Gamma Knife Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy or Targeted Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
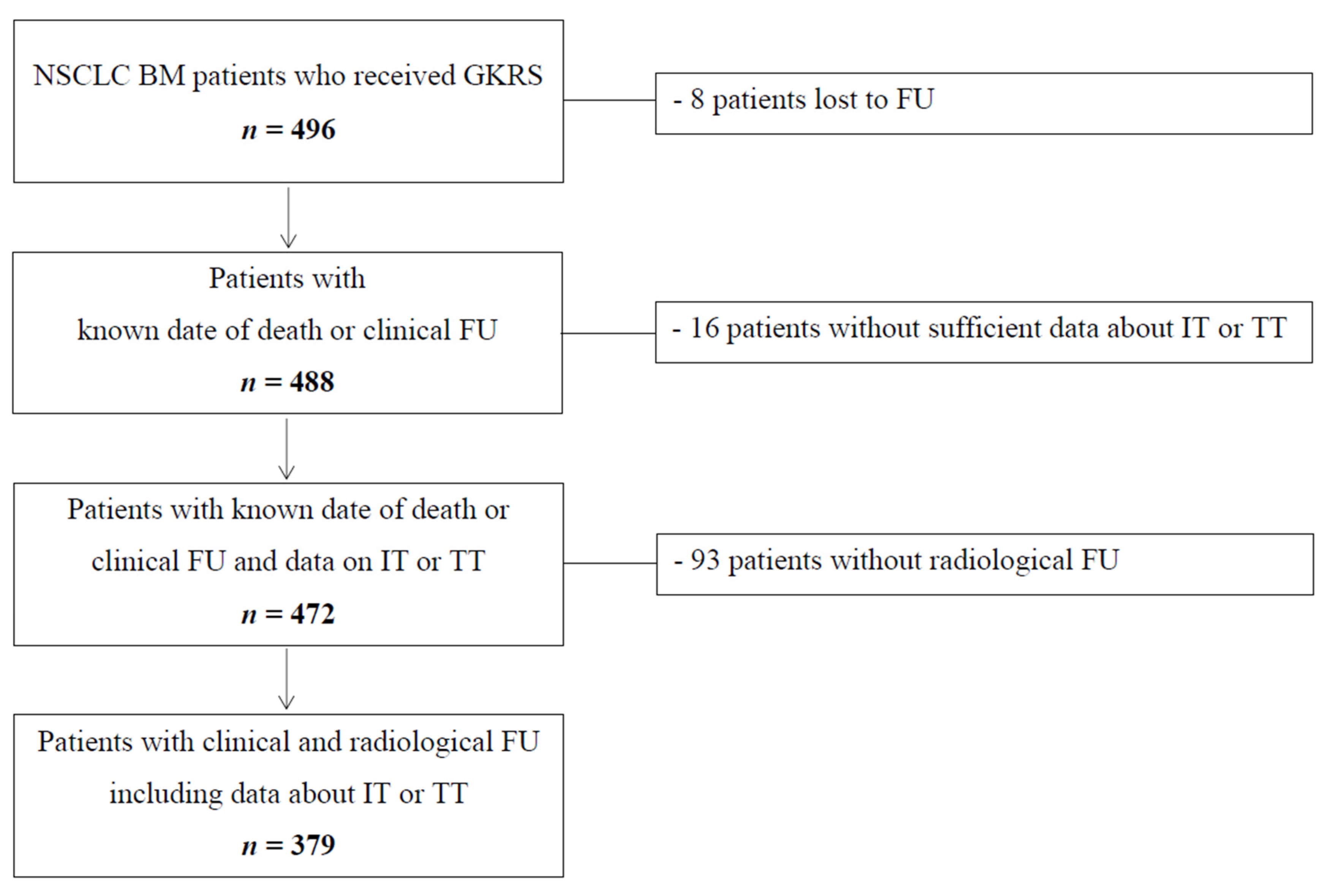
2.1. Patient Characteristics and Overall Follow-Up
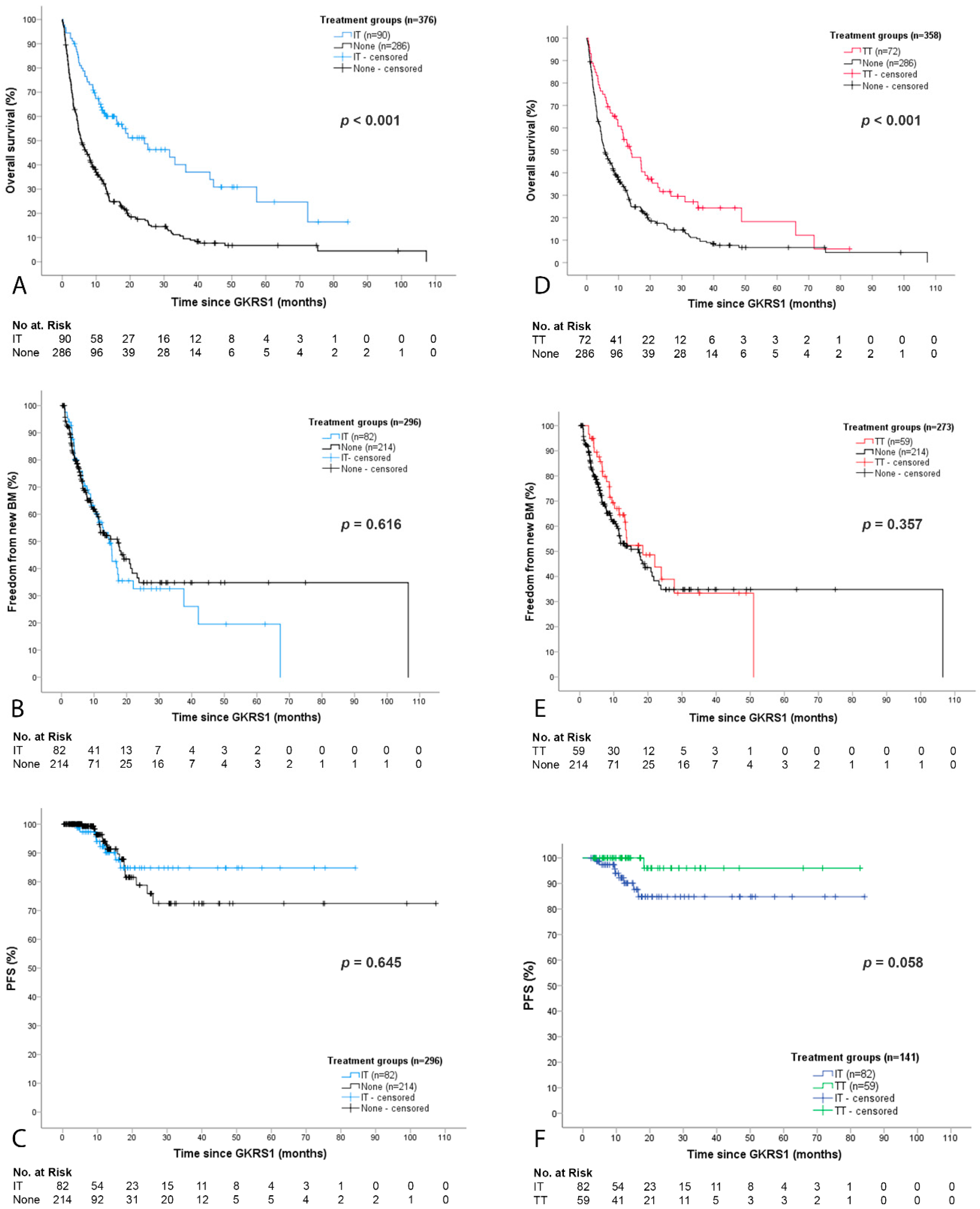
2.2. Overall Outcome and Complications after GKRS and Concurrent Immunotheraphy (IT) or Targeted Therapy (TT)
2.3. Detailed Outcome and Complications after GKRS with Concurrent Immunotherapy (IT Group)
2.4. Detailed Outcome and Complications after GKRS with Concurrent Targeted Therapy (TT Group)
3. Discussion
3.1. Survival after GKRS in Relation to IT or TT
3.2. Local and Distant Cerebral Tumor Control after GKRS in Relation to IT or TT
3.3. Complications after GKRS in Relation to IT or TT
3.4. The Role of Radiosurgery in the Era of IT or TT
4. Materials and Methods
4.1. Patient Sample and Data Evaluation
4.2. Radiosurgery Technique
4.3. Follow-Up and Outcome Evaluation
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Alexander, B.M.; Redig, A.J.; Schoenfeld, J.D.; Aizer, A.A. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018, 4, 1123–1124. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, S.G.; Fritz, C.; Hoyer, M.; Lo, S.S.; Ricardi, U.; Sahgal, A.; Stahel, R.; Stupp, R.; Guckenberger, M. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: A systematic review. Cancer Treat. Rev. 2017, 53, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Douglass, J.; Kleinberg, L.; Ye, X.; Marciscano, A.E.; Forde, P.M.; Brahmer, J.; Lipson, E.; Sharfman, W.; Hammers, H.; et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: A retrospective multi-institutional analysis. J. Clin. Oncol. 2017, 35, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Gatterbauer, B.; Hirschmann, D.; Eberherr, N.; Untersteiner, H.; Cho, A.; Shaltout, A.; Gobl, P.; Fitschek, F.; Dorfer, C.; Wolfsberger, S.; et al. Toxicity and efficacy of Gamma Knife radiosurgery for brain metastases in melanoma patients treated with immunotherapy or targeted therapy—A retrospective cohort study. Cancer Med. 2020, 9, 4026–4036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepard, M.J.; Xu, Z.; Donahue, J.; Eluvathingal Muttikkal, T.J.; Cordeiro, D.; Hansen, L.; Mohammed, N.; Gentzler, R.D.; Larner, J.; Fadul, C.E.; et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: A matched cohort study. J. Neurosurg. 