Simple Summary
High lymphocytic infiltration (TILs) seem to reflect favorable host antitumor immune responses. In breast cancer, the variation of TILs before and after neoadjuvant chemotherapy (NAC) according to BRCA status has been poorly described. Little data is available on their value after treatment. We investigated TIL levels before and after NAC and response to treatment in 267 paired biopsy and surgical specimens. In our study, luminal BCs were associated with pathologic complete response (pCR) and higher TIL levels after chemotherapy completion in patients with BRCA pathogenic mutations. Our data supports that (i) NAC should be reconsidered in luminal BCs with BRCA pathogenic mutation, (ii) TILs could be a biomarker for response to immune checkpoint blockade in luminal BCs with BRCA pathogenic variant who did not achieve a pCR and (iii) exploiting the antitumor immune response in luminal BCs could be an area of active research.
Abstract
Introduction: Five to 10% of breast cancers (BCs) occur in a genetic predisposition context (mainly BRCA pathogenic variant). Nevertheless, little is known about immune tumor infiltration, response to neoadjuvant chemotherapy (NAC), pathologic complete response (pCR) and adverse events according to BRCA status. Material and Methods: Out of 1199 invasive BC patients treated with NAC between 2002 and 2012, we identified 267 patients tested for a germline BRCA pathogenic variant. We evaluated pre-NAC and post-NAC immune infiltration (TILs). Response to chemotherapy was assessed by pCR rates. Association of clinical and pathological factors with TILs, pCR and survival was assessed by univariate and multivariate analyses. Results: Among 1199 BC patients: 46 were BRCA-deficient and 221 BRCA-proficient or wild type (WT). At NAC completion, pCR was observed in 84/266 (31%) patients and pCR rates were significantly higher in BRCA-deficient BC (p = 0.001), and this association remained statistically significant only in the luminal BC subtype (p = 0.006). The interaction test between BC subtype and BRCA status was nearly significant (Pinteraction = 0.056). Pre and post-NAC TILs were not significantly different between BRCA-deficient and BRCA-proficient carriers; however, in the luminal BC group, post-NAC TILs were significantly higher in BRCA-deficient BC. Survival analysis were not different between BRCA-carriers and non-carriers. Conclusions: BRCA mutation status is associated with higher pCR rates and post-NAC TILs in patients with luminal BC. BRCA-carriers with luminal BCs may represent a subset of patients deriving higher benefit from NAC. Second line therapies, including immunotherapy after NAC, could be of interest in non-responders to NAC.
1. Introduction
Neoadjuvant or pre-operative chemotherapy (NAC) is classically administered to patients with inflammatory or locally advanced breast cancer (BC). Beyond increasing breast-conserving surgery rates [1], it also serves as an in vivo chemosensitivity test and the analysis of residual tumor burden may help understanding treatment resistance mechanisms [2]. In addition, it helps refining the prognosis of patients after NAC, as pathological complete response (pCR) after NAC is associated with a better long-term survival [1,3].
Nearly 5% of breast cancers occur in a context of genetic predisposition, mostly represented by monoallelic pathogenic variants of BRCA1, BRCA2 or PALB2 genes [4]. Patients with loss-of-function of the BRCA1 or 2 proteins have a higher cumulated breast cancer risk, with a cumulated lifetime risk at eighty years old of 72% (BRCA1) and 69% (BRCA2) [5]. The peak incidence for BRCA1 mutation carriers occurs between 41 and 50 years old (28.3 per 1000 person-years), whereas it occurs ten years later for BRCA2 mutation carriers (30.6 per 1000 person-years between 51 and 60) [5]. BRCA1 and BRCA2 are tumor-suppressor genes that code for proteins involved in homologous recombination (HR) repair. HR deficiency (HRD) occurs when the second allele is inactivated by allelic deletion (often detected by LOH), genic alteration or promoter methylation (for BRCA1 only). Biallelic BRCA1/2 inactivation results in genomic instability and theoretically increases the somatic mutational load [6].
Tumors associated with germline or somatic BRCA1/2 pathogenic mutations display different patterns when compared with sporadic BCs. Cancers occurring among BRCA1 carriers are more frequently classified as medullary [7], whereas histological subtypes among BRCA2 carriers tend to be more heterogeneous [8]. In addition, BRCA1 carriers are more frequently ER-negative, PR-negative and lack HER2 amplification (i.e., display a triple negative (TNBCs) phenotype [9]) whereas in BRCA2 carriers, a similar prevalence of ER-positive tumors has been described when compared with sporadic controls [10,11,12,13].
Most of patients with TNBCs receive chemotherapy [14,15]. Due to the alteration of BRCA1 and BRCA2 proteins in tumor cells, BRCA-mutated cells are unable to properly repair double-strand breaks, classically induced by DNA-alkylating agents [16]. Hence, BRCA deficiency has sometimes been associated with a higher sensitivity to platinum agents when compared to other types of neoadjuvant chemotherapy regimens [17,18,19]. In a recent meta-analysis of platinum-based neoadjuvant chemotherapy in TNBC, the addition of carboplatin was not associated with significantly increased pCR rate in BRCA-mutated patients (OR = 1.17, CI95% [0.51–2.67], p = 0.711) [20]. So far, the benefit of adding a platinum agent in BRCA-mutated patients receiving standard neoadjuvant chemotherapy remains a matter of debate. Nevertheless, beyond the controversy upon platinum-based agents in BRCA-deficient tumors, the effectiveness of standard NAC in all BC subtypes associated with BRCA pathogenic variants compared to controls has been poorly explored so far.
The role of tumor infiltrating lymphocytes (TILs) in BC has been extensively studied over the last decade. High levels of TILs before NAC are associated with higher pCR rates and better survival, especially for TNBC and HER2-positive BCs [21,22]. However, despite a growing interest in the field of immunity and oncology, characterization and quantification of TILs across all BC subtypes according to BRCA status has not been extensively described. Similarly, no study has evaluated so far, the evolution of immune infiltration after NAC according to BRCA status.
The objective of the current study is to determine if pre and post-NAC TILs, chemosensitivity and prognosis differ according to BRCA status in a cohort of BC patients treated with NAC.
2. Results
2.1. Study Population and Tumors Characteristics
The total number of patients included in the neoadjuvant cohort was 1199. Among the whole population, germline BRCA pathogenic variant status was available for 267 patients (22.3%), and was not obtained for 932 patients (77.73%, Figure S1). Median age of cohort’s population was 48 years old (range 24–80) and most patients (n = 747, 62%) were premenopausal. Median BMI index was 24.74, and 25.8% had direct family history of breast cancer. Patients repartition by subtype was as follows: luminal (n = 518, 44%), TNBC (n = 376, 31%), HER2-positive (n = 295, 25%).
Patients with available BRCA status were significantly different from patients with BRCA status unknown. They were younger, had lower body mass index, were more likely to be diagnosed with grade III, TNBC of no specific type (NST), and to receive standard anthracyclines-taxanes containing regimens than patients not screened (p < 0.001) (Table 1, Figure S2).

Table 1.
Patients’ characteristics among the whole population.
Among the 267 screened patients, the distribution of BRCA status was as follows: BRCA-proficient n = 221 (83%); BRCA-deficient, n = 46 (17%) (BRCA1-deficient, n = 31 (67.39%); BRCA2-deficient, n = 14 (30.43%) and BRCA1+2-deficient, n = 1 (2.17%)). Median age at diagnosis for patient with available BRCA mutation status was 40 years old (range 24–70) and most patients (n = 227, 85%) were premenopausal. Patients repartition by subtype was as follows: luminal (n = 90, 33.7%), TNBC (n = 110, 41.2%), HER2-positive (n = 67, 25.1%) (Table S1, Figure S2).
Carriers of a BRCA pathogenic variant were more likely to have familial history of breast cancer (73.9% (34/46) vs. 52.3% (114/218), p = 0.012), and to be diagnosed with TNBC (58.7% (27/46) versus 37.6% (83/221), p = 0.006) than BRCA-proficient patients (Table 1). No other pattern among age, body mass index, histology, tumor size, nor proliferation indices (grade, mitotic index, KI67) was significantly different according to BRCA variant status. These results were substantially similar after the subgroup analysis of BC subtype (Table S2).
