The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
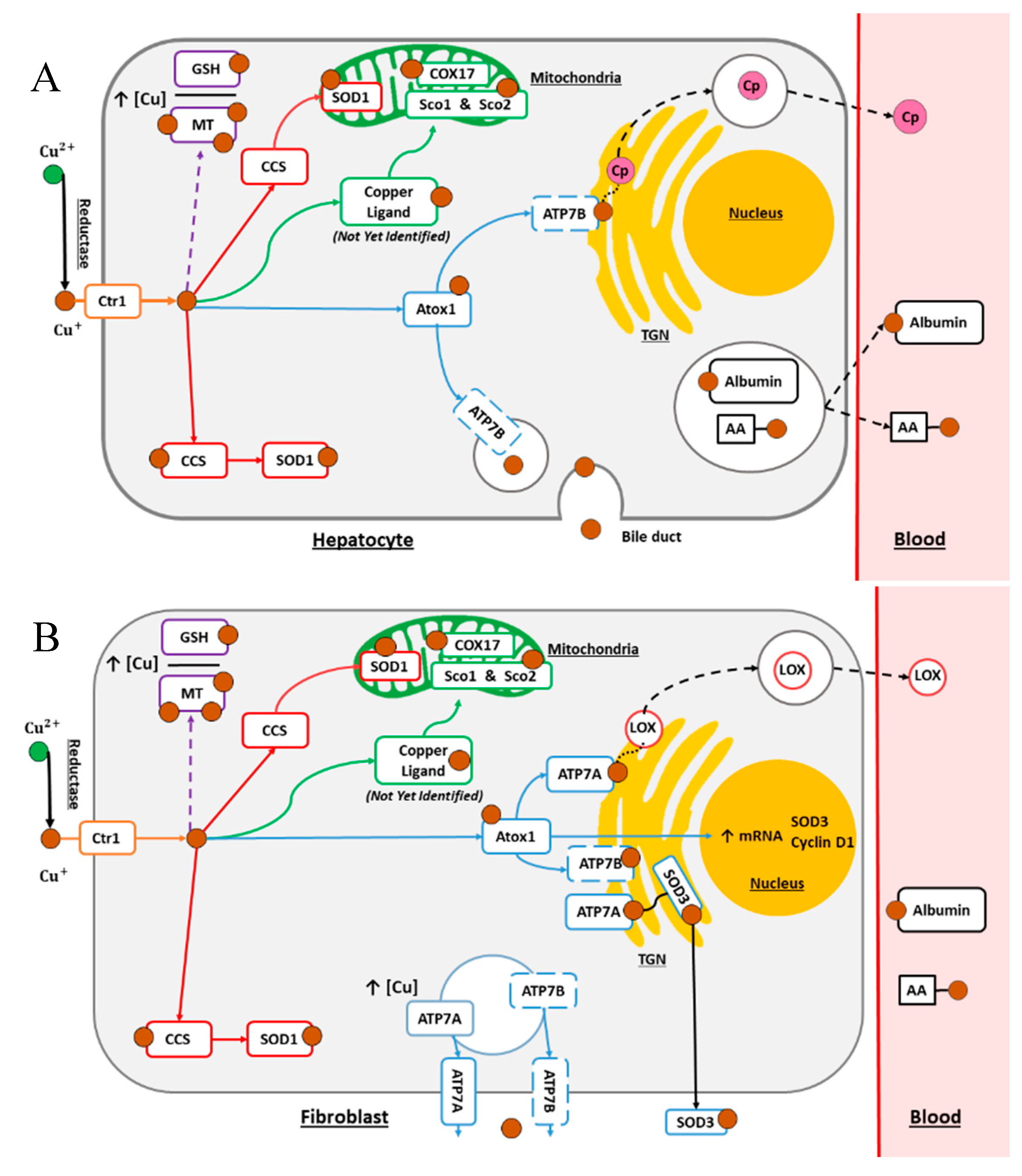
2. Copper Normal Metabolism
2.1. The Secretory Pathway
2.2. The Cytosolic Pathway
2.3. The Mitochondrial Pathway
2.4. Cellular Processes for Metal Detoxification
3. Copper Metabolism in Cancers
4. The Use of Copper Proteins as Cancer Biomarkers
4.1. Involvement of Copper Metabolism Proteins in Metastasis Formation
4.2. Role of Copper Metabolism in Drug Resistance
4.3. Implication of Copper Homeostasis Protein in the Proliferation and Growth of Cancer Cells
4.4. Deregulation of Other Copper-Dependent Proteins in Cancers
5. Copper as a Target or Bullet for Cancer Treatment
5.1. Copper Chelation-Based Treatment Strategies
5.2. Copper-Based Nanoparticles and Metal-Based Strategies
5.3. Targeting Copper Metabolism Proteins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turnlund, J.R.; Keyes, W.R.; Anderson, H.L.; Acord, L.L. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am. J. Clin. Nutr. 1989, 49, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. Ceruloplasmin and other copper binding components of blood plasma and their functions: An update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Celauro, E.; Hildesjö, C.; Feizi, A.; Stål, O.; Fierro-González, J.C.; Wittung-Stafshede, P. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics 2017, 9, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.F.; Sudhahar, V.; Youn, S.W.; Das, A.; Cho, J.; Kamiya, T.; Urao, N.; McKinney, R.D.; Surenkhuu, B.; Hamakubo, T.; et al. Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function. Sci. Rep. 2015, 5, 14780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiber, I.; Dringen, R.; Mercer, J.F.B. Copper: Effects of Deficiency and Overload. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 359–387. ISBN 978-94-007-7499-5. [Google Scholar]
- vandenBerghe, P.V.E.; Folmer, D.E.; Malingré, H.E.M.; vanBeurden, E.; Klomp, A.E.M.; vandeSluis, B.; Merkx, M.; Berger, R.; Klomp, L.W.J. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem. J. 2007, 407, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Liebig, J.K.; Tsigelny, I.F.; Howell, S.B. The copper transporter 1 (CTR1) is required to maintain the stability of copper transporter 2 (CTR2). Metallomics 2015, 7, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Öhrvik, H.; Nose, Y.; Wood, L.K.; Kim, B.E.; Gleber, S.C.; Ralle, M.; Thiele, D.J. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. USA 2013, 110, E4279–E4288. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, T.; Takeuchi, K.; Fukudome, S.; Hara, H.; Adachi, T. Copper chaperone antioxidant-1, Atox-1, is involved in the induction of SOD3 in THP-1 cells. BioMetals 2018, 31, 61–68. [Google Scholar] [CrossRef]
- Panchenko, M.V.; Stetler-Stevenson, W.G.; Trubetskoy, O.V.; Gacheru, S.N.; Kagan, H.M. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. J. Biol. Chem. 1996, 271, 7113–7119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trackman, P.C.; Bedell-Hogan, D.; Tang, J.; Kagan, H.M. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J. Biol. Chem. 1992, 267, 8666–8671. [Google Scholar] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem. Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Scheiber, I.F.; Brůha, R.; Dušek, P. Pathogenesis of Wilson disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 142, pp. 43–55. ISBN 978-0-444-63625-6. [Google Scholar]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef] [PubMed]
- Vashchenko, G.; MacGillivray, R. Multi-Copper Oxidases and Human Iron Metabolism. Nutrients 2013, 5, 2289–2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, Z.L.; Durley, A.P.; Man, T.K.; Gitlin, J.D. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl. Acad. Sci. USA 1999, 96, 10812–10817. [Google Scholar] [CrossRef] [Green Version]
- Turski, M.L.; Thiele, D.J. New Roles for Copper Metabolism in Cell Proliferation, Signaling, and Disease. J. Biol. Chem. 2009, 284, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primer 2018, 4, 21. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef]
- Grubman, A.; White, A.R. Copper as a key regulator of cell signalling pathways. Expert Rev. Mol. Med. 2014, 16, e11. [Google Scholar] [CrossRef] [PubMed]
- Baker, Z.N.; Cobine, P.A.; Leary, S.C. The mitochondrion: A central architect of copper homeostasis. Met. Integr. Biometal Sci. 2017, 9, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.A.; Pierrel, F.; Bestwick, M.L.; Winge, D.R. Mitochondrial Matrix Copper Complex Used in Metallation of Cytochrome Oxidase and Superoxide Dismutase. J. Biol. Chem. 2006, 281, 36552–36559. [Google Scholar] [CrossRef] [Green Version]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018, 293, 1887–1896. [Google Scholar] [CrossRef] [Green Version]
- Zischka, H.; Einer, C. Mitochondrial copper homeostasis and its derailment in Wilson disease. Int. J. Biochem. Cell Biol. 2018, 102, 71–75. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Polishchuk, R.S. The emerging role of lysosomes in copper homeostasis. Met. Integr. Biometal Sci. 2016, 8, 853–862. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Concilli, M.; Iacobacci, S.; Chesi, G.; Pastore, N.; Piccolo, P.; Paladino, S.; Baldantoni, D.; van IJzendoorn, S.C.D.; Chan, J.; et al. Wilson Disease Protein ATP7B Utilizes Lysosomal Exocytosis to Maintain Copper Homeostasis. Dev. Cell 2014, 29, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef]
- Gomes, I.M.; Maia, C.J.; Santos, C.R. STEAP Proteins: From Structure to Applications in Cancer Therapy. Mol. Cancer Res. 2012, 10, 573–587. [Google Scholar] [CrossRef] [Green Version]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J.H. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am. J. Physiol. Cell Physiol. 2013, 304, C768–C779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.T.; Bourassa, D.; Harankhedkar, S.; McCallum, A.M.; Zlatic, S.A.; Calvo, J.S.; Meloni, G.; Faundez, V.; Fahrni, C.J. Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells. Proc. Natl. Acad. Sci. USA 2019, 116, 12167–12172. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Pierdomenico, S.D.; Costantini, F.; Romano, F.; De Cesare, D.; Cuccurullo, F.; Imbastaro, T.; Riario-Sforza, G.; Di Giacomo, F.; Zuliani, G.; et al. Copper/zinc ratio and systemic oxidant load: Effect of aging and aging-related degenerative diseases. Free Radic. Biol. Med. 1998, 25, 676–681. [Google Scholar] [CrossRef]
- Mao, S.; Huang, S. Zinc and Copper Levels in Bladder Cancer: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2013, 153, 5–10. [Google Scholar] [CrossRef]
