases and malignancies. Crit. Rev. Oncol. Hematol. 2009, 70, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, Y.; Li, M.; Song, J.; Gao, Y.; Cheng, S.; Xiao, T. Systems biology analysis of the lung cancerrelated secretome. Oncol. Rep. 2018, 40, 1103–1118. [Google Scholar] [CrossRef]
- Shin, J.; Song, S.Y.; Ahn, H.S.; An, B.C.; Choi, Y.D.; Yang, E.G.; Na, K.J.; Lee, S.T.; Park, J.I.; Kim, S.Y.; et al. Integrative analysis for the discovery of lung cancer serological markers and validation by MRM-MS. PLoS ONE 2017, 12, e0183896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baradaran, A.; Nasri, H. Comment on: Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin. Exp. Nephrol. 2014, 18, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Ogata, H.; Takeshige, Y.; Takeshima, A.; Yoshida, N.; Yamamoto, M.; Ito, H.; Kinugasa, E. Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin. Exp. Nephrol. 2013, 17, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Furuya, F.; Takahashi, K.; Shikata, M.; Takamura, T.; Kobayashi, H.; Miyazaki, A.; Morinaga, J.; Terada, K.; Oike, Y.; et al. Angiopoietin-Like Protein 2 Promotes the Progression of Diabetic Kidney Disease. J. Clin. Endocrinol. Metab. 2019, 104, 172–180. [Google Scholar] [CrossRef]
- Grgurevic, L.; Macek, B.; Healy, D.R.; Brault, A.L.; Erjavec, I.; Cipcic, A.; Grgurevic, I.; Rogic, D.; Galesic, K.; Brkljacic, J.; et al. Circulating bone morphogenetic protein 1–3 isoform increases renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Markmann, A.; Hausser, H.; Schonherr, E.; Kresse, H. Influence of decorin expression on transforming growth factor-beta-mediated collagen gel retraction and biglycan induction. Matrix Biol. 2000, 19, 631–636. [Google Scholar] [CrossRef]
- Klemis, V.; Ghura, H.; Federico, G.; Wurfel, C.; Bentmann, A.; Gretz, N.; Miyazaki, T.; Grone, H.J.; Nakchbandi, I.A. Circulating fibronectin contributes to mesangial expansion in a murine model of type 1 diabetes. Kidney Int. 2017, 91, 1374–1385. [Google Scholar] [CrossRef] [Green Version]
- Eardley, K.S.; Zehnder, D.; Quinkler, M.; Lepenies, J.; Bates, R.L.; Savage, C.O.; Howie, A.J.; Adu, D.; Cockwell, P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006, 69, 1189–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poluzzi, C.; Nastase, M.V.; Zeng-Brouwers, J.; Roedig, H.; Hsieh, L.T.; Michaelis, J.B.; Buhl, E.M.; Rezende, F.; Manavski, Y.; Bleich, A.; et al. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 2019, 95, 540–562. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.B.; Holler, S.; Cui, Y.; Hudkins, K.L.; Eitner, F.; Fogo, A.; Alpers, C.E. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000, 57, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Lesher, A.M.; Song, W.C. Review: Complement and its regulatory proteins in kidney diseases. Nephrology (Carlton) 2010, 15, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Depret, F.; Amzallag, J.; Pollina, A.; Fayolle-Pivot, L.; Coutrot, M.; Chaussard, M.; Santos, K.; Hartmann, O.; Jully, M.; Fratani, A.; et al. Circulating dipeptidyl peptidase-3 at admission is associated with circulatory failure, acute kidney injury and death in severely ill burn patients. Crit. Care 2020, 24, 168. [Google Scholar] [CrossRef] [Green Version]
- Jha, S.; Taschler, U.; Domenig, O.; Poglitsch, M.; Bourgeois, B.; Pollheimer, M.; Pusch, L.M.; Malovan, G.; Frank, S.; Madl, T.; et al. Dipeptidyl peptidase 3 modulates the renin-angiotensin system in mice. J. Biol. Chem. 2020, 295, 13711–13723. [Google Scholar] [CrossRef]
