Prognostic Value of Red Blood Cell Distribution Width in Resected pN1 Lung Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
| Author (ref. n.) | Year | RDW Relation with | Findings |
|---|---|---|---|
| Wang L [1] | 2020 | Ischemic stroke | RDW was an independent predictor of 3-month functional outcome, and a trend of dose-dependent relationship between RDW and 3-month death was detected. |
| Jia L [3] | 2020 | Acute kidney injury | RDW is positively correlated to survival time of 4-year follow-up in critically ill patients with acute kidney injury, and RDW is an independent prognostic factor of long-term outcomes of these patients. |
| Wang [6] | 2020 | Lung cancer | A higher value of pre-treatment RDW indicated worse survival of patients with lung cancer. RDW may serve as a reliable and economical marker for prediction of lung cancer prognosis |
| Toyokawa G [7] | 2020 | Lung cancer | RDW was shown to be associated with a worse long-term prognosis in resected pathologic stage I NSCLC patients |
| Seretis C [13] | 2013 | Breast cancer | RDW was significantly higher in patients with breast cancer, when compared to the enrolled patients with fibroadenomas. |
| Wang F [14] | 2014 | Renal cell carcinoma | RDW significantly higher in patients with renal cell carcinoma (RCC) than those in controls, and the baseline RDW was independently associated with RCC. |
| Clarke K [22] | 2008 | Inflammatory bowel disease | RDW is an effective differentiating test between Crohn’s disease and ulcerative colitis. |
| Li N [23] | 2017 | Cardio/cerebro vascular disease | Hypothetical and potential epidemiological associations between RDW and cardiovascular diseases. |
| Tonelli M [24] | 2008 | Coronary disease | Independent relation between higher levels of RDW and the risk of death and cardiovascular events in people with prior myocardial infarction. |
| Skjelbakken T [25] | 2014 | Myocardial infarction | RDW is associated with incident myocardial infarction in a general population independent of anemia and cardiovascular risk factors. |
| Zyczkowski M [26] | 2017 | Renal cell carcinoma | Cancer specific survival in patients receiving nephrectomy for renal cell carcinoma was significantly lower in patients with RDW ≥ 13.9%. |
| Qin Y [27] | 2017 | Ovarian cancer | RDW is associated with ovarian cancer and is a potential marker of its progression. |
| Chen GP [28] | 2015 | Esophageal cancer | RDW was an independent prognostic factor in patients with esophageal squamous cell carcinoma. |
| Wei TT [29] | 2017 | Gastric cancer | Patients with gastric cancer had significantly higher RDW than healthy controls. |
| Kemal Y [30] | 2015 | Endometrial cancer | Grade II and above endometrial cancer patients had higher levels of RDW than Grade I patients |
2. Material and Methods
Statistical Methods
3. Results