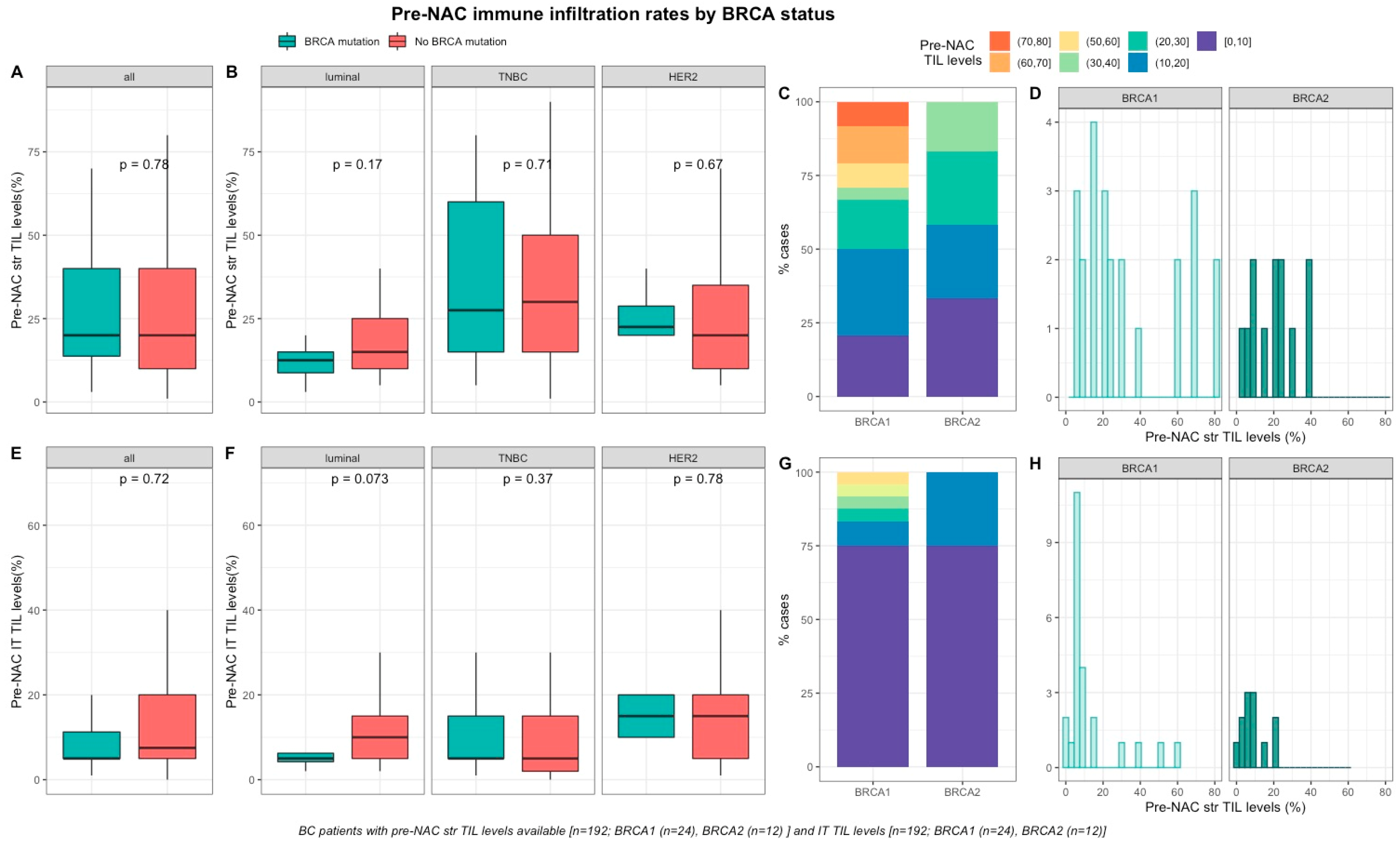
Baseline TILs were available for 192 out of 267 screened patients (72%). Neither pre-NAC str TIL levels (Figure 1A–D), nor IT TILs (Figure 1E–H) were significantly different by BRCA status (Table S1), nor in each BC subtype (Table S2). There was a strong, positive, linear relationship between stromal and intra-tumoral TILs (Spearman correlation coefficient of 0.74, p < 0.001, Figure S3)
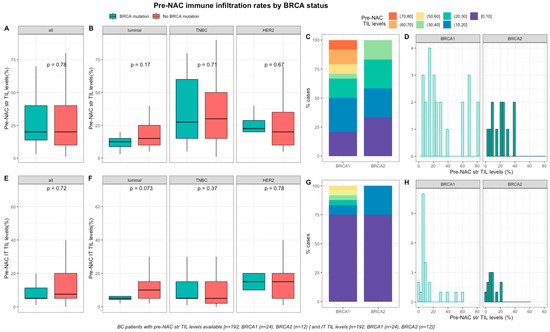
Figure 1.
Associations between pre-NAC TILs and BRCA status in whole population, and by breast cancer subtype. Bottom and top bars of the boxplots represent the first and third quartiles, respectively, the medium bar is the median, and whiskers extend to 1.5 times the interquartile range. (A) stromal lymphocytes among the whole population (All (n = 192), BRCA mutation (n = 36), BRCA wild-type (n = 156)). (B) stromal lymphocytes in each BC subtype (Luminal (n = 52), BRCA mutation (n = 8), BRCA wild-type (n = 44); TNBC (n = 97), BRCA mutation (n = 24), BRCA wild-type (n = 73); HER2 (n = 43), BRCA mutation (n = 4), BRCA wild-type (n = 39)). (C) percentage of tumor according to pre-NAC stromal lymphocytes levels binned by 10% increment in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (D) distribution of pre-NAC stromal lymphocytes by gene mutations (histogram plot) in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (E) intratumoral lymphocytes among the whole population (All (n = 192), BRCA mutation (n = 36), BRCA wild-type (n = 156)). (F) intratumoral lymphocytes in each BC subtype (Luminal (n = 52), BRCA mutation (n = 8), BRCA wild-type (n = 44); TNBC (n = 97), BRCA mutation (n = 24), BRCA wild-type (n = 73); HER2 (n = 43), BRCA mutation (n = 4), BRCA wild-type (n = 39)). (G) Percentage of tumor according to pre-NAC intratumoral lymphocytes levels binned by 10% increment in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (H) distribution of pre-NAC intratumoral lymphocytes by gene mutations (histogram plot) in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)).
2.2. Response to Treatment and Post-NAC Immune Infiltration
2.2.1. Response to Treatment
At NAC completion, pCR was observed in 84 out of 266 (31%) patients and pCR rates were significantly different by BC subtype (luminal: 10% (9/89), TNBC: 45% (49/110) and HER2-positive 39% (26/67), p < 0.001). Pre-NAC str TIL levels were significantly higher in tumors for which pCR was achieved (p < 0.001) and there was a significant association between pre-NAC TIL levels and pCR status in the whole population (all: OR = 1.03, CI95% [1.02–1.05], p < 0.001; Figure S3) and in the TNBC subgroup (luminal: OR = 1.03, CI95% [1–1.09], p = 0.21; TNBC: OR = 1.03; CI95% [1–1.04], p = 0.007; HER2-positive: OR = 1.02, CI95% [0.99–1.06], p = 0.23; Figure S4).
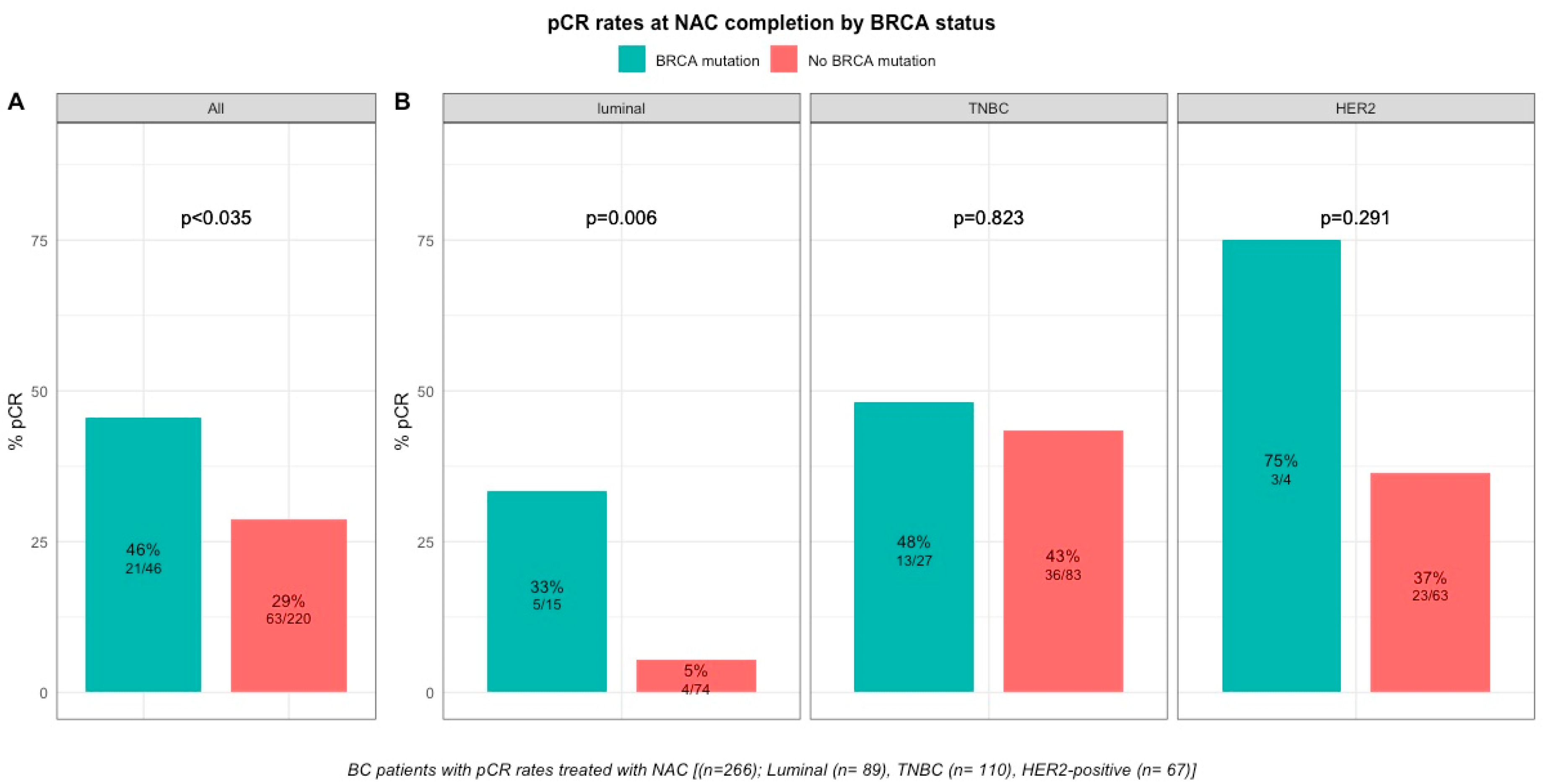
pCR rates were significantly higher in patients with BRCA-deficient breast cancers (45.7% (21/46) versus 28% (63/221) in BRCA-proficient, p < 0.035, Table S1, Figure 2). After the subgroup analysis of BC subtype, this was confirmed only in the luminal BC subtype (33.3% (5/15), p = 0.006), but not in TNBC and HER2-positive BCs (48.1% (13/27), p = 0.823 and 75% (3/4), p = 0.291, respectively, Table S2, Figure 2). The interaction test between BC subtype and BRCA status was nearly significant (Pinteraction = 0.056). There were no differences in pCR rates by BRCA1 or BRCA2 mutation status in patients with BRCA-deficient tumors (BRCA1, 42% (13/31) versus BRCA2, 50% (7/14), p = 0.7; Figure S5) but the effective of the subpopulations were limited.