- Juloski, J.T.; Rakic, A.; Ćuk, V.V.; Ćuk, V.M.; Stefanović, S.; Nikolić, D.; Janković, S.; Trbovich, A.M.; De Luka, S.R. Colorectal cancer and trace elements alteration. J. Trace Elem. Med. Biol. 2020, 59, 126451. [Google Scholar] [CrossRef]
- Zowczak, M.; Iskra, M.; Torliński, L.; Cofta, S. Analysis of Serum Copper and Zinc Concentrations in Cancer Patients. Biol. Trace Elem. Res. 2001, 82, 001–008. [Google Scholar] [CrossRef]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yücel, I.; Arpaci, F.; Özet, A.; Döner, B.; Karayilanoĝlu, T.; Sayar, A.; Berk, Ö. Serum copper and zinc levels and copper/zinc ratio in patients with breast cancer. Biol. Trace Elem. Res. 1994, 40, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel, Z.; Naghibalhossaini, F.; Abdollahi, K.; Shojaei, S.; Moradi, M.; Malekzadeh, M. Serum Copper and Zinc Levels Among Iranian Colorectal Cancer Patients. Biol. Trace Elem. Res. 2016, 170, 294–299. [Google Scholar] [CrossRef]
- Fabris, C.; Farini, R.; Del Favero, G.; Gurrieri, G.; Piccoli, A.; Sturniolo, G.C.; Panucci, A.; Naccarato, R. Copper, zinc and copper/zinc ratio in chronic pancreatitis and pancreatic cancer. Clin. Biochem. 1985, 18, 373–375. [Google Scholar] [CrossRef]
- Cunzhi, H.; Jiexian, J.; Xianwen, Z.; Jingang, G.; Shumin, Z.; Lili, D. Serum and Tissue Levels of Six Trace Elements and Copper/Zinc Ratio in Patients with Cervical Cancer and Uterine Myoma. Biol. Trace Elem. Res. 2003, 94, 113–122. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Gózdz, S. Selenium, Copper, and Zinc Concentrations in Intestinal Cancer Tissue and in Colon and Rectum Polyps. Biol. Trace Elem. Res. 2003, 92, 1–10. [Google Scholar] [CrossRef]
- Jouybari, L.; Kiani, F.; Islami, F.; Sanagoo, A.; Sayehmiri, F.; Hosnedlova, B.; Doşa, M.D.; Kizek, R.; Chirumbolo, S.; Bjørklund, G. Copper Concentrations in Breast Cancer: A Systematic Review and Meta-Analysis. Curr. Med. Chem. 2019, 26. [Google Scholar] [CrossRef]
- Lavilla, I.; Costas, M.; Miguel, P.S.; Millos, J.; Bendicho, C. Elemental fingerprinting of tumorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis. BioMetals 2009, 22, 863–875. [Google Scholar] [CrossRef]
- Díez, M.; Arroyo, M.; Cerdàn, F.J.; Muñoz, M.; Martin, M.A.; Balibrea, J.L. Serum and Tissue Trace Metal Levels in Lung Cancer. Oncology 1989, 46, 230–234. [Google Scholar] [CrossRef]
- Yoshida, D.; Ikeda, Y.; Nakazawa, S. Quantitative analysis of copper, zinc and copper/zinc ratio in selected human brain tumors. J. Neurooncol. 1993, 16, 109–115. [Google Scholar] [CrossRef]
- Callejón-Leblic, B.; Gómez-Ariza, J.L.; Pereira-Vega, A.; García-Barrera, T. Metal dyshomeostasis based biomarkers of lung cancer using human biofluids. Metallomics 2018, 10, 1444–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blockhuys, S.; Malmberg, P.; Wittung-Stafshede, P. Copper distribution in breast cancer cells detected by time-of-flight secondary ion mass spectrometry with delayed extraction methodology. Biointerphases 2018, 13, E412. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Chatterjee, S.; Pal, S.; Biswas, J.; Efferth, T.; Choudhuri, S.K. The role of copper in drug-resistant murine and human tumors. BioMetals 2009, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Télouk, P.; Puisieux, A.; Fujii, T.; Balter, V.; Bondanese, V.P.; Morel, A.P.; Clapisson, G.; Lamboux, A.; Albarede, F. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics 2015, 7, 299–308. [Google Scholar] [CrossRef]
- Barresi, V.; Trovato-Salinaro, A.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis of copper homeostasis genes reveals coordinated upregulation of SLC31A1, SCO 1, and COX11 in colorectal cancer. FEBS Open Bio. 2016, 6, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Bond, G.J.; Tsang, T.; Posimo, J.M.; Busino, L.; Brady, D.C. Copper chaperone ATOX1 is required for MAPK signaling and growth in BRAF mutation-positive melanoma. Metallomics 2019, 11, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.; Das, A.; Krett, N.L.; Guzman, G.; Thomas, A.; Mancinelli, G.; Bauer, J.; Ushio-Fukai, M.; Fukai, T.; Jung, B. Nuclear translocation of Atox1 potentiates activin A-induced cell migration and colony formation in colon cancer. PLoS ONE 2020, 15, e0227916. [Google Scholar] [CrossRef] [Green Version]
- Crnogorac–Jurcevic, T.; Gangeswaran, R.; Bhakta, V.; Capurso, G.; Lattimore, S.; Akada, M.; Sunamura, M.; Prime, W.; Campbell, F.; Brentnall, T.A.; et al. Proteomic Analysis of Chronic Pancreatitis and Pancreatic Adenocarcinoma. Gastroenterology 2005, 129, 1454–1463. [Google Scholar] [CrossRef]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Qiu, M.; Zeng, Z.; Luo, H.; Wu, W.; Wang, F.; Wang, Z.; Zhang, D.; Li, Y.; Xu, R. Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC). J. Transl. Med. 2012, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Owatari, S.; Akune, S.; Komatsu, M.; Ikeda, R.; Firth, S.D.; Che, X.F.; Yamamoto, M.; Tsujikawa, K.; Kitazono, M.; Ishizawa, T.; et al. Copper-Transporting P-Type ATPase, ATP7A, Confers Multidrug Resistance and Its Expression Is Related to Resistance to SN-38 in Clinical Colon Cancer. Cancer Res. 2007, 67, 4860–4868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samimi, G.; Safaei, R.; Katano, K.; Holzer, A.K.; Rochdi, M.; Tomioka, M.; Goodman, M.; Howell, S.B. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 4661–4669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samimi, G.; Varki, N.M.; Wilczynski, S.; Safaei, R.; Alberts, D.S.; Howell, S.B. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin. Cancer Res. 2003, 9, 5853–5859. [Google Scholar] [PubMed]
- Higashimoto, M.; Kanzaki, A.; Shimakawa, T.; Konno, S.; Naritaka, Y.; Nitta, Y.; Mori, S.; Shirata, S.; Yoshida, A.; Terada, K.; et al. Expression of copper-transporting P-type adenosine triphosphatase in human esophageal carcinoma. Int. J. Mol. Med. 2003, 11, 337–341. [Google Scholar] [CrossRef]
- Aida, T.; Takebayashi, Y.; Shimizu, T.; Okamura, C.; Higasimoto, M.; Kanzaki, A.; Nakayama, K.; Terada, K.; Sugiyama, T.; Miyazaki, K.; et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a prognostic factor in human endometrial carcinoma. Gynecol. Oncol. 2005, 97, 41–45. [Google Scholar] [CrossRef]
- Kanzaki, A.; Toi, M.; Neamati, N.; Miyashita, H.; Oubu, M.; Nakayama, K.; Bando, H.; Ogawa, K.; Mutoh, M.; Mori, S.; et al. Copper-transporting P-Type Adenosine Triphosphatase (ATP7B) Is Expressed in Human Breast Carcinoma. Jpn. J. Cancer Res. 2002, 93, 70–77. [Google Scholar] [CrossRef]
- Miyashita, H.; Nitta, Y.; Mori, S.; Kanzaki, A.; Nakayama, K.; Terada, K.; Sugiyama, T.; Kawamura, H.; Sato, A.; Morikawa, H.; et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker in human oral squamous cell carcinoma treated with cisplatin. Oral Oncol. 2003, 39, 157–162. [Google Scholar] [CrossRef]