4. Comments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, C.; Wu, S.; Li, Y.; Guo, W.; Liu, M. Red blood cell distribution width is associated with mortality after acute ischemic stroke: A cohort study and systematic review. Ann. Transl. Med. 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Buttarello, M. Laboratory diagnosis of anemia: Are the old and new red cell parameters useful in classification and treatment, how? Int. J. Lab. Hematol. 2016, 38 (Suppl. 1), 123–132. [Google Scholar] [CrossRef]
- Jia, L.; Cui, S.; Yang, J.; Jia, Q.; Hao, L.; Jia, R.; Zhang, H. Red blood cell distribution width predicts long-term mortality in critically ill patients with acute kidney injury: A retrospective database study. Sci. Rep. 2020, 10, 4563. [Google Scholar] [CrossRef] [PubMed]
- Peirovy, A.; Mahdavi, A.M.; Khabbazi, A.; Hajialilo, M.; Sakhinia, E.; Rashtchizadeh, N. Clinical Usefulness of Hematologic Indices as Predictive Parameters for Systemic Lupus Erythematosus. Lab. Med. 2020, 51, 519–558. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Borné, Y.; Engström, G. The relationship between red cell distribution width and all-cause and cause-specific mortality in a general population. Sci. Rep. 2019, 9, 16208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Zhou, K.; Li, J.; Che, G. Prognostic value of pre-treatment red blood cell distribution width in lung cancer: A meta-analysis. Biomarkers 2020, 25, 241–247. [Google Scholar] [CrossRef]
- Toyokawa, G.; Shoji, F.; Yamazaki, K.; Shimokawa, M.; Takeo, S. Significance of the Red Blood Cell Distribution Width in Resected Pathologic Stage I Nonsmall Cell Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 1036–1045. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv1–iv21. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.; Wang, X.; Gu, L.; Pei, H.; Kuai, S.; Zhang, Y.; Shang, Z. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics 2015, 70, 524–530. [Google Scholar] [CrossRef]
- Toda, M.; Tsukioka, T.; Izumi, N.; Komatsu, H.; Okada, S.; Hara, K.; Miyamoto, H.; Ito, R.; Shibata, T.; Nishiyama, N. Platelet-to-lymphocyte ratio predicts the prognosis of patients with non-small cell lung cancer treated with surgery and postoperative adjuvant chemotherapy. Thorac. Cancer 2018, 9, 112–119. [Google Scholar] [CrossRef]
- Watanabe, K.; Yasumoto, A.; Amano, Y.; Kage, H.; Goto, Y.; Yatomi, Y.; Takai, D.; Nagase, T. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PLoS ONE 2018, 13, e0203625. [Google Scholar] [CrossRef] [PubMed]
- Warwick, R.; Mediratta, N.; Shackcloth, M.; Shaw, M.; McShane, J.; Poullis, M. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2014, 45, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Seretis, C.; Seretis, F.; Lagoudianakis, E.; Gemenetzis, G.; Salemis, N.S. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J. Clin. Med. Res. 2013, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-M.; Xu, G.; Zhang, Y.; Ma, L.-L. Red cell distribution width is associated with presence, stage, and grade in patients with renal cell carcinoma. Dis. Markers 2014, 2014, 860419. [Google Scholar] [CrossRef]
- Douglas, S.W.; Adamson, J.W. The anemia of chronic disorders: Studies of marrow regulation and iron metabolism. Blood 1975, 45, 55–65. [Google Scholar] [CrossRef]