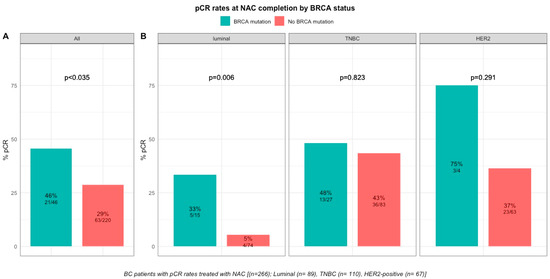
Figure 2.
Barplot of associations between response to treatment and BRCA status in whole population, and by breast cancer subtype. (A), among the whole population (All (n = 266), BRCA mutation (n = 46), BRCA wild-type (n = 220)). (B), by BC subtype (Luminal (n = 89), BRCA mutation (n = 15), BRCA wild-type (n = 74); TNBC (n = 110), BRCA mutation (n = 27), BRCA wild-type (n = 83); HER2 (n = 67), BRCA mutation (n = 4), BRCA wild-type (n = 63)).
However, BRCA status was not significantly associated with pCR after multivariate analysis, and only BC subtype (TNBC, OR = 7.14, CI95% [3.39–16.57], p < 0.001; HER2-positive, OR = 5.64, CI95% [2.5–13.78], p = 0.001), tumor size (T2, OR = 0.37, CI95% [0.16–0.83], p = 0.017; T3, OR = 0.21, CI95% [0.08–0.55], p = 0.002) and pre-NAC str and IT TILs (OR = 1.03, CI95% [1.02–1.05], p = 0.001 and OR = 1.04, CI95% [1.02–1.07], p = 0.002) were independent predictors of pCR (Table S3).
2.2.2. Post-NAC Immune Infiltration by BRCA Status
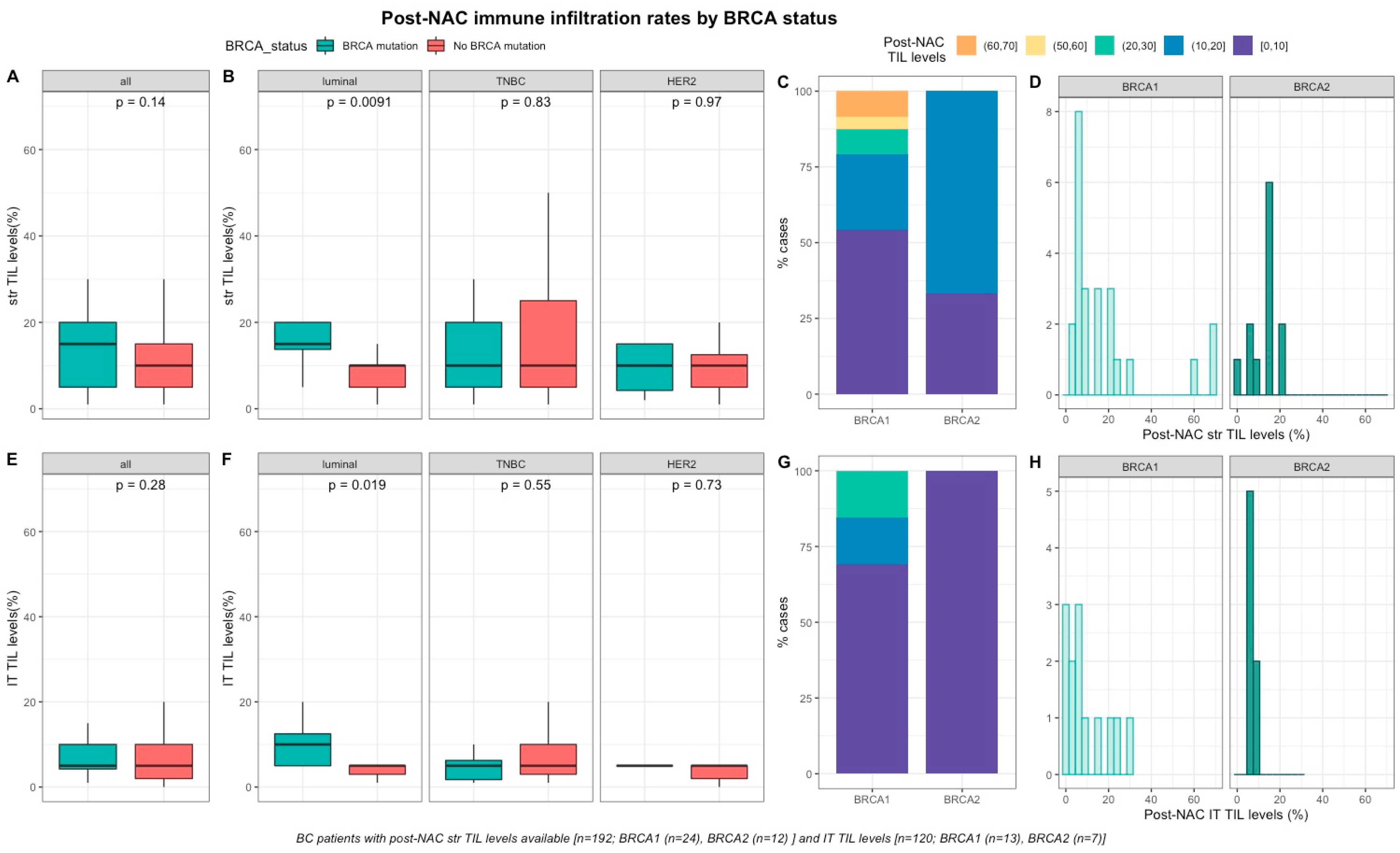
After NAC, str and IT TILs were available in 192 (72%) and 120 (45%) patients respectively. Post-NAC immune infiltration (whether intra-tumoral or stromal) was not significantly different between BRCA-deficient and BRCA-proficient carriers (Table S1, Figure 3A–E). However, both str and IT TIL levels were significantly higher in tumors with BRCA pathogenic mutations when compared with wild-type tumors in luminal BCs (median str TIL levels: 15% vs. 10%, p = 0.009 and median IT TIL levels: 10% vs. 5%, p = 0.019, respectively, Table S2, Figure 3).
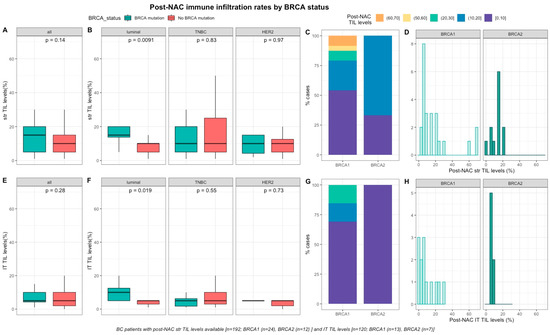
Figure 3.