- Ohbu, M.; Ogawa, K.; Konno, S.; Kanzaki, A.; Terada, K.; Sugiyama, T.; Takebayashi, Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human gastric carcinoma. Cancer Lett. 2003, 189, 33–38. [Google Scholar] [CrossRef]
- Sugeno, H.; Takebayashi, Y.; Higashimoto, M.; Ogura, Y.; Shibukawa, G.; Kanzaki, A.; Terada, K.; Sugiyama, T.; Watanabe, K.; Katoh, R.; et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) in human hepatocellular carcinoma. Anticancer Res. 2004, 24, 1045–1048. [Google Scholar]
- Zhang, J.; Zhang, L.; Li, C.; Yang, C.; Li, L.; Song, S.; Wu, H.; Liu, F.; Wang, L.; Gu, J. LOX-1 is a poor prognostic indicator and induces epithelial-mesenchymal transition and metastasis in pancreatic cancer patients. Cell. Oncol. 2018, 41, 73–84. [Google Scholar] [CrossRef]
- González-Chavarría, I.; Fernandez, E.; Gutierrez, N.; González-Horta, E.E.; Sandoval, F.; Cifuentes, P.; Castillo, C.; Cerro, R.; Sanchez, O.; Toledo, J.R. LOX-1 activation by oxLDL triggers an epithelial mesenchymal transition and promotes tumorigenic potential in prostate cancer cells. Cancer Lett. 2018, 414, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Cox, T.R.; Bird, D.; Lang, G.; Murray, G.I.; Sun, X.F.; Southall, S.M.; Wilson, J.R.; Erler, J.T. The Role of Lysyl Oxidase in SRC-Dependent Proliferation and Metastasis of Colorectal Cancer. J. Natl. Cancer Inst. 2011, 103, 407–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.C.L.; Tse, A.P.W.; Huang, Y.P.; Zhu, Y.T.; Chiu, D.K.C.; Lai, R.K.H.; Au, S.L.K.; Kai, A.K.L.; Lee, J.M.F.; Wei, L.L.; et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology 2014, 60, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Kirschmann, D.A.; Seftor, E.A.; Fong, S.F.T.; Nieva, D.R.C.; Sullivan, C.M.; Edwards, E.M.; Sommer, P.; Csiszar, K.; Hendrix, M.J.C. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002, 62, 4478–4483. [Google Scholar]
- Erler, J.T.; Bennewith, K.L.; Cox, T.R.; Lang, G.; Bird, D.; Koong, A.; Le, Q.T.; Giaccia, A.J. Hypoxia-Induced Lysyl Oxidase Is a Critical Mediator of Bone Marrow Cell Recruitment to Form the Premetastatic Niche. Cancer Cell 2009, 15, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Postovit, L.M.; Abbott, D.E.; Payne, S.L.; Wheaton, W.W.; Margaryan, N.V.; Sullivan, R.; Jansen, M.K.; Csiszar, K.; Hendrix, M.J.C.; Kirschmann, D.A. Hypoxia/reoxygenation: A dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J. Cell. Biochem. 2008, 103, 1369–1378. [Google Scholar] [CrossRef]
- Woznick, A.R.; Braddock, A.L.; Dulai, M.; Seymour, M.L.; Callahan, R.E.; Welsh, R.J.; Chmielewski, G.W.; Zelenock, G.B.; Shanley, C.J. Lysyl oxidase expression in bronchogenic carcinoma. Am. J. Surg. 2005, 189, 297–301. [Google Scholar] [CrossRef]
- Kaneda, A.; Wakazono, K.; Tsukamoto, T.; Watanabe, N.; Yagi, Y.; Tatematsu, M.; Kaminishi, M.; Sugimura, T.; Ushijima, T. Lysyl Oxidase Is a Tumor Suppressor Gene Inactivated by Methylation and Loss of Heterozygosity in Human Gastric Cancers. Cancer Res. 2004, 64, 6410–6415. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.T.; Harris, J.; Magliocco, A.M.; Kong, C.S.; Diaz, R.; Shin, B.; Cao, H.; Trotti, A.; Erler, J.T.; Chung, C.H.; et al. Validation of Lysyl Oxidase As a Prognostic Marker for Metastasis and Survival in Head and Neck Squamous Cell Carcinoma: Radiation Therapy Oncology Group Trial 90-03. J. Clin. Oncol. 2009, 27, 4281–4286. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xiao, Q.; Ma, H.; Li, L.; Liu, J.; Feng, Y.; Fang, Z.; Wu, J.; Han, X.; Zhang, J.; et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 18892–18897. [Google Scholar] [CrossRef] [Green Version]
- Wilgus, M.L.; Borczuk, A.C.; Stoopler, M.; Ginsburg, M.; Gorenstein, L.; Sonett, J.R.; Powell, C.A. Lysyl oxidase: A lung adenocarcinoma biomarker of invasion and survival. Cancer 2011, 117, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Albinger-Hegyi, A.; Stoeckli, S.J.; Schmid, S.; Storz, M.; Iotzova, G.; Probst-Hensch, N.M.; Rehrauer, H.; Tinguely, M.; Moch, H.; Hegyi, I. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC). Int. J. Cancer 2010. [Google Scholar] [CrossRef] [Green Version]
- Bouez, C. The Lysyl Oxidase LOX Is Absent in Basal and Squamous Cell Carcinomas and Its Knockdown Induces an Invading Phenotype in a Skin Equivalent Model. Clin. Cancer Res. 2006, 12, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassar, M.J.J.G.; Devitt, G.; Brosius, M.; Rinnab, L.; Prang, J.; Schradin, T.; Simon, J.; Petersen, S.; Kopp-Schneider, A.; Zöller, M. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br. J. Cancer 2001, 85, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Guo, Z.; Chang, X.; Kim, M.S.; Nagpal, J.K.; Liu, J.; Maki, J.M.; Kivirikko, K.I.; Ethier, S.P.; Trink, B.; et al. LOXL1 and LOXL4 Are Epigenetically Silenced and Can Inhibit Ras/Extracellular Signal-Regulated Kinase Signaling Pathway in Human Bladder Cancer. Cancer Res. 2007, 67, 4123–4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef]
- Bell, A.; Bell, D.; Weber, R.S.; El-Naggar, A.K. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer 2011, 117, 2898–2909. [Google Scholar] [CrossRef] [Green Version]
- Peinado, H.; Moreno-Bueno, G.; Hardisson, D.; Perez-Gomez, E.; Santos, V.; Mendiola, M.; de Diego, J.I.; Nistal, M.; Quintanilla, M.; Portillo, F.; et al. Lysyl Oxidase-Like 2 as a New Poor Prognosis Marker of Squamous Cell Carcinomas. Cancer Res. 2008, 68, 4541–4550. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Bueno, G.; Salvador, F.; Martín, A.; Floristán, A.; Cuevas, E.P.; Santos, V.; Montes, A.; Morales, S.; Castilla, M.A.; Rojo-Sebastián, A.; et al. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol. Med. 2011, 3, 528–544. [Google Scholar] [CrossRef] [Green Version]
- Hollosi, P.; Yakushiji, J.K.; Fong, K.S.K.; Csiszar, K.; Fong, S.F.T. Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int. J. Cancer 2009, 125, 318–327. [Google Scholar] [CrossRef]
- Brekhman, V.; Lugassie, J.; Zaffryar-Eilot, S.; Sabo, E.; Kessler, O.; Smith, V.; Golding, H.; Neufeld, G. Receptor activity modifying protein-3 mediates the protumorigenic activity of lysyl oxidase-like protein-2. FASEB J. 2011, 25, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric inhibition of lysyl oxidase–like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Kim Differential expression of the LOX family genes in human colorectal adenocarcinomas. Oncol. Rep. 2009, 22. [CrossRef] [Green Version]