- Ferrucci, L.; Guralnik, J.M.; Woodman, R.C.; Bandinelli, S.; Lauretani, F.; Corsi, A.M.; Chaves, P.H.; Ershler, W.B.; Longo, D.L. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am. J. Med. 2005, 118, 1288. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F.R. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Banzet, S.; Sanchez, H.; Chapot, R.; Bigard, X.; Vaulont, S.; Koulmann, N. Interleukin-6 contributes to hepcidin mRNA increase in response to exercise. Cytokine 2012, 58, 158–161. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef]
- Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Clarke, K.; Sagunarthy, R.; Kansal, S. RDW as an Additional Marker in inflammatory bowel disease/undifferentiated colitis. Dig. Dis. Sci. 2008, 53, 2521–2523. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhou, H.; Tang, Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis. Markers 2017, 2017, 7089493. [Google Scholar] [CrossRef]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008, 117, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Sung, J.F.; Chien, K.-L.; Hsu, H.-C.; Su, T.-C.; Lee, Y.-T. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am. J. Epidemiol. 2010, 171, 214–220. [Google Scholar] [CrossRef]
- Zyczkowski, M.; Rajwa, P.; Gabrys, E.; Jakubowska, K.; Jantos, E.; Paradysz, A. The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin. Genitourin. Cancer 2018, 16, e677–e683. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, P.; Huang, Z.; Huang, G.; Tang, J.; Guo, Y.; Huang, P.; Lai, Z.; Lin, F. The value of red cell distribution width in patients with ovarian cancer. Medicine 2017, 96, e6752. [Google Scholar] [CrossRef]
- Chen, G.-P.; Huang, Y.; Yang, X.; Feng, J.-F. A Nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediat. Inflamm. 2015, 2015, 854670. [Google Scholar] [CrossRef]
- Wei, T.-T.; Wang, L.-L.; Yin, J.-R.; Liu, Y.-T.; Qin, B.-D.; Li, J.-Y.; Yin, X.; Zhou, L.; Zhong, R.-Q. Relationship between red blood cell distribution width, bilirubin, and clinical characteristics of patients with gastric cancer. Int. J. Lab. Hematol. 2017, 39, 497–501. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Skjelbakken, T.; Lappegård, J.; Ellingsen, T.S.; Barrett-Connor, E.; Brox, J.; Løchen, M.; Njølstad, I.; Wilsgaard, T.; Mathiesen, E.B.; Brækkan, S.K.; et al. Red Cell Distribution Width Is Associated With Incident Myocardial Infarction in a General Population: The Tromsø Study. J. Am. Heart Assoc. 2014, 3, e001109. [Google Scholar] [CrossRef] [PubMed]
- Kemal, Y.; Demirag, G.; Baş, B.; Önem, S.; Teker, F.; Yücel, I. The value of red blood cell distribution width in endometrial cancer. Clin. Chem. Lab. Med. 2015, 53, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-P.; Ma, R.-M.; Xiang, Y.-Q. Utility of Red Cell Distribution Width as a Prognostic Factor in Young Breast Cancer Patients. Medicine 2016, 95, e3430. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, J.; Murakawa, T.; Kawashima, M.; Nagayama, K.; Nitadori, J.-I.; Anraku, M.; Nakajima, J. Prognostic significance of red cell distribution width in elderly patients undergoing resection for non-small cell lung cancer. J. Thorac. Dis. 2016, 8, 3658–3666. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, T.; Li, J.; Yang, W.; Liu, E.; Li, G. Elevated red cell distribution width level is associated with oxidative stress and inflammation in a canine model of rapid atrial pacing. Int. J. Cardiol. 2014, 174, 174–176. [Google Scholar] [CrossRef]