Associations between post-NAC TILs and BRCA status in whole population, and after stratification by breast cancer subtype. Bottom and top bars of the boxplots represent the first and Table 1. 5 times the interquartile range. (A) stromal lymphocytes among the whole population (All (n = 192), BRCA mutation (n = 36), BRCA wild-type (n = 156)). (B) stromal lymphocytes in each BC subtype (Luminal (n = 52), BRCA mutation (n = 8), BRCA wild-type (n = 44); TNBC (n = 97), BRCA mutation (n = 24), BRCA wild-type (n = 73); HER2 (n = 43), BRCA mutation (n = 4), BRCA wild-type (n = 39)). (C) Percentage of tumor according to post-NAC stromal lymphocytes levels binned by 10% increment in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (D) distribution of post-NAC stromal lymphocytes by gene mutations (histogram plot) in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (E) intratumoral lymphocytes among the whole population (All (n = 120), BRCA mutation (n = 20), BRCA wild type (n = 100)). (F) intratumoral lymphocytes in each BC subtype (Luminal (n = 44), BRCA mutation (n = 7), BRCA wild-type (n = 37); TNBC (n = 50), BRCA mutation (n = 12), BRCA wild-type (n = 38); HER2 (n = 26), BRCA mutation (n = 1), BRCA wild-type (n = 25)). (G) percentage of tumor according to post-NAC intratumoral lymphocytes levels binned by 10% increment in patients with BRCA-deficient (BRCA1 (n = 13), BRCA2 (n = 7)). (H) distribution of post-NAC intratumoral lymphocytes by gene mutations (histogram plot) in patients with BRCA-deficient (BRCA1 (n = 13), BRCA2 (n = 7)).
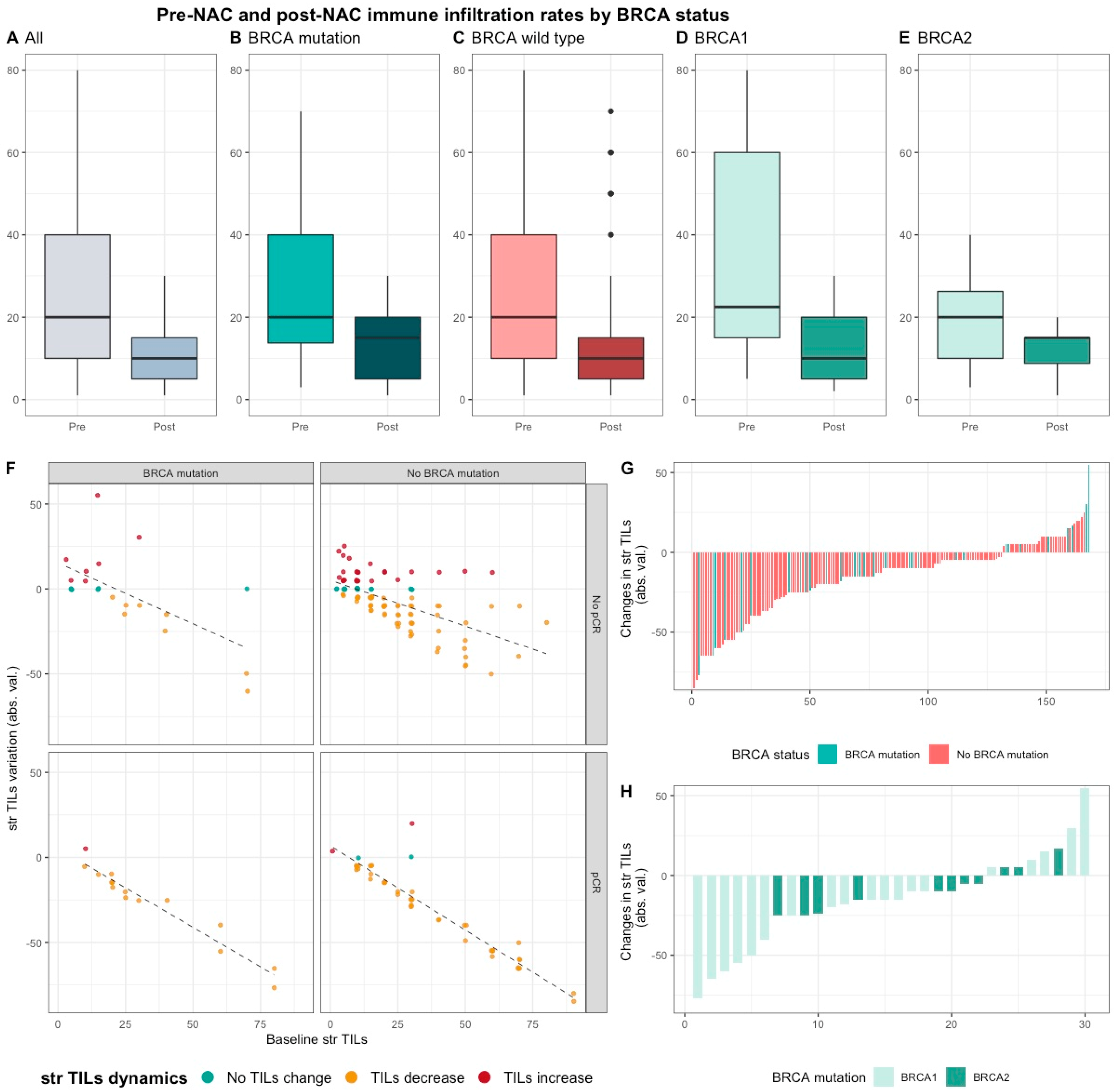
Median pre-NAC str TIL were higher than after NAC (20% vs. 10%, 11.95%), also according to BRCA status and type (Table S1, Figure 4). There was no correlation between pre and post NAC str TILs (correlation coefficient of 0.13 and p < 0.06, Figure S6A) and there was a weak, positive, linear relationship between pre and post NAC IT TIL levels (correlation coefficient of 0.31 and p < 0.001, Figure S6B).
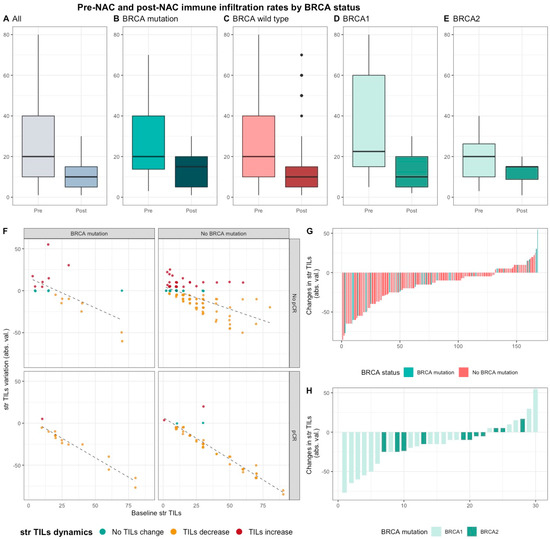
Figure 4.
Pre-NAC and post-NAC stromal immune infiltration rates in the whole population and by BRCA status. (A–E) bar plots of str TIL levels before and after NAC in the whole population and in BRCA pathogenic variant. Bottom and top bars of the boxplots represent the first and third quartiles, respectively, the medium bar is the median, and whiskers extend to 1.5 times the interquartile range. (All (n = 192); BRCA mutation (n = 36), BRCA wild-type (n = 156); BRCA1 (n = 24), BRCA2 (12)). (F) variation of str TIL levels according to the pre-NAC str TIL levels binned by BRCA status and response to chemotherapy. Points represent the difference between pre- and post-NAC paired TIL levels values of a given patient and are colored according to TIL variation category (TIL level decrease: yellow/no change: green/increase: red) (All (n = 191), BRCA mutation (n = 36), BRCA wild-type (n = 155)). (G–H) waterfall plot representing the variation of TIL levels according to BRCA-deficient (BRCA1-deficient, BRCA2-deficient); each bar represents one sample, and samples are ranked by increasing order of TIL level change. Paired samples for which no change was observed have been removed from the graph. (All (n = 191), BRCA mutation [(n = 36), BRCA1, n = 24; BRCA2 = 12)], BRCA wild-type (n = 155)).
2.2.3. Survival Analysis
After a median of follow-up of 90.4 months (range from 0.2 to 187 months), 73 patients experienced relapse, and 38 died. RFS and OS were not significantly different between carriers of a BRCA pathogenic variant and BRCA-proficient patients, neither were they in screened population nor after the subgroup analysis of BC subtype (Figures S7 and S8).
3. Discussion
In the current study, we did not identify any association between BRCA status and immune infiltration, whatever the type of TILs (IT, str). We found a better response to neoadjuvant chemotherapy in tumors associated with a germline BRCA pathogenic variant when compared to BRCA-WT, however the latter was limited to the restricted group of luminal BCs (BRCA-proficient n = 75; BRCA-deficient, n = 15) and was not statistically significant after multivariate analysis, possibly due to the small sample size of the population. Probably in relation, we recovered higher post-NAC lymphocyte infiltration in BRCA-deficient tumors in the luminal BC subgroup.
Regarding pre-treatment immune infiltration, Sønderstrup and colleagues [23] analyzed str TIL levels in a nationwide cohort of BRCA1 and BRCA2 carriers with primary BCs. They found a greater prevalence of high stromal TILs (defined as TILs-positive tumors with ≥ 60% str TILs) in BRCA1-deficient tumors (n = 243) when compared with BRCA2-deficient tumors (n = 168) (36% versus 15% respectively, p < 0.0001). However, no control group with BRCA-WT tumors was available in this study. In a small study of 85 TNBC patients, Solinas and colleagues [24] investigated the distribution of TILs subpopulations. The tumors of patients in the BRCA1 or BRCA2-mutated group displayed a higher prevalence of TILs-positive tumors (defined as tumors with ≥ 10% str or IT TILs) when compared with the BRCA-WT (93.2% versus 75.6% respectively, p = 0.037). No other statistically significant differences were identified between BRCA-carriers and non-carriers, neither in TILs subpopulations nor their location. More recently, Telli and colleagues [25] investigated the association between TILs, homologous recombination deficiency (HDR) and BRCA1/2 status in a cohort of 161 TNBC patients pooled from five phase II neoadjuvant clinical trials of platinum-based therapy. They found that IT TILs and str TILs density were not associated with BRCA1/2 status (p = 0.312 and p = 0.391, respectively). Consistently with Telli et al., we did not observe any difference in baseline immune infiltration according to BRCA status.