2019, 133, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.; Kano, H.; Caparosa, E.; Park, S.H.; Niranjan, A.; Flickinger, J.; Lunsford, L.D. Gamma knife radiosurgery for the management of cerebral metastases from non-small cell lung cancer. J. Neurosurg. 2015, 122, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Wang, R.; Ye, T.; Yu, S.; Hu, H.; Shen, X.; Li, Y.; Ji, H.; Sun, Y.; Chen, H. MET exon 14 skipping defines a unique molecular class of non-small cell lung cancer. Oncotarget 2016, 7, 41691–41702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Weltman, E.; Salvajoli, J.V.; e Oliveira, V.C.; Brandt, R.A.; da Cruz, J.C.; de Oliveira Borges, S.R.; Anselmo, R.T.R.; Wajsbrot, D.B. Score Index for stereotactic radiosurgery of brain metastases. J. Radiosurg. 1998, 1, 89–97. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef]
- Singh, C.; Qian, J.M.; Yu, J.B.; Chiang, V.L. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J. Neurosurg. 2019, 132, 512–517. [Google Scholar] [CrossRef]
- Foster, C.C.; Sher, D.J.; Rusthoven, C.G.; Verma, V.; Spiotto, M.T.; Weichselbaum, R.R.; Koshy, M. Overall survival according to immunotherapy and radiation treatment for metastatic non-small-cell lung cancer: A National Cancer Database analysis. Radiat. Oncol. 2019, 14, 18. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Ahluwalia, M.S. Targeted therapy of brain metastases: Latest evidence and clinical implications. Ther. Adv. Med. Oncol. 2017, 9, 781–796. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.A.; McDermott, D.M.; Mattes, M.D. Impact of systemic therapy type and timing on intracranial tumor control in patients with brain metastasis from non-small-cell lung cancer treated with stereotactic radiosurgery. World Neurosurg. 2020, 144, e813–e823. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Chung, T.S.; Ting, L.L.; Tsai, J.T.; Chen, S.W.; Chiou, J.F.; Leung, H.W.; Liu, H.E. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat. Oncol. 2012, 7, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, N. Optimizing outcomes in EGFR mutation-positive NSCLC: Which tyrosine kinase inhibitor and when? Future Oncol. 2018, 14, 1117–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneed, P.K.; Mendez, J.; Vemer-van den Hoek, J.G.; Seymour, Z.A.; Ma, L.; Molinaro, A.M.; Fogh, S.E.; Nakamura, J.L.; McDermott, M.W. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J. Neurosurg. 