- Eichhorn, F.; Klotz, L.; Muley, T.; Kobinger, S.; Winter, H.; Eichhorn, M. Prognostic relevance of regional lymph-node distribution in patients with N1-positive non-small cell lung cancer: A retrospective single-center analysis. Lung Cancer 2019, 138, 95–101. [Google Scholar] [CrossRef]
- Semba, R.D.; Patel, K.V.; Ferrucci, L.; Sun, K.; Roy, C.N.; Guralnik, J.M.; Fried, L.P. Serum antioxidants and inflammation predict red cell distribution width in older women: The women’s health and aging study I. Clin. Nutr. 2010, 29, 600–604. [Google Scholar] [CrossRef]
- Banfi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar]
- Park, B.J.; Cho, J.H.; Lee, J.H.; Shin, S.; Kim, H.K.; Choi, Y.S.; Zo, J.I.; Shim, Y.M.; Sun, J.-M.; Lee, S.-H.; et al. Temporal and regional distribution of initial recurrence site in completely resected N1-stage II lung adenocarcinoma: The effect of postoperative adjuvant chemotherapy. Lung Cancer 2018, 117, 7–13. [Google Scholar] [CrossRef]
- Nakagawa, T.; Okumura, N.; Kokado, Y.; Miyoshi, K.; Matsuoka, T.; Kameyama, K. Retrospective study of patients with pathologic N1-stage II non-small cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 474–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petrella, F.; Rizzo, S.; Radice, D.; Borri, A.; Galetta, D.; Gasparri, R.; Solli, P.; Veronesi, G.; Bellomi, M.; Spaggiari, L. Predicting prolonged air leak after standard pulmonary lobectomy: Computed tomography assessment and risk factors stratification. Surgeon 2011, 9, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hao, Y.; Zhu, L.; Li, S.; Zuo, Y.; Zhang, Y.; Song, H.; Xue, Y. Monocyte to lymphocyte ratio predicts survival in patients with advanced gastric cancer undergoing neoadjuvant chemotherapy. Onco Targets Ther. 2017, 10, 4007–4016. [Google Scholar] [CrossRef]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Level | Statistics a | |
|---|---|---|---|
| Age at Surgery, years | 67.7 (8.6) | ||
| Tumor Size, mm | 46.5 (25.9) | ||
| Female Gender | 24 (35.8) | ||
| Smoke | Yes | 60 (89.6) | |
| Surgery | Open | 50 (74.6) | |
| MIS b | 17 (25.4) | ||
| Pathological Stage | T1-2 | 39 (58.2) | |
| T3-4 | 28 (41.8) | ||
| N1 Status | N1A (single station) | 55 (82.1) | |
| N1B (multiple stations) | 12 (17.9) | ||
| Stage | 3A | 28 (41.8) | |
| 2B | 39 (58.2) | ||
| Grading | 1 | 1 (1.7) | |
| 2 | 20 (34.5) | ||
| 3 | 37 (63.8) | ||
| Adjuvant Treatments | No | 42 (62.9) | |
| CT | 18 (26.9) | ||
| RT | 5 (7.5) | ||
| CT/RT | 1 (1.5) | ||
| CT/RT/Other | 1 (1.5) | ||
| Neoadjuvant Treatments | No | 58 (86.6) | |
| CT | 9 (13.4) | ||
| Procedures | Upper Lobectomy | Left | 18 (26.9) |
| Right | 17 (25.4) | ||