Some retrospective studies suggested that tumors displayed higher chemosensitivity according to BRCA-mutation status [17,18,19,26,27,28,29,30,31,32]. Arun et al. [30] compared pCR rates after NAC between BRCA1 or BRCA2-carriers (n = 57 and n = 23, respectively) and WT controls (n = 237). The majority of patients (82%) received an anthracycline-taxane containing regimen as NAC. The authors found that BRCA1 mutation was an independent positive predictor of pCR (OR = 3.16, 95%CI 1.55–6.42, p = 0.002). In the largest study so far, Wunderle et al. [18] investigated efficacy of chemotherapy among a cohort of 355 patients composed with 16.6% (59/355) of BRCA-carriers. Across all BC subtypes, 64.4% of patients with a BRCA1/2 pathogenic variant received anthracycline-based treatments, while the rest received carboplatin. pCR was observed in 54.3% (32/59) of all BRCA1/2 mutation carriers, and in 39.5% (15/34) of the BRCA-carriers versus 13% of the WT BCs in the anthracycline-regimen (Table 2). In our cohort, we found similar results after univariate analysis, and we additionally evidenced a nearly significant interaction with BC subtype. In addition, ongoing trials should determine whether PARP inhibitors might improve outcome when administered in the adjuvant or neoadjuvant setting in early luminal breast cancer patients with BRCA1/2 mutation [33]. The fact that our results were no longer significant after multivariate analysis is possibly due to a lack of statistical power.
Furthermore, we found that both str and IT TIL levels were higher after NAC completion in the luminal BCs. Whether this difference in post treatment TILs is a cause, a consequence, or unrelated to response to chemotherapy remains unknown. Indeed, post-NAC TIL levels have been shown to be strongly related to response to chemotherapy in BC cohorts including all BC subtypes [34,35,36,37] and response to checkpoint inhibitors (IC) in early TNBC [37]. Moreover, Anurag et al. [38] identified upregulation of the targetable immune-checkpoint components (IDO1, LAG3 and PD1) in AI-resistant luminal B tumors suggesting that luminal BC could also be immunologically “hot”. Besides, only a few studies have investigated the dynamic of TIL levels in response to NAC. Hamy et al. [36] noticed that mean TIL levels decreased after chemotherapy completion across all the BC subtype (pre-NAC TILs: 24.1% vs. post-NAC TILs: 13.0%, p < 0.001).

Table 2.
Literature Review.
Table 2.
Literature Review.
| Study | Setting/Design | Control Group | Number of Patients (n) | TNBC (n) | HER2-Positive (n) | Luminal (n) | BRCA1 | BRCA2 | BRCA 1 and 2 | Chemotherapy Regimen | sTILS Evaluation | pCR in BRCA-Carriers vs. Non-Carriers | Survival Analyses | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Byrski (2014) [26] {BCRT | Neoadjuvant epidemiologic prospective cohort | No | 10 | 10 | 0 | 10 | 0 | 0 | 0 | Cis | No | 90% | No | 90% (9/10) in BRCA1-mutated BC patients achieved a pCR after NAC with cisplatin chemotherapy |
| Byrski (2015) [27] HCCP | Neoadjuvant epidemiologic prospective cohort | No | 107 | 82 | 2 | NA | 107 | 0 | 0 | Cis | No | 61% | No | 61% (65/107) in BRCA1-mutated BC patients achieved pCR after NAC with cisplatin chemotherapy. In this study of BRCA1-mutation carriers, a pCR was also achieved in 56% of 16 patients with ER-positive BC. No survival analysis were provided in the current study. |
| Hanhnen (2017) [28] JAMA Oncology | Neoadjuvant secondary analysis of the GeparSixto randomized clinical trial | Yes | 291 | 291 | 0 | 0 | 50 | 0 | P + Dox + Bev ± Cb | No | 66.7% vs. 36.4% | Yes | Patients with BRCA-mutation did not derive a pCR benefit from the addition of carboplatine (65.4% vs. 66.7%) compared to non-BRCA carriers (55% vs. 36.4%). No significant difference in overall prognosis observed in the BRCA-mutated subgroup. | |
| Sharma (2017) [39] CCR | Neoadjuvant prospective, multicenter, non-randomized trial | Yes | 190 | 190 | 0 | 0 | 30 | 0 | Cb + D | No | 59% vs. 56% | No | No significative difference in pCR between BRCA-carriers and WT TNBC (59% and 56%, respectively (p = 0.83)). The Cb-D regimen was well tolerated and yielded high pCR rates in both BRCA associated and WT TNBC. These results are comparable to pCR of previous studies (who investigated pCR after NAC with addition of Cb to AT regimen in TNBC cohort). | |
| Poggio (2018) [20] Annals of Oncology | Neoadjuvant meta-analysis of nine randomized controlled trials | No | 96 | 96 | 0 | 0 | 96 | 0 | P + Dox + Bev ± Cb P + AC ± Cb | No | 54.3% | No | Among 96 BRCA-mutated patients included in 2 controlled trials, the addition of carboplatin was not associated with increased pCR rate (OR 1.17, 95% CI 0.51–2.67, p = 0.711). No survival analyses were available according to BRCA status. | |
| Telli (2019) [25] CCR | Five randomized controlled trials | Yes | 161 | 161 | 0 | 0 | 34 | 0 | Cb + Gem + Iniparib; Cis; Cis + Bev; Cb + Eribulin; Cb + nab-P ± Vorinostat | Yes | No | No | pCR was achieved in 51 (31.7%) patients. In patients with TNBC treated with neoadjuvant platinum-based therapy, iTIL and sTIL densities were not significantly associated with BRCA1/2-mutated tumor status (p = 0.312 and p = 0.391). In multivariate analyses, sTIL density (OR 1.23, 95% CI 0.94–1.61, p = 0.139) was not associated with pCR, but was associated with RCB 0/I status (OR 1.62, 95% CI 1.20–2.28, p = 0.001). | |
| Sønderstrup (2019) [23] Acta Oncologica | Epidemiologic prospective mulitcentric cohort (nationwide) | No | 411 | NA | 24 | NA | 243 | 168 | 0 | NA | Yes | No | Yes | High sTILs (defined as TILs > 60%) were observed in 36% in BRCA1- and 15% in BRCA2-mutated tumors (p < 0.0001). Significant association with survival (OS and DFS) was observed in BRCA1 subgroup. sTILs are an important prognostic factor in BRCA BC and increasing sTILs is associated with a better prognosis. |
| Byrski (2009) [17] JCO | Neoadjuvant Epidemiologic epidemiologic retrospective cohort | No | 102 | NA | 6 | NA | 102 | 0 | 0 | CMF; AT; AC FAC or Cis | No | 23.5% | No | pCR was achieved in 23.5% of 102 patients with a BRCA1 mutation who received NAC. Especially, a complete pCR was observed in 8% (2/25) with AT- regimen (standard of care) compared to 83% (10/12) with cisplatin. |
| Chappuis (2002) [29] JMG | Neoadjuvant Retrospective retrospective multicentric clinical trial | Yes | 38 | NA | NA | NA | 7 | 4 | 0 | FAC; AC; CEF AC + CMF AC + D | No | 44% vs. 4% | No | pCR was achieved in 44% (4/11) of the BRCA-carriers and 4%(1/27) of the non-carriers (p = 0.009). No survival analysis were experienced in this study. |
| Arun (2011) [30] JCO | Neoadjuvant Epidemiologic epidemiologic retrospective cohort | Yes | 317 | 77 | 60 | NA | 57 | 23 | 0 | A-single agent; AT or T-single-agent | No | 46% vs. 22% | Yes | pCR was achieved in 46% of BRCA1-carriers and 13% of BRCA2-carriers and 22% of BRCA non-carriers (<0.001). In the multivariate logistic model, BRCA1 status (OR = 1.96, p = 0.03) remained as independant significant predictors of a pCR. No significant difference in overall prognosis. |
| Wang (2014) [40] Annals of Oncology | Neoadjuvant Epidemiologic retrospective cohort | Yes | 652 | 652 | 0 | 0 | 52 | NA | 0 | A-single agent; AT or T-single-agent | No | 53.8% vs. 29.7% | Yes | The pCR rate was 31.6% in the 652 patients who received NAC. BRCA1 carriers had a significantly higher pCR rate than non-carriers (BRCA1 carriers versus non-carriers, 53.8% versus 29.7%, p < 0.001). Among women treated with anthracycline with or without taxane regimens, the pCR rate was 57.1% for BRCA1 carriers, 29.0% for non-carriers (p < 0.001). The RFS was similar according to BRCA status. |
| Paluch-Shimon(2016) [31] BCRT | Neoadjuvant epidemiologic retrospective cohort | Yes | 80 | 80 | 0 | 0 | 34 | 0 | 0 | AT | No | 68% vs. 37% | Yes | The BRCA1-carriers had pCR rate of 68% compared with 37% among non-carriers, p = 0.01. Yet this did not translate into superior survival for BRCA1 carriers compared with non-carriers. |
| Bignon (2017) [41] Breast | Neoadjuvant epidemiologic retrospective cohort | No | 53 | 53 | 0 | 0 | 46 | 6 | 1 | A-single agent or AT | No | 66% | Yes | The pCR rate was 38.3% [95% CI, 26%–55%] among BRCA1 mutation carriers, and 66% among the 6 BRCA2 mutation carriers. 15 relapses and 6 s cancers were recorded during the follow-up period. 11 deaths occurred, all of which were in the non-pCR group. DFS (p < 0.01) and OS (p < 0.01) were significantly better in the pCR group than the non-pCR group. |
| Wunderle (2018) [18] BCRT | Neoadjuvant Epidemiologic retrospective cohort | Yes | 355 | 138 | 58 | 159 | 43 | 16 | 0 | AT; Cb | No | 54.3% vs. 12.6% | Yes | pCR was observed in 54.3% of BRCA1/2 mutation carriers, but only in 12.6% of non-carriers. The adjusted odds ratio was 2.48 (95% CI 1.26–4.91) for BRCA1/2 carriers versus non-carriers. No difference in overall survival was observed. |