- Lusco, M.A.; Chen, Y.P.; Cheng, H.; Dong, H.R.; Najafian, B.; Alpers, C.E.; Fogo, A.B. AJKD Atlas of Renal Pathology: Fibronectin Glomerulopathy. Am. J. Kidney. Dis. 2017, 70, e21–e22. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Kuo, P.L. The Role of Galectin-3 in the Kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Tang, Y.; Lv, J.; Wang, X.H.; Yang, H.; Tang, P.M.K.; Huang, X.R.; He, Z.J.; Zhou, Z.J.; Huang, Q.Y.; et al. Macrophage migration inhibitory factor promotes renal injury induced by ischemic reperfusion. J. Cell. Mol. Med. 2019, 23, 3867–3877. [Google Scholar] [CrossRef] [Green Version]
- Tesch, G.H.; Schwarting, A.; Kinoshita, K.; Lan, H.Y.; Rollins, B.J.; Kelley, V.R. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J. Clin. Investig. 1999, 103, 73–80. [Google Scholar] [CrossRef] [Green Version]
- van Roeyen, C.R.C.; Martin, I.V.; Drescher, A.; Schuett, K.A.; Hermert, D.; Raffetseder, U.; Otten, S.; Buhl, E.M.; Braun, G.S.; Kuppe, C.; et al. Identification of platelet-derived growth factor C as a mediator of both renal fibrosis and hypertension. Kidney Int. 2019, 95, 1103–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floege, J.; Eitner, F.; Alpers, C.E. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 2008, 19, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitner, F.; Bucher, E.; van Roeyen, C.; Kunter, U.; Rong, S.; Seikrit, C.; Villa, L.; Boor, P.; Fredriksson, L.; Backstrom, G.; et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2008, 19, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, N.; Ahlawat, R.S. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: New evidence linking inflammation, glycemic control, and microalbuminuria. Iran J. Kidney Dis. 2008, 2, 72–79. [Google Scholar]
- Lee, S.J.; Borsting, E.; Decleves, A.E.; Singh, P.; Cunard, R. Podocytes express IL-6 and lipocalin 2/neutrophil gelatinase-associated lipocalin in lipopolysaccharide-induced acute glomerular injury. Nephron Exp. Nephrol. 2012, 121, e86–e96. [Google Scholar] [CrossRef] [Green Version]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Eddy, A.A. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int. Suppl. 2014, 4, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Ding, H.; Miao, H.; Garcia, J.M.; Li, Y.P. Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci. Rep. 2017, 7, 2273. [Google Scholar] [CrossRef] [Green Version]
- Zager, R.A. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996, 49, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Belliere, J.; Casemayou, A.; Ducasse, L.; Zakaroff-Girard, A.; Martins, F.; Iacovoni, J.S.; Guilbeau-Frugier, C.; Buffin-Meyer, B.; Pipy, B.; Chauveau, D.; et al. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J. Am. Soc. Nephrol. 2015, 26, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Nasr, S.H.; Fogo, A.B. New developments in the diagnosis of fibrillary glomerulonephritis. Kidney Int. 2019, 96, 581–592. [Google Scholar] [CrossRef]
- Rops, A.; Jansen, E.; van der Schaaf, A.; Pieterse, E.; Rother, N.; Hofstra, J.; Dijkman, H.; van de Logt, A.E.; Wetzels, J.; van der Vlag, J.; et al. Interleukin-6 is essential for glomerular immunoglobulin A deposition and the development of renal pathology in Cd37-deficient mice. Kidney Int. 2018, 93, 1356–1366. [Google Scholar] [CrossRef]
- Rosales, I.A.; Colvin, R.B. Glomerular disease with idiopathic linear immunoglobulin deposition: A rose by any other name would be atypical. Kidney Int. 2016, 89, 750–752. [Google Scholar] [CrossRef] [Green Version]