| Lower Lobectomy | Left | 6 (9.0) | |
| Right | 9 (13.4) | ||
| Pneumonectomy | Left | 4 (6.0) | |
| Right | 7 (10.5) | ||
| Upper Sleeve Lobectomy | Left | 1 (1.5) | |
| Right | 1 (1.5) | ||
| Lower bilobectomy | 2 (3.0) | ||
| Culminectomy | 1 (1.5) | ||
| Middle Lobectomy | 1 (1.5) |
| Mean (IQR) a | Median (Min, Max) | |
|---|---|---|
| Hemoglobin, g/dL | 13.3 (12.5–14.5) | 13.4 (9.1,16.1) |
| Neutrophils, ×103/µL | 4.86 (3.60–5.62) | 4.41 (1.80,9.82) |
| Lymphocytes, ×103/µL | 1.85 (1.35–2.17) | 1.69 (0.82,6.20) |
| RDW (%) | 14.1 (12.9–14.8) | 13.7 (11.3,18.9) |
| Neutrophil/Lymphocytes Ratio | 3.05 (1.88–3.66) | 2.70 (0.58,11.3) |
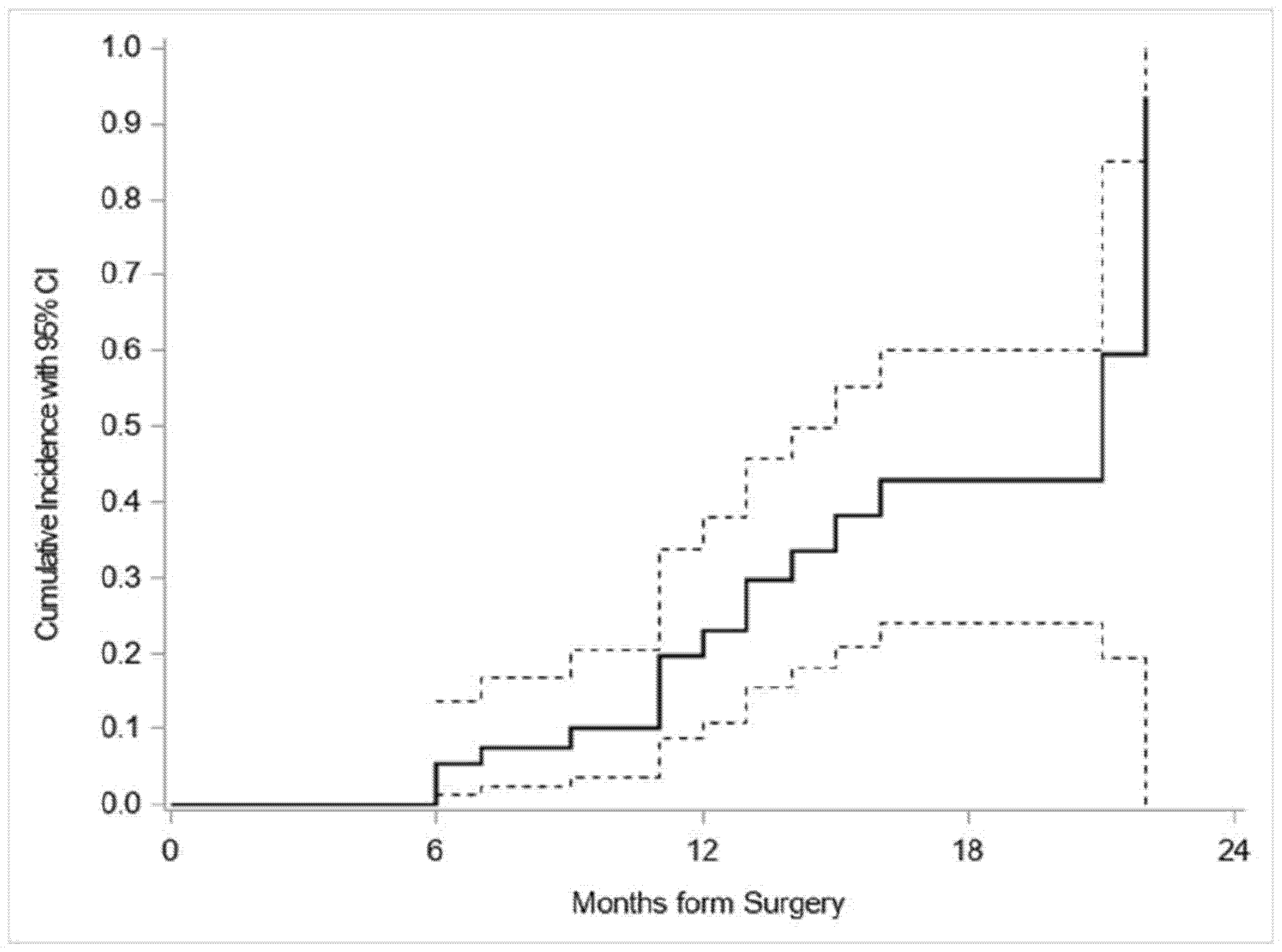
| Months from Surgery | Patients Relapsed (Failed Events) | CIF a (%) |
|---|---|---|
| 0 | 0 | 0 |
| 6 | 3 | 5.4 |
| 12 | 9 | 23.0 |
| 22 | 16 | 93.6 |
| Risk Factor at Surgery | HR (95% CI) | p-Value | |
|---|---|---|---|
| Age | 0.98 a (0.75–1.27) | 0.87 | |
| Tumor Size | 1.06 b (0.86–1.30) | 0.59 | |
| Hemoglobin | 0.77 c (0.53–1.12) | 0.17 | |
| Neutrophiles | 1.08 c (0.83–1.39) | 0.58 | |
| Lymphocytes | 1.01 c (0.78–1.29) | 0.96 | |
| Neutrophil/Lymphocytes Ratio | 1.08 c (0.81–1.43) | 0.62 | |
| RDW % | 1.29 c (1.04–1.59) | 0.02 | |
| N1 Status | N1A | 1 | |
| N1B | 2.63 (0.90–7.64) | 0.08 | |
| Gender | Females | 1 | |
| Males | 0.86 (0.30–2.42) | 0.77 | |
| Smoker | No | 1 | |
| Yes | Not estimable | ||
| Surgery | Open | 1 | |
| MIS d | 0.77 (0.27–2.19) | 0.62 | |
| pT | 1–2 | 1 | |
| 3–4 | 1.83 (0.73-4.59) | 0.20 | |
| Grading | 1–2 | 1 | |
| 3 | 1.21 (0.38–3.86) | 0.75 | |
| Stage | 3A | 1 | |
| 2B | 0.55 (0.22–1.37) | 0.55 | |
| Treatments | No | 1 | |
| Yes | 0.46 (0.13–1.61) | 0.23 | |
| Procedure | Lobectomy | 1 | |
| Pneumonectomy | 0.39 (0.06–2.71) | 0.34 | |
| Risk Factor at Surgery | HR (95% CI) | p-Value | |
|---|---|---|---|
| Age at Surgery | 0.97 a (0.75–1.26) | 0.82 | |
| RDW % | 1.35 b (1.11–1.65) | 0.003 | |
| N1 Status | N1A | 1 | |
| N1B | 3.61 (1.36–9.58) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrella, F.; Casiraghi, M.; Radice, D.; Prisciandaro, E.; Rizzo, S.; Spaggiari, L. Prognostic Value of Red Blood Cell Distribution Width in Resected pN1 Lung Adenocarcinoma. Cancers 2020, 12, 3677. https://doi.org/10.3390/cancers12123677
Petrella F, Casiraghi M, Radice D, Prisciandaro E, Rizzo S, Spaggiari L. Prognostic Value of Red Blood Cell Distribution Width in Resected pN1 Lung Adenocarcinoma. Cancers. 2020; 12(12):3677. https://doi.org/10.3390/cancers12123677
Chicago/Turabian StylePetrella, Francesco, Monica Casiraghi, Davide Radice, Elena Prisciandaro, Stefania Rizzo, and Lorenzo Spaggiari. 2020. "Prognostic Value of Red Blood Cell Distribution Width in Resected pN1 Lung Adenocarcinoma" Cancers 12, no. 12: 3677. https://doi.org/10.3390/cancers12123677
APA StylePetrella, F., Casiraghi, M., Radice, D., Prisciandaro, E., Rizzo, S., & Spaggiari, L. (2020). Prognostic Value of Red Blood Cell Distribution Width in Resected pN1 Lung Adenocarcinoma. Cancers, 12(12), 3677. https://doi.org/10.3390/cancers12123677