| Saether (2018) [32] HCCP | Neoadjuvant Epidemiologic retrospective cohort | No | 12 | NA | NA | NA | 12 | 0 | 0 | Cis + Dox or Cb + D | No | 83% | No | 11 patients received a combination of cisplatin and doxorubicin, and 1 patient received carboplatin and docetaxel. 83% (10/12) of the BRCA1-carriers achieved pCR. This results were comparable to existing results found in similar studies. No information about BC subtype among the study population and the toxicity of the chemotherapy was not evaluated. |
| Sella (2018) [19] Breast | Neoadjuvant Epidemiologic retrospective cohort | Yes | 43 | 43 | 0 | 0 | 14 | 0 | 0 | AT ± Cb | No | 67% vs. 38% | No | pCR was achieved in 38% in BRCA WT compared to 67% in BRCA-associated TNBC (p = 0.232). No benefit from the addition of carboplatine in BRCA-carriers (64.3% vs. 67%) compared to non-BRCA carriers (44.8% vs. 38%) when compared to historic institutional rates with AT. |
| Solinas (2019) [24] Cancer Letters | Epidemiologic retrospective cohort | Yes | 85 | 85 | 0 | 0 | 38 | 6 | 0 | NA | Yes | No | Yes | The BRCA-mutated tumors had a significantly higher incidence of TIL-positive levels compared to WT (44% and 41%, respectively p = 0.037). No significant difference between BRCA-mutated and WT groups neither in TIL subpopulation nor their location. No difference in I-DFS and OS after stratification on TIL infiltration levels. |
| Our study (2020) | Epidemiologic retrospective cohort | Yes | 267 | 110 | 67 | 90 | 31 | 14 | 1 | A-single agent; AT or T-single-agent | Yes | 45.7% vs 28% | Yes | Among the whole population, 84 tumors achieved a pCR (31.5%). After stratification by BC subtype, pCR rates were significantly higher in luminal BRCA-mutated BCs when compared with WT tumors (33.3% vs. 5.4%, p = 0.006).Pre and post-NAC str or IT TILs were not significantly different between BRCA-carriers and non-carriers in whole population. In the luminal BC, both str and IT post-NAC TIL levels were significantly higher in BRCA-mutated tumors when compared with WT tumors but was no longer significant after multivariate analysis. No difference in RFS or OS between BRCA-mutated and BRCA-WT patients. |
Abbreviations: CMF = cisplatine-methotrexate-fluorouracile; AT = doxorubicine-docetaxel; AC = doxorubicine-cyclophosphamide; FAC = fluorouracile-doxorubicin-cyclophophosphamide; CEF = cyclophosphamide-epirubicine-fluorouracile; A = anthracycline; Dox = doxorubicine; D = docetaxel; Cb = carboplatin; Cis = cisplatine; Bev = bevacizumab; Gem = gemcitabine; Nab-P = nabpaclitaxel; P = paclitaxel; T = taxane.
This decrease was strongly associated with high pCR rates, and the variation of TIL levels was strongly inversely correlated with pre-NAC TIL levels (and the variation of TIL levels was strongly inversely correlated with pre-NAC TIL levels (r = −0.80, p < 0.001).
Finally, in line with several recently published clinical studies [42,43,44], we found that survival outcomes were not different between BRCA-carriers and non-carriers. A multivariate study, including 223 BC patients carrying BRCA pathogenic variants and 446 controls with sporadic BC matched for age and year of diagnosis, showed no difference in terms of specific BC survival between BRCA1 or BRCA2 mutation carriers and controls [45]. Templeton et al. evaluated a total of 16 studies comprising data from 10,180 patients and concluded that BRCA pathogenic mutations were not associated with a worse overall survival [46]. The difference between the pCR rate and survival analysis could be due to several factors. First, BRCA mutation carriers are commonly offered additional treatment, including a bilateral mastectomy. Second, the increase of TILs in the surgical piece might reveals a higher immunogenic tumor, which may involve a more sustained response to treatment over time. Besides, carriers of a BRCA pathogenic variant were more likely to be diagnosed with TNBC. Copson et al. [44] have shown that BRCA mutation carriers with triple-negative breast cancer might have a survival advantage during the first few years after diagnosis compared with non-carriers. This benefit might reflect greater sensitivity of BRCA-mutant breast cancers to chemotherapy or the greater visibility to host immune attack.
Limits of our study include its retrospective observational design as well as small effectives potentially leading to a lack of statistical power. Therefore, our results might be submitted to evaluation biases, especially for the time-to-event analysis. Indeed, we present a study of patients with two rare conditions. First, according to French national guidelines, neoadjuvant chemotherapy is currently prescribed only in 15% of the patients with locally advanced breast cancers. Second, screening of inherited BRCA mutation is performed in a highly selected population representing nearly one quarter of breast cancer [47]. Our study design does not allow us to draw firm conclusions and future studies are warranted to confirm the hypotheses generated. Moreover, the incidence of bi-allelic pathogenic alterations in HR-related genes according to somatic origin is well-known and ranches from 1 to 2% [48] but we did not explore somatic mutational status in the tumor tissues in the current study. The study also has several strengths, for instance from being the largest cohort with a BRCA-WT control group, and analyses performed after stratification by BC subtype. Finally, to our knowledge, we provide data on post-NAC immune infiltration according to BRCA status for the first time.
4. Materials and Methods
4.1. Patients and Tumors
The study was performed on a retrospective institutional cohort of 1199 female patients with T1-T3NxM0 invasive BC (NEOREP Cohort, CNIL declaration number 1547270) treated with NAC at Institute Curie (Paris and Saint-Cloud) between 2002 and 2012. The cohort included unifocal, unilateral, non-recurrent, non-metastatic tumors, excluding T4 tumors (inflammatory, chest wall or skin invasion). Approved by the Breast Cancer Study Group of Institute Curie, the study was conducted according to institutional and ethical rules concerning research on tissue specimens and patients. Informed consent from patients was not required.
Information on family history, clinical characteristics (age; menopausal status; body mass index) and tumor characteristics (clinical tumor stage and grade; histology; clinical nodal status; ER, PR and HER2 status; BC subtype; mitotic index; Ki67) were retrieved from electronic medical records. All the patients received NAC, and additional treatments were decided according to national guidelines (see Supplementary Materials).
4.2. Tumors Samples
In accordance with French national guidelines [49], cases were considered estrogen receptor (ER)-positive or progesterone receptor (PR)-positive if at least 10% of tumor cells expressed estrogen and/or progesterone receptors (ER/PR), and endocrine therapy was prescribed when this threshold was exceeded. HER2 negative status was defined as 0 or 1 + on immunohistochemistry (IHC) stained tissue section. IHC 2+ scores were subsequently analyzed by fluorescence in situ hybridization (FISH) to confirm HER2 positivity. Pathological BC were classified into subtypes (TNBC, HER2-positive, and luminal HER2-negative [referred to hereafter as “luminal”]) (see Supplementary Materials).
4.3. TIL Levels, Pathological Complete Response and Pathological Review
TIL levels were evaluated retrospectively for research purposes, by two pathologists (ML and DdC) specialized in breast cancer. TIL levels were assessed on formalin-fixed paraffin-embedded (FFPE) tumor tissue samples from pretreatment core needle biopsies and the corresponding post-NAC surgical specimens, according to the recommendations of the international TILs Working Group before [50] and after NAC [51]. TILs were defined as the presence of a mononuclear cell infiltrate (including lymphocytes and plasma cells, excluding polymorphonuclear leukocytes). TILs in direct contact with tumor cells were counted as intra-tumoral TILs (IT TILs) and those in the peri-tumoral areas as stromal TILs (str TILs). They were evaluated both in the stroma and within tumor scar border, after excluding areas around ductal carcinoma in situ, tumor zones with necrosis and artifacts, and were scored continuously as the average percentage of stroma area occupied by mononuclear cells. We defined pathological complete response (pCR) as the absence of invasive residual tumor from both the breast and axillary nodes (ypT0/is N0).