2015, 123, 373–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.J.; Kummerlowe, M.N.; Redmond, K.J.; Rigamonti, D.; Lim, M.K.; Kleinberg, L.R. Stereotactic radiosurgery: Treatment of brain metastasis without interruption of systemic therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Colaco, R.J.; Martin, P.; Kluger, H.M.; Yu, J.B.; Chiang, V.L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J. Neurosurg. 2016, 125, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartgerink, D.; van der Heijden, B.; De Ruysscher, D.; Postma, A.; Ackermans, L.; Hoeben, A.; Anten, M.; Lambin, P.; Terhaag, K.; Jochems, A.; et al. Stereotactic radiosurgery in the management of patients with brain metastases of non-small cell lung cancer: Indications, decision tools and future directions. Front. Oncol. 2018, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Skrepnik, T.; Sundararajan, S.; Cui, H.; Stea, B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 2017, 6, e1283461. [Google Scholar] [CrossRef] [Green Version]
- Trapani, S.; Manicone, M.; Sikokis, A.; D’Abbiero, N.; Salaroli, F.; Ceccon, G.; Buti, S. Effectiveness and safety of “real” concurrent stereotactic radiotherapy and immunotherapy in metastatic solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2019, 142, 9–15. [Google Scholar] [CrossRef]
- Frischer, J.M.; Fraller, A.; Mallouhi, A.; Vogl, U.M.; Baier, F.; Ertl, A.; Preusser, M.; Knosp, E.; Kitz, K.; Gatterbauer, B. Evaluation of dose-staged gamma knife radiosurgical treatment method for high-risk brain metastases. World Neurosurg. 2016, 94, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Stockham, A.L.; Tievsky, A.L.; Koyfman, S.A.; Reddy, C.A.; Suh, J.H.; Vogelbaum, M.A.; Barnett, G.H.; Chao, S.T. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J. Neuro-Oncol. 2012, 109, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.J.; Iv, M.; Wintermark, M. Imaging of intracranial hemorrhage. J. Stroke 2017, 19, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, W.G., Jr. MR appearance of hemorrhage in the brain. Radiology 1993, 189, 15–26. [Google Scholar] [CrossRef] [PubMed]

| Time of First GKRS, Total Sample (n = 496) | Patients with IT or TT at or after GKRS1 “IT or TT Group” (n = 186 *) | Patients without IT or TT at or after GKRS1 “None Group” (n = 286 *) | IT or TT vs. None Group | |
|---|---|---|---|---|
| Age | 64 | 62 | 65 | p = 0.004 |
| in years, median (range) | (28–87) | (28–87) | (36–87) | |
| Female/male ratio | 258:238 | 102:84 | 141:145 | p = 0.239 |
| KPS | 80 | 80 | 80 | p = 0.001 |