- Offenberg, H.; Brünner, N.; Mansilla, F.; Ørntoft Torben, F.; Birkenkamp-Demtroder, K. TIMP-1 expression in human colorectal cancer is associated with TGF-B1, LOXL2, INHBA1, TNF-AIP6 and TIMP-2 transcript profiles. Mol. Oncol. 2008, 2, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Macartney-Coxson, D.P.; Hood, K.A.; Shi, H.; Ward, T.; Wiles, A.; O’Connor, R.; Hall, D.A.; Lea, R.A.; Royds, J.A.; Stubbs, R.S.; et al. Metastatic susceptibility locus, an 8p hot-spot for tumour progression disrupted in colorectal liver metastases: 13 candidate genes examined at the DNA, mRNA and protein level. BMC Cancer 2008, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Ran, Y.L.; Hu, H.; Yu, L.; Liu, Q.; Zhou, Z.; Sun, Y.M.; Sun, L.C.; Pan, J.; Sun, L.X.; et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis 2009, 30, 1660–1669. [Google Scholar] [CrossRef] [Green Version]
- Zhan, P.; Shen, X.; Qian, Q.; Zhu, J.; Zhang, Y.; Xie, H.Y.; Xu, C.H.; Hao, K.; Hu, W.; Xia, N.; et al. Down-regulation of lysyl oxidase-like 2 (LOXL2) is associated with disease progression in lung adenocarcinomas. Med. Oncol. 2012, 29, 648–655. [Google Scholar] [CrossRef]
- Rückert, F.; Joensson, P.; Saeger, H.D.; Grützmann, R.; Pilarsky, C. Functional analysis of LOXL2 in pancreatic carcinoma. Int. J. Colorectal Dis. 2010, 25, 303–311. [Google Scholar] [CrossRef]
- Schmidt, H.; Semjonow, A.; Csiszar, K.; Korsching, E.; Brandt, B.; Eltze, E. Mapping of a deletion interval on 8p21-22 in prostate cancer by gene dosage PCR. Verh. Dtsch. Ges. Pathol. 2007, 91, 302–307. [Google Scholar]
- Sano, M.; Aoyagi, K.; Takahashi, H.; Kawamura, T.; Mabuchi, T.; Igaki, H.; Tachimori, Y.; Kato, H.; Ochiai, A.; Honda, H.; et al. Forkhead box A1 transcriptional pathway in KRT7-expressing esophageal squamous cell carcinomas with extensive lymph node metastasis. Int. J. Oncol. 2010, 36, 321–330. [Google Scholar] [CrossRef]
- Peinado, H.; del Carmen Iglesias-de la Cruz, M.; Olmeda, D.; Csiszar, K.; Fong, K.S.K.; Vega, S.; Nieto, M.A.; Cano, A.; Portillo, F. A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. EMBO J. 2005, 24, 3446–3458. [Google Scholar] [CrossRef] [Green Version]
- Scola, N.; Görögh, T. LOXL4 as a selective molecular marker in primary and metastatic head/neck carcinoma. Anticancer Res. 2010, 30, 4567–4571. [Google Scholar] [PubMed]
- Holtmeier, C.; Görögh, T.; Beier, U.; Meyer, J.; Hoffmann, M.; Gottschlich, S.; Heidorn, K.; Ambrosch, P.; Maune, S. Overexpression of a novel lysyl oxidase-like gene in human head and neck squamous cell carcinomas. Anticancer Res. 2003, 23, 2585–2591. [Google Scholar] [PubMed]
- Weise, J.B.; Csiszar, K.; Gottschlich, S.; Hoffmann, M.; Schmidt, A.; Weingartz, U.; Adamzik, I.; Heiser, A.; Kabelitz, D.; Ambrosch, P.; et al. Vaccination strategy to target lysyl oxidase-like 4 in dendritic cell based immunotherapy for head and neck cancer. Int. J. Oncol. 2008, 32, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.H.W.; Yan, J.J.; Chen, W.C.; Kuo, M.T.; Lai, Y.H.; Lai, W.W.; Liu, H.S.; Su, W.C. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer 2012, 75, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.Y.; Choi, C.H.; Do, I.G.; Song, S.Y.; Lee, W.; Park, H.S.; Song, T.J.; Kim, M.K.; Kim, T.J.; Lee, J.W.; et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol. Oncol. 2011, 122, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.K.; Samimi, G.; Katano, K.; Naerdemann, W.; Lin, X.; Safaei, R.; Howell, S.B. The Copper Influx Transporter Human Copper Transport Protein 1 Regulates the Uptake of Cisplatin in Human Ovarian Carcinoma Cells. Mol. Pharmacol. 2004, 66, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeoti, M.; Oguntola, A.; Akanni, E.; Agodirin, O.; Oyeyemi, G. Trace elements; copper, zinc and selenium, in breast cancer afflicted female patients in LAUTECH Osogbo, Nigeria. Indian J. Cancer 2015, 52, 106. [Google Scholar] [CrossRef]
- Dragutinović, V.V.; Tatić, S.B.; Nikolić-Mandić, S.D.; Tripković, T.M.; Dunđerović, D.M.; Paunović, I.R. Copper as Ancillary Diagnostic Tool in Preoperative Evaluation of Possible Papillary Thyroid Carcinoma in Patients with Benign Thyroid Disease. Biol. Trace Elem. Res. 2014, 160, 311–315. [Google Scholar] [CrossRef]
- Atakul, T.; Altinkaya, S.O.; Abas, B.I.; Yenisey, C. Serum Copper and Zinc Levels in Patients with Endometrial Cancer. Biol. Trace Elem. Res. 2020, 195, 46–54. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Chen, C.; Zeng, Z.; Yang, H.; Oh, J.; Chen, L.; Lu, S.C. Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver regeneration. FASEB J. 2001, 15, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Teoh-Fitzgerald, M.L.T.; Fitzgerald, M.P.; Jensen, T.J.; Futscher, B.W.; Domann, F.E. Genetic and Epigenetic Inactivation of Extracellular Superoxide Dismutase Promotes an Invasive Phenotype in Human Lung Cancer by Disrupting ECM Homeostasis. Mol. Cancer Res. 2012, 10, 40–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, D.G.; Song, Y.J.; Cho, E.J.; Lee, S.K.; Park, J.B.; Yu, J.H.; Lim, S.P.; Kim, J.M.; Jeon, B.H. Alteration of APE1/ref-1 expression in non-small cell lung cancer: The implications of impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer 2008, 60, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Svensk, A.M.; Soini, Y.; Pääkkö, P.; Hirvikoski, P.; Kinnula, V.L. Differential Expression of Superoxide Dismutases in Lung Cancer. Am. J. Clin. Pathol. 2004, 122, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Teoh-Fitzgerald, M.L.; Fitzgerald, M.P.; Zhong, W.; Askeland, R.W.; Domann, F.E. Epigenetic reprogramming governs EcSOD expression during human mammary epithelial cell differentiation, tumorigenesis and metastasis. Oncogene 2014, 33, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubackova, M.; Vaclavikova, R.; Ehrlichova, M.; Mrhalova, M.; Kodet, R.; Kubackova, K.; Vrána, D.; Gut, I.; Soucek, P. Association of superoxide dismutases and NAD(P)H quinone oxidoreductases with prognosis of patients with breast carcinomas. Int. J. Cancer 2012, 130, 338–348. [Google Scholar] [CrossRef]
- Chaiswing, L.; Zhong, W.; Oberley, T.D. Increasing discordant antioxidant protein levels and enzymatic activities contribute to increasing redox imbalance observed during human prostate cancer progression. Free Radic. Biol. Med. 2014, 67, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Mizokami, A.; Shin, M.; Izumi, K.; Konaka, H.; Kadono, Y.; Kitagawa, Y.; Keller, E.T.; Zhang, J.; Namiki, M. SOD3 acts as a tumor suppressor in PC-3 prostate cancer cells via hydrogen peroxide accumulation. Anticancer Res. 2014, 34, 2821–2831. [Google Scholar]