4.4. BRCA Status
Genetic counseling was offered based on individual or family criteria (see Supplemental material). When constitutional genetic analysis of BRCA1 and BRCA2 genes were required, Denaturing High Performance Liquid Chromatography (DHPLC) and Sanger sequencing were performed to search for point alterations, and Quantitative Multiplex Polymerase Chain Reaction of Short Fluorescent (QMPSF) to research large gene rearrangements between 2002 and 2012. In case of previously known pathogenic familial variants, targeted tests were performed.
4.5. Survival Endpoints
Relapse-free survival (RFS) was defined as the time from surgery to death, loco-regional recurrence or distant recurrence, whichever occurred first. Overall survival (OS) was defined as the time from surgery to death. For patients for whom none of these events were recorded, data was censored at the time of last known contact. Survival cutoff date analysis was 1 February 2019.
4.6. Statistical Analysis
Pre- and post-NAC TIL levels were analyzed as continuous variables. All analyses were performed on the whole population and after stratification by BC subtype. To compare continuous variables among different groups, Wilcoxon-Mann-Whitney test was used for groups including less than 30 patients and for variables displaying multimodal distributions; otherwise, student t-test was used. Association between categorical variables was assessed with chi-square test, or with the Fisher’s exact test if at least one category included less than three patients. In boxplots, lower and upper bars represented the first and third quartile respectively, the medium bar was the median, and whiskers extended to 1.5 times the inter-quartile range. Factors predictive of pCR were introduced in a univariate logistic regression model. Covariates selected for multivariate analysis were those with a p-value no greater than 0.1 after univariate analysis. Survival probabilities were estimated by Kaplan-Meyer method, and survival curves were compared with log-rank tests. Hazard ratios (HR) and their 95% confidence intervals (CI) were calculated with the Cox proportional hazard model. Analyses were performed with R software version 3.1.2(RStudio Team (2018). RStudio Integrated Development for R.RStudio, Inc., Boston, MA URL)). The significance threshold was set at 5%.
5. Conclusions
Although the number of patients BRCA-deficient is restrained, our study raises several hypotheses. First, it generates an unprecedented hypothesis that luminal BC patients with germline BRCA pathogenic variants might represent a subset of luminal BCs that are more likely to benefit from chemotherapy as primary treatment than the whole luminal BC population. It is known that the absolute benefit of chemotherapy is lower in luminal BC than in the other BC subtypes [52]. Genetic signatures have been implemented in the daily clinical practice to complement classic prognostic factors and aid in treatment decisions with luminal-HER2 negative early-stage breast cancer [53]. While providing information on the expression of some genes related to estrogen receptor, proliferation and immunity, such tools enable a better prediction of the response to standard neoadjuvant chemotherapy. Nowadays, genomic tests allow us to scale down or escalate treatments in luminal-HER2 negative early breast cancer with intermediate prognostic factors. If further validated in independent cohorts, germline breast cancer BRCA mutation could be in the future, in a luminal context, an argument to boost a patient to chemotherapy, in addition to multigene assays. Second, patients not achieving pCR may be candidates for post-operative clinical trials exploring alternative therapeutic strategies. As post-NAC immune infiltration seems to be higher in post-NAC specimens of luminal tumors with BRCA pathogenic mutations, we can hypothesize that those tumors would be more likely to respond to checkpoint inhibitors after chemotherapy. Second line trials using immune checkpoint inhibitors (such as anti–PD-1 and anti–PD-L1 antibodies) alone or in combination, together with endocrine therapy could be a relevant strategy for patients failing to reach pCR at NAC completion.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/12/3681/s1, Figure S1: Study flow diagram of included patients and tumors samples available. Figure S2: Patients´ and tumors ‘characteristics by BRCA status. (All (n = 1199), BRCA mutation (n = 36), BRCA wild-type (n = 156), not screened (n = 1007). Figure S3: Variation of pre-NAC str TIL levels according to the pre-NAC IT TIL levels (scatterplot) (str TILs (n = 192), IT TILs (n = 192)). Figure S4: pCR rate by pre-NAC str TIL levels by BRCA status (TILs were binned by increments of 10%). Figure S5: Barplot of associations between response to treatment and BRCA pathogenic mutations treated with NAC. Figure S6: TILs correlation between pre and post-NAC. Figure S7: Relapse free survival curves according BRCA status. Figure S8: Overall survival curves according BRCA status. Table S1: BRCA screened patients ‘characteristics by BRCA status. Table S2: Patients ‘characteristics in each tumor subtype and by BRCA status. Table S3: Association of BRCA status with pCR after univariate and multivariate analysis in the whole population.
Author Contributions
Conceptualization, B.G., C.E., F.R. and A.-S.H.; methodology, B.G., C.E., F.R. and A.-S.H.; validation, E.L., F.C., J.-Y.P., E.B., C.S.(Claire Saule), D.S.-L., S.F., C.S.(Claire Sénéchal), M.L., F.R. and A.-S.H.; formal analysis, B.G., E.D., F.R. and A.-S.H.; investigation, B.G., C.E., F.R. and A.-S.H.; resources, M.L., D.D.C. and G.B.; data curation, I.J., S.R., L.L. and J.G.; writing—original draft preparation, B.G., C.E., F.R. and A.-S.H.; visualization, B.G., E.D., F.R. and A.-S.H.; supervision, M.L., F.R. and A.-S.H. All authors have read and agreed to the published version of the manuscript.
Funding
B.G.R. was supported by Alfonso Martin Escudero Foundation research grant.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Reyal, F.; Hamy, A.S.; Piccart, M.J. Neoadjuvant treatment: The future of patients with breast cancer. ESMO Open 2018, 3, e000371. [Google Scholar] [CrossRef] [PubMed]
- Luangdilok, S.; Samarnthai, N.; Korphaisarn, K. Association between Pathological Complete Response and Outcome Following Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer Patients. J. Breast Cancer 2014, 17, 376–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, K.-L.; Mashl, R.J.; Wu, Y.; Ritter, D.I.; Wang, J.; Oh, C.; Paczkowska, M.; Reynolds, S.; Wyczalkowski, M.A.; Oak, N.; et al. Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 2018, 173, 355–370.e14. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.N.; Brianese, R.C.; de Almeida, R.V.B.; Drummond, R.D.; de Souza, J.E.; da Silva, I.T.; de Souza, S.J.; Carraro, D.M. Influence of BRCA1 Germline Mutations in the Somatic Mutational Burden of Triple-Negative Breast Cancer. Transl. Oncol. 2019, 12, 1453–1460. [Google Scholar] [CrossRef]
- Eisinger, F.; Jacquemier, J.; Charpin, C.; Stoppa-Lyonnet, D.; Bressac-de Paillerets, B.; Peyrat, J.P.; Longy, M.; Guinebretière, J.M.; Sauvan, R.; Noguchi, T.; et al. Mutations at BRCA1: The medullary breast carcinoma revisited. Cancer Res. 1998, 58, 1588–1592. [Google Scholar]
- Phillips, K.A. Immunophenotypic and pathologic differences between BRCA1 and BRCA2 hereditary breast cancers. J. Clin. Oncol. 2000, 18, 107S–112S. [Google Scholar]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomark. Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef]
- Armes, J.E.; Trute, L.; White, D.; Southey, M.C.; Hammet, F.; Tesoriero, A.; Hutchins, A.M.; Dite, G.S.; McCredie, M.R.; Giles, G.G.; et al. Distinct molecular pathogeneses of early-onset breast cancers in BRCA1 and BRCA2 mutation carriers: A population-based study. Cancer Res. 1999, 59, 2011–2017. [Google Scholar] [PubMed]
- Palacios, J.; Honrado, E.; Osorio, A.; Cazorla, A.; Sarrió, D.; Barroso, A.; Rodríguez, S.; Cigudosa, J.C.; Diez, O.; Alonso, C.; et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res. Treat. 2005, 90, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Reis-Filho, J.S.; Fulford, L.; Penault-Llorca, F.; van der Vijver, M.; Parry, S.; Bishop, T.; Benitez, J.; Rivas, C.; Bignon, Y.-J.; et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin. Cancer Res. 2005, 11, 5175–5180. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet Lond. Engl. 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Mancini, P.; Angeloni, A.; Risi, E.; Orsi, E.; Mezi, S. Standard of Care and Promising New Agents for Triple Negative Metastatic Breast Cancer. Cancers 2014, 6, 2187–2223. [Google Scholar] [CrossRef]