| in %, median (range) | (40–100) | (40–100) | (40–90) | |
| KPS groups | p = 0.003 | |||
| ≥80% | 345 (70%) | 143 (77%) | 183 (64%) | |
| <80% | 151 (30%) | 43 (23%) | 103 (36%) | |
| ECM status at time of BM diagnosis | p = 0.019 | |||
| Yes | 321(65%) | 134 (72%) | 176 (62%) | |
| No | 175 (35%) | 52 (28%) | 110 (38%) | |
| NSCLC subtype | p = 0.020 | |||
| Adenocarcinoma | 416 (84%) | 169 (91%) | 225 (79%) | |
| Squamous cell carcinoma | 66 (13%) | 13 (7%) | 51 (18%) | |
| Large cell neuroendocrine carcinoma | 7 (2%) | 2 (1%) | 5 (2%) | |
| Large cell carcinoma | 5 (1%) | 2 (1%) | 3 (1%) | |
| Adenosquamous carcinoma | 1 (0%) | 0 (0%) | 1 (0%) | |
| Polymorphous carcinoma | 1 (0%) | 0 (0%) | 1 (0%) | |
| CNS treatment before GKRS1 | p = 0.637 | |||
| None | 415 (84%) | 159 (85%) | 239 (83%) | |
| WBRT and/or fRT | 36 (7%) | 15 (9%) | 20 (7%) | |
| BM resection without RT | 20 (4%) | 6 (3%) | 11 (4%) | |
| BM resection with WBRT and/or fRT | 25 (5%) | 6 (3%) | 16 (6%) | |
| Localization of BM at initial diagnosis | p = 0.861 | |||
| Multiple | 276 (56%) | 112 (60%) | 150 (52%) | |
| Frontal | 58 (12%) | 20 (11%) | 36 (13%) | |
| Parietal | 22 (4%) | 7 (4%) | 14 (5%) | |
| Temporal | 23 (5%) | 8 (4%) | 15 (5%) | |
| Occipital | 35 (7%) | 11 (6%) | 22 (8%) | |
| Central | 29 (6%) | 9 (5%) | 19 (7%) | |
| Basal ganglia, brainstem, other | 11 (2%) | 4 (2%) | 7 (2%) | |
| Cerebellar | 42 (8%) | 15 (8%) | 23 (8%) | |
| Predicted survival after prognostic scores | ||||
| in months, median (range) | ||||
| GPA general | 3.8 (2.6–11.0) | 3.8 (2.6–11.0) | 3.8 (2.6–11.0) | p = 0.409 |
| GPA specific | 5.5 (3.0–14.8) | 5.5 (3.0–14.8) | 5.5 (3.0–14.8) | p = 0.450 |
| RPA | 4.5 (2.3–7.7) | 4.5 (2.3–7.7) | 4.5 (2.3–7.7) | p = 0.374 |
| SIR | 6.0 (2.1–8.8) | 6.0 (2.1–8.8) | 6.0 (2.1–8.8) | p = 0.009 |
| Lung-molGPA for adenocarcinoma | 13.7 (6.9–46.8) | 13.7 (6.9–46.8) | 13.7 (6.9–46.8) | p = 0.004 |
| Lung-molGPA for nonadenocarcinoma | 9.8 (5.3–12.8) | 9.8 (5.3–12.8) | 9.8 (5.3–12.8) | p = 0.126 |
| Parameters of first GKRS | ||||
| Number of treated BMs, median (range) | 2 (1–13) | 2 (1–9) | 2 (1–13) | p = 0.001 |
| Treatment volume in cm3, median (range) | 0.6 (0.1–27.7) | 0.8 (0.1–27.7) | 0.6 (0.1–13.8) | p < 0.001 |
| Isodose in %, median (range) | 50 (40–90) | 50 (40–90) | 50 (40–90) | p < 0.001 |
| Prescription dose in Gy, median (range) | 18 (8–20) | 18 (8–20) | 18 (10–20) | p = 0.010 |
| Central dose in Gy, median (range) | 32 (13–45) | 34 (16–44) | 30 (13–45) | p < 0.001 |