- Selvaskandan, H.; Cheung, C.K.; Muto, M.; Barratt, J. New strategies and perspectives on managing IgA nephropathy. Clin. Exp. Nephrol. 2019, 23, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Devasahayam, J.; Erode-Singaravelu, G.; Bhat, Z.; Oliver, T.; Chandran, A.; Zeng, X.; Dakshinesh, P.; Pillai, U. C1q Nephropathy: The Unique Underrecognized Pathological Entity. Anal. Cell. Pathol. 2015, 2015, 490413. [Google Scholar] [CrossRef] [Green Version]
- Xavier, S.; Sahu, R.K.; Bontha, S.V.; Mass, V.; Taylor, R.P.; Megyesi, J.; Thielens, N.M.; Portilla, D. Complement C1r serine protease contributes to kidney fibrosis. Am. J. Physiol. Renal Physiol. 2019, 317, F1293–F1304. [Google Scholar] [CrossRef]
- Boudhabhay, I.; Poillerat, V.; Grunenwald, A.; Torset, C.; Leon, J.; Daugan, M.V.; Lucibello, F.; El Karoui, K.; Ydee, A.; Chauvet, S.; et al. Complement activation is a crucial driver of acute kidney injury in rhabdomyolysis. Kidney Int. 2020. [Google Scholar] [CrossRef]
- Cosio, F.G.; Bakaletz, A.P. Abnormal plasma fibronectin levels in patients with proteinuria. J. Lab. Clin. Med. 1984, 104, 867–872. [Google Scholar]
- Jhaveri, K.D.; Shah, H.H.; Patel, C.; Kadiyala, A.; Stokes, M.B.; Radhakrishnan, J. Glomerular diseases associated with cancer, chemotherapy, and hematopoietic stem cell transplantation. Adv. Chronic Kidney Dis. 2014, 21, 48–55. [Google Scholar] [CrossRef]


| Gene Name | Protein Name | Signaling Pathway in Renal Pathogenesis | Kidney Disease Association | Ref. |
|---|---|---|---|---|
| ANGPL2 | Angiopoietin-related protein 2 | TGF-β pathway | Renal fibrosis | [37] |
| BGN | Biglycan | TLR-2/4 signaling | Inflammation | [39,42,43] |
| BMP1 | Bone morphogenetic protein1 | TGF-β pathway | Renal fibrosis | [38] |
| C1r | Complement C1r | Complement system | Renal fibrosis | [44] |
| C1s | Complement C1s | Complement system | Renal fibrosis | [44] |
| DCN | Decorin | TGF-β pathway | Renal fibrosis | [39,43] |
| DPP3 | Dipeptidyl peptidase 3 | Renin-angiotensin system | Inflammation | [45,46] |
| FN1 | Fibronectin | TGF-β pathway | Glomerulopathy, mesangial expansion, renal fibrosis | [40,47] |
| LGALS3 | Galectin-3 | Interstitial fibrosis, ciliopathy | [48] | |
| MIF | Macrophage migration inhibitory factor | TLR-4-NF-β B signaling axis | Acute kidney injury, inflammation | [49] |
| MCP1 | Monocyte chemoattractant protein 1 | TGF-β pathway | Inflammation, glomerular injury, renal fibrosis | [41,50] |
| PDGFC | Platelet-derived growth factor C | PDGF signaling | Renal interstitial fibrosis | [51,52,53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.-C.; Zhen, Y.-Y.; Niu, S.-W.; Hsu, J.-F.; Lee, T.-H.; Chuang, H.-H.; Wang, P.-H.; Lee, S.-C.; Lin, P.-C.; Chiu, Y.-W.; et al. Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice. Cancers 2020, 12, 3561. https://doi.org/10.3390/cancers12123561
Hung C-C, Zhen Y-Y, Niu S-W, Hsu J-F, Lee T-H, Chuang H-H, Wang P-H, Lee S-C, Lin P-C, Chiu Y-W, et al. Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice. Cancers. 2020; 12(12):3561. https://doi.org/10.3390/cancers12123561
Chicago/Turabian StyleHung, Chi-Chih, Yen-Yi Zhen, Sheng-Wen Niu, Jui-Feng Hsu, Tai-Huang Lee, Hsiang-Hao Chuang, Pei-Hui Wang, Su-Chu Lee, Pi-Chen Lin, Yi-Wen Chiu, and et al. 2020. "Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice" Cancers 12, no. 12: 3561. https://doi.org/10.3390/cancers12123561
APA StyleHung, C.-C., Zhen, Y.-Y., Niu, S.-W., Hsu, J.-F., Lee, T.-H., Chuang, H.-H., Wang, P.-H., Lee, S.-C., Lin, P.-C., Chiu, Y.-W., Wu, C.-H., Huang, M.-S., Hsiao, M., Chen, H.-C., & Yang, C.-J. (2020). Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice. Cancers, 12(12), 3561. https://doi.org/10.3390/cancers12123561