- O’Leary, B.R.; Fath, M.A.; Bellizzi, A.M.; Hrabe, J.E.; Button, A.M.; Allen, B.G.; Case, A.J.; Altekruse, S.; Wagner, B.A.; Buettner, G.R.; et al. Loss of SOD3 (EcSOD) Expression Promotes an Aggressive Phenotype in Human Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2015, 21, 1741–1751. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xu, Y.; Meng, Q.; Zheng, Q.; Wu, J.; Wang, C.; Jia, W.; Figeys, D.; Chang, Y.; Zhou, H. Proteomic analysis of minute amount of colonic biopsies by enteroscopy sampling. Biochem. Biophys. Res. Commun. 2016, 476, 286–292. [Google Scholar] [CrossRef]
- Parascandolo, A.; Rappa, F.; Cappello, F.; Kim, J.; Cantu, D.A.; Chen, H.; Mazzoccoli, G.; Hematti, P.; Castellone, M.D.; Salvatore, M.; et al. Extracellular Superoxide Dismutase Expression in Papillary Thyroid Cancer Mesenchymal Stem/Stromal Cells Modulates Cancer Cell Growth and Migration. Sci. Rep. 2017, 7, 41416. [Google Scholar] [CrossRef] [Green Version]
- Subbannayya, Y.; Mir, S.A.; Renuse, S.; Manda, S.S.; Pinto, S.M.; Puttamallesh, V.N.; Solanki, H.S.; Manju, H.C.; Syed, N.; Sharma, R.; et al. Identification of differentially expressed serum proteins in gastric adenocarcinoma. J. Proteom. 2015, 127, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, C.; Daigo, Y.; Kikuchi, T.; Katagiri, T.; Nakamura, Y. Identification of COX17 as a therapeutic target for non-small cell lung cancer. Cancer Res. 2003, 63, 7038–7041. [Google Scholar] [PubMed]
- Prusinkiewicz, M.A.; Gameiro, S.F.; Ghasemi, F.; Dodge, M.J.; Zeng, P.Y.F.; Maekebay, H.; Barrett, J.W.; Nichols, A.C.; Mymryk, J.S. Survival-Associated Metabolic Genes in Human Papillomavirus-Positive Head and Neck Cancers. Cancers 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blockhuys, S.; Wittung-Stafshede, P. Copper chaperone Atox1 plays role in breast cancer cell migration. Biochem. Biophys. Res. Commun. 2017, 483, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Brady, D.C.; Wittung-Stafshede, P. Evaluation of copper chaperone ATOX1 as prognostic biomarker in breast cancer. Breast Cancer 2020, 27, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Blockhuys, S.; Zhang, X.; Wittung-Stafshede, P. Single-cell tracking demonstrates copper chaperone Atox1 to be required for breast cancer cell migration. Proc. Natl. Acad. Sci. USA 2020, 117, 2014–2019. [Google Scholar] [CrossRef] [Green Version]
- Ashino, T.; Kohno, T.; Sudhahar, V.; Ash, D.; Ushio-Fukai, M.; Fukai, T. Copper transporter ATP7A interacts with IQGAP1, a Rac1 binding scaffolding protein: Role in PDGF-induced VSMC migration and vascular remodeling. Am. J. Physiol. Cell Physiol. 2018, 315, C850–C862. [Google Scholar] [CrossRef]
- An Online Survival Analysis Tool to Rapidly Assess the Effect of 22,277 Genes on Breast Cancer Prognosis Using Microarray Data of 1,809 Patients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20020197/ (accessed on 3 November 2020).
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. 2012, 5, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.M.; Bird, D.; Lang, G.; Cox, T.R.; Erler, J.T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 2013, 32, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.L.; Fogelgren, B.; Hess, A.R.; Seftor, E.A.; Wiley, E.L.; Fong, S.F.T.; Csiszar, K.; Hendrix, M.J.C.; Kirschmann, D.A. Lysyl Oxidase Regulates Breast Cancer Cell Migration and Adhesion through a Hydrogen Peroxide–Mediated Mechanism. Cancer Res. 2005, 65, 11429–11436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lian, S.; Cui, X.; Meng, K.; Győrffy, B.; Jin, T.; Huang, D. Potential options for managing LOX+ ER− breast cancer patients. Oncotarget 2016, 7, 32893–32901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanbhag, V.; Jasmer-McDonald, K.; Zhu, S.; Martin, A.L.; Gudekar, N.; Khan, A.; Ladomersky, E.; Singh, K.; Weisman, G.A.; Petris, M.J. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 6836–6841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Musulén, E.; Ginés, A.; Manzano, J.L.; Aranda, E.; Plasencia, C.; Neamati, N.; Abad, A. Increased levels of copper efflux transporter ATP7B are associated with poor outcome in colorectal cancer patients receiving oxaliplatin-based chemotherapy. Int. J. Cancer 2009, 124, 2905–2910. [Google Scholar] [CrossRef]
- Nakagawa, T.; Inoue, Y.; Kodama, H.; Yamazaki, H.; Kawai, K.; Suemizu, H.; Masuda, R.; Iwazaki, M.; Yamada, S.; Ueyama, Y.; et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) correlates with cisplatin resistance in human non-small cell lung cancer xenografts. Oncol. Rep. 2008, 20, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K. Prognostic Value of the Cu-Transporting ATPase in Ovarian Carcinoma Patients Receiving Cisplatin-Based Chemotherapy. Clin. Cancer Res. 2004, 10, 2804–2811. [Google Scholar] [CrossRef] [Green Version]
- Godwin, A.K.; Meister, A.; O’Dwyer, P.J.; Huang, C.S.; Hamilton, T.C.; Anderson, M.E. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl. Acad. Sci. USA 1992, 89, 3070–3074. [Google Scholar] [CrossRef] [Green Version]
- Jamali, B.; Nakhjavani, M.; Hosseinzadeh, L.; Amidi, S.; Nikounezhad, N.; Shirazi, F.H. Intracellular GSH Alterations and Its Relationship to Level of Resistance following Exposure to Cisplatin in Cancer Cells. Iran. J. Pharm. Res. 2015, 14, 513–519. [Google Scholar]
- Holzer, A.K.; Varki, N.M.; Le, Q.T.; Gibson, M.A.; Naredi, P.; Howell, S.B. Expression of the Human Copper Influx Transporter 1 in Normal and Malignant Human Tissues. J. Histochem. Cytochem. 2006, 54, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Song, I.S.; Savaraj, N.; Siddik, Z.H.; Liu, P.; Wei, Y.; Wu, C.J.; Kuo, M.T. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol. Cancer Ther. 2004, 3, 1543–1549. [Google Scholar] [PubMed]
- Liang, Z.D.; Stockton, D.; Savaraj, N.; Tien Kuo, M. Mechanistic Comparison of Human High-Affinity Copper Transporter 1-Mediated Transport between Copper Ion and Cisplatin. Mol. Pharmacol. 2009, 76, 843–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, S.; McCormick, F.; Smith-McCune, K.; Hanahan, D. Enhancing Tumor-Specific Uptake of the Anticancer Drug Cisplatin with a Copper Chelator. Cancer Cell 2010, 17, 574–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, S.B.; Safaei, R.; Larson, C.A.; Sailor, M.J. Copper Transporters and the Cellular Pharmacology of the Platinum-Containing Cancer Drugs. Mol. Pharmacol. 2010, 77, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, S.; Wang, E.; Tong, Y.; Ma, G.; Wei, K.; Liu, Y. Platinum transfer from hCTR1 to Atox1 is dependent on the type of platinum complex. Metallomics 2017, 9, 546–555. [Google Scholar] [CrossRef]
- Arnesano, F.; Banci, L.; Bertini, I.; Felli, I.C.; Losacco, M.; Natile, G. Probing the Interaction of Cisplatin with the Human Copper Chaperone Atox1 by Solution and In-Cell NMR Spectroscopy. J. Am. Chem. Soc. 2011, 133, 18361–18369. [Google Scholar] [CrossRef]