| Treatment Group (n = 488) | Number of Patients (%) |
|---|---|
| IT group (90/488, 18%) | 90 |
| Nivolumab 1 | 38 (42%) |
| Pembrolizumab 1 | 29 (32%) |
| Atezolizumab 2 | 15 (17%) |
| Durvalumab 2 | 5 (6%) |
| Unknown therapy types, only documented as IT (external study patients) | 3 (3%) |
| TT group (72/488, 15%) | 72 |
| Erlotinib 3 | 14 (19%) |
| Gefitinib 3 | 11 (15%) |
| Afatinib 3 | 11 (15%) |
| Alectinib 4 | 4 (6%) |
| Crizotinib 5 | 4 (6%) |
| Osimertinib 3 | 3 (4%) |
| Nintedanib 6 | 3 (4%) |
| Brigatinib 4 | 2 (3%) |
| Ceritinib 4 | 1 (1%) |
| Osimertinib and Afatinib | 9 (13%) |
| Combinations of TT | 10 (14%) |
| Other/IT and TT combination group (24/488, 5%) | 24 |
| Multiple combinations | 20 (83%) |
| Bevacizumab | 4 (17%) |
| None group (286/488, 59%) | 286 |
| No data on IT or TT (16/488, 3%) | 16 |
| IT/TT at or after GKRS1 (n) | Intralesional Hemorrhage after GKRS1 (n = 379) | Radiation Reaction after GKRS1 (n = 379) | Radiation Necrosis after GKRS1 (n = 379) | |||
|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | |
| IT (n = 82) | 0 | 82 (100) | 9 (11) | 73 (89) | 8 (10) | 74 (90) |
| TT (n = 59) | 0 | 59 (100) | 3 (5) | 56 (95) | 3 (5) | 56 (95) |
| Other/Combination (n = 24) | 0 | 24 (100) | 1 (4) | 23 (96) | 2 (8) | 22 (92) |
| None (n = 214) | 2 (1) | 212 (99) | 23 (11) | 191 (89) | 15 (7) | 199 (93) |
| Total (n = 379) | 2 (1) | 377 (99) | 36 (9) | 343 (91) | 28 (7) | 351 (93) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, A.; Untersteiner, H.; Hirschmann, D.; Shaltout, A.; Göbl, P.; Dorfer, C.; Rössler, K.; Marik, W.; Kirchbacher, K.; Kapfhammer, I.; et al. Gamma Knife Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy or Targeted Therapy. Cancers 2020, 12, 3668. https://doi.org/10.3390/cancers12123668
Cho A, Untersteiner H, Hirschmann D, Shaltout A, Göbl P, Dorfer C, Rössler K, Marik W, Kirchbacher K, Kapfhammer I, et al. Gamma Knife Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy or Targeted Therapy. Cancers. 2020; 12(12):3668. https://doi.org/10.3390/cancers12123668
Chicago/Turabian StyleCho, Anna, Helena Untersteiner, Dorian Hirschmann, Abdallah Shaltout, Philipp Göbl, Christian Dorfer, Karl Rössler, Wolfgang Marik, Klaus Kirchbacher, Irene Kapfhammer, and et al. 2020. "Gamma Knife Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy or Targeted Therapy" Cancers 12, no. 12: 3668. https://doi.org/10.3390/cancers12123668