- Godet, I.; Gilkes, D.M. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr. Cancer Sci. Ther. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wiśniowski, R.; Siolek, M.; et al. Pathologic Complete Response Rates in Young Women With BRCA1-Positive Breast Cancers After Neoadjuvant Chemotherapy. J. Clin. Oncol. 2009, 28, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, M.; Gass, P.; Häberle, L.; Flesch, V.M.; Rauh, C.; Bani, M.R.; Hack, C.C.; Schrauder, M.G.; Jud, S.M.; Emons, J.; et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res. Treat. 2018, 171, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sella, T.; Gal Yam, E.N.; Levanon, K.; Rotenberg, T.S.; Gadot, M.; Kuchuk, I.; Molho, R.B.; Itai, A.; Modiano, T.M.; Gold, R.; et al. Evaluation of tolerability and efficacy of incorporating carboplatin in neoadjuvant anthracycline and taxane based therapy in a BRCA1 enriched triple-negative breast cancer cohort. Breast Edinb. Scotl. 2018, 40, 141–146. [Google Scholar] [CrossRef]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; De Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Solinas, C.; Ceppi, M.; Lambertini, M.; Scartozzi, M.; Buisseret, L.; Garaud, S.; Fumagalli, D.; de Azambuja, E.; Salgado, R.; Sotiriou, C.; et al. Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: A meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2017, 57, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast Edinb. Scotl. 2017, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Sønderstrup, I.M.H.; Jensen, M.B.; Ejlertsen, B.; Eriksen, J.O.; Gerdes, A.M.; Kruse, T.A.; Larsen, M.J.; Thomassen, M.; Laenkholm, A.V. Evaluation of tumor-infiltrating lymphocytes and association with prognosis in BRCA-mutated breast cancer. Acta Oncol. Stockh. Swed. 2019, 58, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Marcoux, D.; Garaud, S.; Vitória, J.R.; Van den Eynden, G.; de Wind, A.; De Silva, P.; Boisson, A.; Craciun, L.; Larsimont, D.; et al. BRCA gene mutations do not shape the extent and organization of tumor infiltrating lymphocytes in triple negative breast cancer. Cancer Lett. 2019, 450, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Chu, C.; Badve, S.S.; Vinayak, S.; Silver, D.P.; Isakoff, S.J.; Kaklamani, V.; Gradishar, W.; Stearns, V.; Connolly, R.M.; et al. Association of Tumor Infiltrating Lymphocytes with Homologous Recombination Deficiency and BRCA1/2 Status in Patients with Early Triple-Negative Breast Cancer: A Pooled Analysis. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Marczyk, E.; Jasiowka, M.; Gronwald, J.; Jakubowicz, J.; Cybulski, C.; Wisniowski, R.; Godlewski, D.; et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 2014, 147, 401–405. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Marczyk, E.; Jasiowka, M.; Gronwald, J.; Jakubowicz, J.; Cybulski, C.; Wisniowski, R.; Godlewski, D.; et al. Pathological complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Hered. Cancer Clin. Pract. 2015, 13, A8. [Google Scholar] [CrossRef][Green Version]
- Hahnen, E.; Lederer, B.; Hauke, J.; Loibl, S.; Kröber, S.; Schneeweiss, A.; Denkert, C.; Fasching, P.A.; Blohmer, J.U.; Jackisch, C.; et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer. JAMA Oncol. 2017, 3, 1378–1385. [Google Scholar] [CrossRef]
- Chappuis, P.; Goffin, J.; Wong, N.; Perret, C.; Ghadirian, P.; Tonin, P.; Foulkes, W. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. J. Med. Genet. 2002, 39, 608–610. [Google Scholar] [CrossRef]
- Arun, B.; Bayraktar, S.; Liu, D.D.; Gutierrez Barrera, A.M.; Atchley, D.; Pusztai, L.; Litton, J.K.; Valero, V.; Meric-Bernstam, F.; Hortobagyi, G.N.; et al. Response to Neoadjuvant Systemic Therapy for Breast Cancer in BRCA Mutation Carriers and Noncarriers: A Single-Institution Experience. J. Clin. Oncol. 2011, 29, 3739–3746. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef] [PubMed]
- Sæther, N.H.; Skuja, E.; Irmejs, A.; Maksimenko, J.; Miklasevics, E.; Purkalne, G.; Gardovskis, J. Platinum-based neoadjuvant chemotherapy in BRCA1-positive breast cancer: A retrospective cohort analysis and literature review. Hered. Cancer Clin. Pract. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Bertucci, A.; Bertucci, F. PARP Inhibitors in the Treatment of Early Breast Cancer: The Step Beyond? Cancers 2020, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Dariush, A.; Provenzano, E.; Bardwell, H.; Abraham, J.E.; Iddawela, M.; Vallier, A.-L.; Hiller, L.; Dunn, J.A.; Bowden, S.J.; et al. Computational pathology of pre-treatment biopsies identifies lymphocyte density as a predictor of response to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. BCR 2016, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Dariush, A.; Thomas, J.; Provenzano, E.; Dunn, J.; Hiller, L.; Vallier, A.-L.; Abraham, J.; Piper, T.; Bartlett, J.M.S.; et al. Lymphocyte density determined by computational pathology validated as a predictor of response to neoadjuvant chemotherapy in breast cancer: Secondary analysis of the ARTemis trial. Ann. Oncol. 2017, 28, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Hamy, A.-S.; Bonsang-Kitzis, H.; De Croze, D.; Laas, E.; Darrigues, L.; Topciu, L.; Menet, E.; Vincent-Salomon, A.; Lerebours, F.; Pierga, J.-Y.; et al. Interaction between Molecular Subtypes and Stromal Immune Infiltration before and after Treatment in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Clin. Cancer Res. 2019, 25, 6731–6741. [Google Scholar] [CrossRef] [PubMed]
- Loi: LBA13Relationship between Tumor Infiltrating. Available online: https://scholar.google.com/scholar_lookup?hl=en&volume=28&publication_year=2017&pages=v605-v649&journal=Ann+Oncol&issue=suppl+5&author=S+Loi&author=S+Adams&author=P+Schmid&title=Relationship+between+tumor+infiltrating+lymphocyte+%28TIL%29+levels+and+response+to+pembrolizumab+%28pembro%29+in+metastatic+triple%E2%80%90negative+breast+cancer+%28mTNBC%29%3A+results+from+KEYNOTE%E2%80%90086 (accessed on 22 November 2020).
- Anurag, M.; Zhu, M.; Huang, C.; Vasaikar, S.; Wang, J.; Hoog, J.; Burugu, S.; Gao, D.; Suman, V.; Zhang, X.H.; et al. Immune checkpoint profiles in luminal B breast cancer (Alliance). J. Natl. Cancer Inst. 2020, 112, 737–746. [Google Scholar] [CrossRef]
- Sharma, P.; López-Tarruella, S.; García-Saenz, J.A.; Ward, C.; Connor, C.S.; Gómez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; et al. Efficacy of neoadjuvant carboplatin plus docetaxel in triple negative breast cancer: Combined analysis of two cohorts. Clin. Cancer Res. 2017, 23, 649–657. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xie, Y. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann. Oncol. 2015, 26, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Bignon, L.; Fricker, J.-P.; Nogues, C.; Mouret-Fourme, E.; Stoppa-Lyonnet, D.; Caron, O.; Lortholary, A.; Faivre, L.; Lasset, C.; Mari, V.; et al. Efficacy of anthracycline/taxane-based neo-adjuvant chemotherapy on triple-negative breast cancer in BRCA1/BRCA2 mutation carriers. Breast J. 2018, 24, 269–277. [Google Scholar] [CrossRef]
- Yadav, S.; Ladkany, R.; Yadav, D.; Alhalabi, O.; Khaddam, S.; Isaac, D.; Cardenas, P.Y.; Zakalik, D. Impact of BRCA Mutation Status on Survival of Women With Triple-negative Breast Cancer. Clin. Breast Cancer 2018, 18, e1229–e1235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gou, Q.; Wang, Q.; Zhong, X.; Zheng, H. The role of BRCA status on prognosis in patients with triple-negative breast cancer. Oncotarget 2017, 8, 87151–87162. [Google Scholar] [CrossRef] [PubMed]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef]
- Brekelmans, C.T.M.; Tilanus-Linthorst, M.M.A.; Seynaeve, C.; vd Ouweland, A.; Menke-Pluymers, M.B.E.; Bartels, C.C.M.; Kriege, M.; van Geel, A.N.; Burger, C.W.; Eggermont, A.M.M.; et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur. J. Cancer Oxf. Engl. 1990 2007, 43, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Gonzalez, L.D.; Vera-Badillo, F.E.; Tibau, A.; Goldstein, R.; Šeruga, B.; Srikanthan, A.; Pandiella, A.; Amir, E.; Ocana, A. Interaction between Hormonal Receptor Status, Age and Survival in Patients with BRCA1/2 Germline Mutations: A Systematic Review and Meta-Regression. PLoS ONE 2016, 11, e0154789. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.W.; Ward, K.C.; Howlader, N.; Deapen, D.; Hamilton, A.S.; Mariotto, A.; Miller, D.; Penberthy, L.S.; Katz, S.J. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J. Clin. Oncol. 2019, 37, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Blecua, P.; Lim, R.S.; Shen, R.; Higginson, D.S.; Weinhold, N.; Norton, L.; Weigelt, B.; Powell, S.N.; Reis-Filho, J.S. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat. Commun. 2017, 8, 857. [Google Scholar] [CrossRef]
- Recommendations for the immunohistochemistry of the hormonal receptors on paraffin sections in breast cancer. Update 1999. Group for Evaluation of Prognostic Factors using Immunohistochemistry in Breast Cancer (GEFPICS-FNCLCC). Ann. Pathol. 1999, 19, 336–343.
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. ESMO Guidelines Committee Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).