- Boal, A.K.; Rosenzweig, A.C. Crystal Structures of Cisplatin Bound to a Human Copper Chaperone. J. Am. Chem. Soc. 2009, 131, 14196–14197. [Google Scholar] [CrossRef] [Green Version]
- Tadini-Buoninsegni, F.; Bartolommei, G.; Moncelli, M.R.; Inesi, G.; Galliani, A.; Sinisi, M.; Losacco, M.; Natile, G.; Arnesano, F. Translocation of Platinum Anticancer Drugs by Human Copper ATPases ATP7A and ATP7B. Angew. Chem. Int. Ed. 2014, 53, 1297–1301. [Google Scholar] [CrossRef] [Green Version]
- Dolgova, N.V.; Nokhrin, S.; Yu, C.H.; George, G.N.; Dmitriev, O.Y. Copper chaperone Atox1 interacts with the metal-binding domain of Wilson’s disease protein in cisplatin detoxification. Biochem. J. 2013, 454, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruzzelli, R.; Polishchuk, R.S. Activity and Trafficking of Copper-Transporting ATPases in Tumor Development and Defense against Platinum-Based Drugs. Cells 2019, 8, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katano, K.; Kondo, A.; Safaei, R.; Holzer, A.; Samimi, G.; Mishima, M.; Kuo, Y.M.; Rochdi, M.; Howell, S.B. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002, 62, 6559–6565. [Google Scholar] [PubMed]
- Kalayda, G.V.; Wagner, C.H.; Buss, I.; Reedijk, J.; Jaehde, U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 2008, 8, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.P.; Fofana, M.; Chan, J.; Chang, C.J.; Howell, S.B. Copper transporter 2 regulates intracellular copper and sensitivity to cisplatin. Met. Integr. Biometal Sci. 2014, 6, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Mariniello, M.; Petruzzelli, R.; Wanderlingh, L.G.; La Montagna, R.; Carissimo, A.; Pane, F.; Amoresano, A.; Ilyechova, E.Y.; Galagudza, M.M.; Catalano, F.; et al. Synthetic Lethality Screening Identifies FDA-Approved Drugs that Overcome ATP7B-Mediated Tolerance of Tumor Cells to Cisplatin. Cancers 2020, 12, 608. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Luo, C.; Shan, C.; You, Q.; Lu, J.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J.L.; Fan, J.; et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 2015, 7, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liang, R.; Zhang, X.; Wang, J.; Shan, C.; Liu, S.; Li, L.; Zhang, S. Copper Chaperone for Superoxide Dismutase Promotes Breast Cancer Cell Proliferation and Migration via ROS-Mediated MAPK/ERK Signaling. Front. Pharmacol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Cai, H.; Peng, F. Knockdown of copper chaperone antioxidant-1 by RNA interference inhibits copper-stimulated proliferation of non-small cell lung carcinoma cells. Oncol. Rep. 2013, 30, 269–275. [Google Scholar] [CrossRef]
- Griess, B.; Tom, E.; Domann, F.; Teoh-Fitzgerald, M. Extracellular superoxide dismutase and its role in cancer. Free Radic. Biol. Med. 2017, 112, 464–479. [Google Scholar] [CrossRef]
- Soini, Y.; Kallio, J.; Hirvikoski, P.; Helin, H.; Kellokumpu-Lehtinen, P.; Tammela, T.; Peltoniemi, M.; Martikainen, P.; Kinnula, L. Antioxidant enzymes in renal cell carcinoma. Histol. Histopathol. 2005, 157–165. [Google Scholar] [CrossRef]
- Laukkanen, M.O. Extracellular Superoxide Dismutase: Growth Promoter or Tumor Suppressor? Oxid. Med. Cell. Longev. 2016, 2016, 3612589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues From Patients With Wilson Disease and From ATP7B-Deficient Animals, Protects Hepatocytes From Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carretero, J.; Obrador, E.; Anasagasti, M.J.; Martin, J.J.; Vidal-Vanaclocha, F.; Estrela, J.M. Growth-associated changes in glutathione content correlate with liver metastatic activity of B16 melanoma cells. Clin. Exp. Metastasis 1999, 17, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Reznik, E.; Lee, C.H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.J.; Sittadjody, S.; Thangasamy, T.; Mendoza, E.E.; Limesand, K.H.; Burd, R. Exploiting Tyrosinase Expression and Activity in Melanocytic Tumors: Quercetin and the Central Role of p53. Integr. Cancer Ther. 2011, 10, 328–340. [Google Scholar] [CrossRef]
- Yigit, E.; Gönüllü, G.; Yücel, İ.; Turgut, M.; Erdem, D.; Çakar, B. Relation between hemostatic parameters and prognostic/predictive factors in breast cancer. Eur. J. Intern. Med. 2008, 19, 602–607. [Google Scholar] [CrossRef]
- Auwerda, J.J.A.; Sonneveld, P.; de Maat, M.P.M.; Leebeek, F.W.G. Prothrombotic coagulation abnormalities in patients with newly diagnosed multiple myeloma. Haematologica 2007, 92, 279–280. [Google Scholar] [CrossRef] [Green Version]
- Battistelli, S.; Stefanoni, M.; Lorenzi, B.; Dell’avanzato, R.; Varrone, F.; Pascucci, A.; Petrioli, R.; Vittoria, A. Coagulation factor levels in non-metastatic colorectal cancer patients. Int. J. Biol. Markers 2008, 23, 36–41. [Google Scholar] [CrossRef]
- Minnema, M.C.; Fijnheer, R.; De Groot, P.G.; Lokhorst, H.M. Extremely high levels of von Willebrand factor antigen and of procoagulant factor VIII found in multiple myeloma patients are associated with activity status but not with thalidomide treatment: High levels of VWF-ag, FVIII and thalidomide. J. Thromb. Haemost. 2003, 1, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Gieseler, F.; Lühr, I.; Kunze, T.; Mundhenke, C.; Maass, N.; Erhart, T.; Denker, M.; Beckmann, D.; Tiemann, M.; Schulte, C.; et al. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb. Haemost. 2007, 97, 1023–1030. [Google Scholar] [CrossRef]
- Weekley, C.M.; He, C. Developing drugs targeting transition metal homeostasis. Curr. Opin. Chem. Biol. 2017, 37, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Pearson, H.B.; Clatworthy, S.A.S.; Smith, Z.M.; Francis, P.S.; Llanos, R.M.; Volitakis, I.; Phillips, W.A.; Meggyesy, P.M.; Masaldan, S.; et al. Copper as a target for prostate cancer therapeutics: Copper-ionophore pharmacology and altering systemic copper distribution. Oncotarget 2016, 7, 37064–37080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Saura, R.; Hirohata, K.; Ziff, M. Inhibition of human endothelial cell proliferation in vitro and neovascularization in vivo by D-penicillamine. J. Clin. Investig. 1989, 83, 158–167. [Google Scholar] [CrossRef]
- Yoshii, J.; Yoshiji, H.; Kuriyama, S.; Ikenaka, Y.; Noguchi, R.; Okuda, H.; Tsujinoue, H.; Nakatani, T.; Kishida, H.; Nakae, D.; et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer 2001, 94, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, M.; Nakajima, T.; Kimura, H.; Watanabe, T.; Takashima, H.; Mitsumoto, Y.; Katagishi, T.; Okanoue, T.; Kagawa, K. The copper chelator trientine has an antiangiogenic effect against hepatocellular carcinoma, possibly through inhibition of interleukin-8 production. Int. J. Cancer 2002, 102, 445–452. [Google Scholar] [CrossRef]
- Turski, M.L.; Brady, D.C.; Kim, H.J.; Kim, B.E.; Nose, Y.; Counter, C.M.; Winge, D.R.; Thiele, D.J. A Novel Role for Copper in Ras/Mitogen-Activated Protein Kinase Signaling. Mol. Cell. Biol. 2012, 32, 1284–1295. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Greenberg, D.N.; Counter, C.M. Copper Chelation Inhibits BRAF V600E -Driven Melanomagenesis and Counters Resistance to BRAF V600E and MEK1/2 Inhibitors. Cancer Res. 2017, 77, 6240–6252. [Google Scholar] [CrossRef] [Green Version]
- Baldari, S.; Di Rocco, G.; Heffern, M.C.; Su, T.A.; Chang, C.J.; Toietta, G. Effects of Copper Chelation on BRAFV600E Positive Colon Carcinoma Cells. Cancers 2019, 11, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, S.M. Bleomycin: New Perspectives on the Mechanism of Action 1. J. Nat. Prod. 2000, 63, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stubbe, J. Bleomycins: Towards better therapeutics. Nat. Rev. Cancer 2005, 5, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H. Curing metastatic testicular cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 4592–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J.H. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Helsel, M.E.; Franz, K.J. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans. 2015, 44, 8760–8770. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Schimmer, A. The toxicology of Clioquinol. Toxicol. Lett. 2008, 182, 1–6. [Google Scholar] [CrossRef]
- Khan, R.; Khan, H.; Abdullah, Y.; Dou, Q.P. Feasibility of Repurposing Clioquinol for Cancer Therapy. Recent Patents Anticancer Drug Discov. 2020, 15, 14–31. [Google Scholar] [CrossRef]
- Allensworth, J.L.; Evans, M.K.; Bertucci, F.; Aldrich, A.J.; Festa, R.A.; Finetti, P.; Ueno, N.T.; Safi, R.; McDonnell, D.P.; Thiele, D.J.; et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol. Oncol. 2015, 9, 1155–1168. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Hannafon, B.N.; Zhang, R.R.; Fung, K.M.; Ding, W.Q. Docosahexaenoic acid and disulfiram act in concert to kill cancer cells: A mutual enhancement of their anticancer actions. Oncotarget 2017, 8, 17908–17920. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, D.; Ringler, J.; Chen, W.; Cui, Q.C.; Ethier, S.P.; Dou, Q.P.; Wu, G. Disulfiram Treatment Facilitates Phosphoinositide 3-Kinase Inhibition in Human Breast Cancer Cells In vitro and In vivo. Cancer Res. 2010, 70, 3996–4004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safi, R.; Nelson, E.R.; Chitneni, S.K.; Franz, K.J.; George, D.J.; Zalutsky, M.R.; McDonnell, D.P. Copper Signaling Axis as a Target for Prostate Cancer Therapeutics. Cancer Res. 2014, 74, 5819–5831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cater, M.A.; Pearson, H.B.; Wolyniec, K.; Klaver, P.; Bilandzic, M.; Paterson, B.M.; Bush, A.I.; Humbert, P.O.; La Fontaine, S.; Donnelly, P.S.; et al. Increasing Intracellular Bioavailable Copper Selectively Targets Prostate Cancer Cells. ACS Chem. Biol. 2013, 8, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Mumper, R.J. Intracellular Delivery of the Reactive Oxygen Species Generating Agent d-Penicillamine upon Conjugation to Poly-l-glutamic Acid. Mol. Pharm. 2010, 7, 854–862. [Google Scholar] [CrossRef]
- Luo, C.Q.; Xing, L.; Cui, P.F.; Qiao, J.B.; He, Y.J.; Chen, B.A.; Jin, L.; Jiang, H.L. Curcumin-coordinated nanoparticles with improved stability for reactive oxygen species-responsive drug delivery in lung cancer therapy. Int. J. Nanomed. 2017, 12, 855–869. [Google Scholar] [CrossRef] [Green Version]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [Green Version]
- Norum, O.J.; Fremstedal, A.S.V.; Weyergang, A.; Golab, J.; Berg, K. Photochemical delivery of bleomycin induces T-cell activation of importance for curative effect and systemic anti-tumor immunity. J. Control. Release 2017, 268, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef]
- Li, N.; Sun, Q.; Yu, Z.; Gao, X.; Pan, W.; Wan, X.; Tang, B. Nuclear-Targeted Photothermal Therapy Prevents Cancer Recurrence with Near-Infrared Triggered Copper Sulfide Nanoparticles. ACS Nano 2018, 12, 5197–5206. [Google Scholar] [CrossRef]
- Goswami, U.; Dutta, A.; Raza, A.; Kandimalla, R.; Kalita, S.; Ghosh, S.S.; Chattopadhyay, A. Transferrin–Copper Nanocluster–Doxorubicin Nanoparticles as Targeted Theranostic Cancer Nanodrug. ACS Appl. Mater. Interfaces 2018, 10, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Martini, P.; Janevik-Ivanovska, E.; Duatti, A. The emerging role of copper-64 radiopharmaceuticals as cancer theranostics. Drug Discov. Today 2018, 23, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Shokeen, M.; Wadas, T.J. The Development of Copper Radiopharmaceuticals for Imaging and Therapy. Med. Chem. 2011, 7, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Laforest, R.; Buettner, T.L.; Song, S.K.; Fujibayashi, Y.; Connett, J.M.; Welch, M.J. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone): An agent for radiotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 1206–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knogler, K.; Grünberg, J.; Zimmermann, K.; Cohrs, S.; Honer, M.; Ametamey, S.; Altevogt, P.; Fogel, M.; Schubiger, P.A.; Novak-Hofer, I. Copper-67 Radioimmunotherapy and Growth Inhibition by Anti–L1-Cell Adhesion Molecule Monoclonal Antibodies in a Therapy Model of Ovarian Cancer Metastasis. Clin. Cancer Res. 2007, 13, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Daniel, K.G.; Gupta, P.; Harbach, R.H.; Guida, W.C.; Dou, Q.P. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem. Pharmacol. 2004, 67, 1139–1151. [Google Scholar] [CrossRef]
- Kunjachan, S.; Kotb, S.; Pola, R.; Pechar, M.; Kumar, R.; Singh, B.; Gremse, F.; Taleeli, R.; Trichard, F.; Motto-Ros, V.; et al. Selective Priming of Tumor Blood Vessels by Radiation Therapy Enhances Nanodrug Delivery. Sci. Rep. 2019, 9, 15844. [Google Scholar] [CrossRef] [Green Version]
- Bulin, A.; Broekgaarden, M.; Chaput, F.; Baisamy, V.; Garrevoet, J.; Busser, B.; Brueckner, D.; Youssef, A.; Ravanat, J.; Dujardin, C.; et al. Radiation Dose-Enhancement Is a Potent Radiotherapeutic Effect of Rare-Earth Composite Nanoscintillators in Preclinical Models of Glioblastoma. Adv. Sci. 2020, 7, 2001675. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins—From superoxide dismutation to H2O2-driven pathways. Redox Biol. 2015, 5, 43–65. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.A.; Lopez, K.M. Lysyl oxidase in cancer inhibition and metastasis. Cancer Lett. 2018, 417, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Trackman, P.C. Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert Opin. Ther. Targets 2016, 20, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cell Membrane | Intracellular Vesicles | Endoplasmic Reticulum | Cytoplasm | Extracellular Space | |
|---|---|---|---|---|---|
| ATP7A | TYR | MOXD1 | Atox1 | AOC1 | SOD3 |
| SLC31A1 | TYRP1 | MOXD2P | CCS | Cp | AFP |
| SLC31A2 | COMMD1 | DBH | ALB | ||
| AOC2 | Nucleus | Mitochondrion | CUTC | ENOX1 | F5 |
| AOC3 | COMMD1 | COX11 | LOXL3 | ENOX2 | GPC1 |
| ENOX1 | CUTC | COX17 | MAP2K1 | LOX | LTF |
| ENOX2 | LOXL2 | Sco1 | MEMO1 | LOXL1 | MT3 |
| HEPH | MAP2K1 | Sco2 | SOD1 | LOXL2 | MT4 |
| HEPHL1 | MEMO1 | MT-CO1 | MT3 | LOXL3 | S100A12/A13 |
| PAM | PARK7 | MT-CO2 | PRNP | LOXL4 | SNCA |
| PARK7 | SOD1 | PARK7 | S100A12/A13 | PAM | SPARC |
| APP | LTF | PRNP | SNCA | ||
| GPC1 | PRNP | CUTA | Golgi apparatus | ||
| PRNP | S100A5 | Cytoskeleton | ATP7A | ||
| S100A12 | S100B | MAP2K1 | ATP7B | ||
| SNCA | SNCA | S100A12 | PRNP | ||
| Altered Player | Regulation | Sample | Cancer | Prognostic | Ref. |
|---|---|---|---|---|---|
| Atox1 | + | Tissue | Breast Invasive Cancer | poor | [3] |
| Atox1 | + | Tissue | Melanoma | poor | [58] |
| Atox1 | + | Tissue | Skin Cancer | [58] | |
| Atox1 | + | Blood | [58] | ||
| Atox1 | + | Tissue | Colorectal Cancer | poor | [59] |
| ATP7A | + | Tissue | Pancreatic Cancer | [60] | |
| ATP7A | + | Tissue | Breast Invasive Cancer | poor | [61] |
| ATP7A | + | Tissue/cell lines | Lung Cancer | poor | [62] |
| ATP7A | + | Tissue | Colorectal Cancer | [57,63] | |
| ATP7A | + | Tissue/cell lines | Ovarian Cancer | poor | [64,65] |
| ATP7B | + | Tissue | Esophageal Carcinoma | poor | [66] |
| ATP7B | + | Tissue | Endometrial Carcinoma | poor | [67] |
| ATP7B | + | Tissue | Breast Invasive Cancer | poor | [68] |
| ATP7B | + | Tissue | Oral Squamous Cell Carcinoma | poor | [69] |
| ATP7B | + | Tissue | Gastric Carcinoma | poor | [70] |
| ATP7B | + | Tissue | Hepatocellular Carcinoma | poor | [71] |
| LOX-1 | + | Tissue | Pancreatic Cancer | poor | [72] |
| LOX-1 | + | Tissue | Prostate Cancer | poor | [73] |
| LOX | + | Tissue | Colorectal Cancer | poor | [74] |
| LOX-2 | + | Tissue/Serum | Hepatocellular Carcinoma | poor | [75] |
| LOX | + | Tissue | Breast Invasive Cancer | poor | [76,77,78] |
| LOX | − | Tissue | Bronchogenic Carcinoma | poor | [79] |
| LOX | − | Tissue/cell lines | Gastric Cancers | [80] | |
| LOX | + | Tissue | Head and Neck Squamous Cell Carcinoma | poor | [81] |
| LOX | + | Tissue/cell lines | Lung Adenocarcinoma | poor | [82,83] |
| LOX | + | Cell lines | Melanoma | poor | [76] |
| LOX | + | Tissue | Oral and Oropharyngeal Squamous Cell | poor | [84] |
| LOX | − | Tissue/cell lines | Basal and Squamous Cell Carcinomas | [85] | |
| LOX | + | Tissue | Renal Cell Carcinoma | [86] | |
| LOXL1 | − | Cell lines | Bladder Cancer | [87] | |
| LOXL4 | − | Cell lines | Bladder Cancer | [87] | |
| LOXL1 | + | Cell lines | Lung Adenocarcinoma | [88] | |
| LOXL1 | + | Tissue | Salivary Gland Adenoid Cystic Carcinoma | [89] | |
| LOXL2 | + | Tissue/cell lines | Breast Invasive Cancer | poor | [90,91,92,93,94] |
| LOXL2 | + | Tissue | Colorectal Cancer | poor | [93,95,96,97] |
| LOXL2 | + | Tissue/cell lines | Gastric Cancers | poor | [93,98] |
| LOXL2 | + | Tissue | Endometrial Cancer | poor | [94] |
| LOXL2 | + | Tissue | Testicular Cancer | poor | [94] |
| LOXL2 | + | Tissue | Hepatocellular Carcinoma | poor | [94] |
| LOXL2 | + | Tissue | Lung Squamous Cell Carcinoma | poor | [90,99] |
| LOXL2 | + | Cell lines | Melanoma | poor | [76] |
| LOXL2 | + | Tissue/cell lines | Pancreatic Cancer | poor | [94,100] |
| LOXL2 | + | Tissue | Prostate Cancer | poor | [101] |
| LOXL2 | + | Tissue | Renal Cell Carcinoma | poor | [94] |
| LOXL2 | + | Tissue | Laryngeal Cancer | poor | [90,94] |
| LOXL2 | + | Tissue | Esophageal Squamous Cell | poor | [94,102] |
| LOXL3 | + | Cell lines | Breast Invasive Cancer | [103] | |
| LOXL3 | + | Cell lines | Melanoma | [76,103] | |
| LOXL4 | + | Tissue | Colorectal Adenocarcinoma | [95] | |
| LOXL4 | + | Tissue/cell lines | Head and Neck Squamous Cell Carcinoma | [104,105,106] | |
| Ctr1 | + | Tissue | Colorectal Cancer | [57] | |
| Sco1 | + | Tissue | Colorectal Cancer | [57] | |
| COX11 | + | Tissue | Colorectal Cancer | [57] | |
| Ctr1 | + | Tissue | Non-Small-Cell Lung Cancer | good | [107] |
| Ctr1 | Variable | Tissue | Ovarian Cancer | [108] | |
| Ctr2 | Variable | Tissue | Ovarian Cancer | [108] | |
| Ctr1 | + | Cell lines | Ovarian Cancer | [109] | |
| Ctr1 | + | Tissue | Breast Invasive Cancer | [3] | |
| Cu | + | Serum | Bladder Cancer | [40] | |
| Zn | − | Serum | Bladder Cancer | [40] | |
| Cu | + | Serum | Breast Invasive Cancer | [42,44,49,110] | |
| Cu/Zn | + | Serum | Breast Invasive Cancer | [42,44,110] | |
| Cu | + | Serum/Tissue | Lung Cancer | [51,53] | |
| Cu/Zn | + | Serum | Lung Cancer | [51] | |
| Cu | ± | Serum/Tissue | Colorectal Cancer | [41,45,48] | |
| Cu/Zn | + | Serum/Tissue | Colorectal Cancer | [41,45,48] | |
| Cu/Zn | + | Serum | Pancreatic Cancer | [46] | |
| Cu | − | Serum | Pancreatic Cancer | [46] | |
| Cu | + | Serum | Cervical Cancer and Uterine Myoma | [47] | |
| Cu/Zn | + | Serum | Cervical Cancer and Uterine Myoma | [47] | |
| Cu | + | Serum | Prostate Cancer | [43] | |
| Cu | + | Serum | Papillary Thyroid Carcinoma | [111] | |
| Cu | − | Serum | Endometrial Cancer | [112] | |
| GSH | + | Tissue | Hepatocellular Carcinoma | [113] | |
| CCS | + | Tissue | Brain Cancer | [58] | |
| CCS | + | Tissue | Ovarian Cancer | [58] | |
| CCS | − | Tissue | Hepatocellular Carcinoma | [58] | |
| CCS | − | Tissue | Prostate Cancer | [58] | |
| SOD3 | − | Tissue | Lung Cancer | poor | [114,115,116] |
| SOD3 | − | Tissue | Breast Invasive Cancer | poor | [117,118] |
| SOD3 | − | Tissue | Prostate Cancer | poor | [119,120] |
| SOD3 | − | Tissue | Pancreatic Cancer | poor | [121] |
| SOD3 | − | Tissue | Colorectal Cancer | [122] | |
| SOD3 | − | Cell lines | Thyroid Cancer | [123] | |
| SOD3 | + | Serum | Gastric Adenocarcinoma | [124] | |
| COX17 | + | Cell lines | Lung Cancer | [125] | |
| COX17 | + | Tissue | Head and Neck Squamous Cell Carcinomas | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. https://doi.org/10.3390/cancers12123594
Lelièvre P, Sancey L, Coll J-L, Deniaud A, Busser B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers. 2020; 12(12):3594. https://doi.org/10.3390/cancers12123594
Chicago/Turabian StyleLelièvre, Pierre, Lucie Sancey, Jean-Luc Coll, Aurélien Deniaud, and Benoit Busser. 2020. "The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy" Cancers 12, no. 12: 3594. https://doi.org/10.3390/cancers12123594
APA StyleLelièvre, P., Sancey, L., Coll, J.-L., Deniaud, A., & Busser, B. (2020). The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers, 12(12), 3594. https://doi.org/10.3390/cancers12123594







