Role of Natural Killer Cells in Uveal Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Overview of Primary Uveal Melanoma Risk Stratification
3. Limitations to Treatment of MUM
4. NK Cells: An Introduction
5. Role of NK Cells in Primary Uveal Melanoma
5.1. NK Cell Suppression within Ocular Environment
5.2. Infiltrating Immune Cells in Primary Uveal Melanoma
5.3. NK Cell Suppression in Primary Uveal Melanoma Microenvironment
6. Circulating NK Cell Control of Uveal Melanoma Metastasis
6.1. Role of Tumor HLA Expression
6.2. NK Cell Ligand Expression on Uveal Melanoma Cells
6.3. Potential Relevance of Chromosome 6 Aberrations
7. Role of NK Cells in Uveal Melanoma Liver Metastasis
7.1. Uveal Melanoma Dormancy of Hepatic Micro-Metastasis
7.2. Evidence for Role of NK Cells in Controlling Uveal Melanoma Liver Metastasis
8. Tumor Specific Suppression of NK Cells in Uveal Melanoma Metastasis
9. Hepatic Immune Tolerance and Its Role in Suppressing NK Cell Function
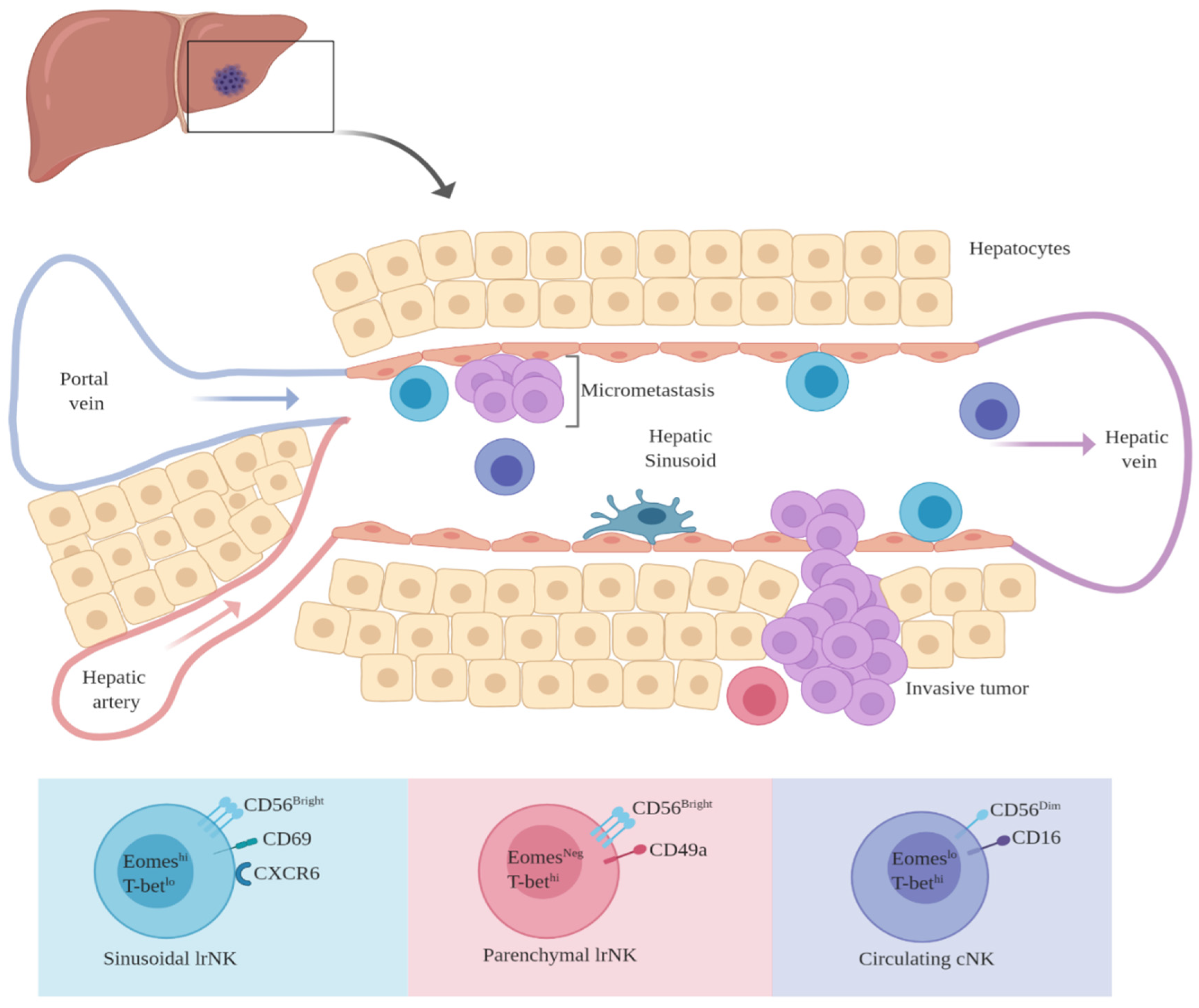
10. Liver Resident NK Cells: Their Potential Immunomodulatory Role in MUM
Hepatic NK Cells: Conventional vs. Liver-Resident NK Cells
11. Future Direction
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.D.; Bergman, L.; Seregard, S. Uveal melanoma: Epidemiologic aspects. Ophthalmol. Clin. N. Am. 2005, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.-M.; Paci, E. Incidence of Uveal Melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, S. Mode of presentation and time to treatment of uveal melanoma in Finland. Br. J. Ophthalmol. 2002, 86, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of Uveal Melanoma Millimeter-by-Millimeter in 8033 Consecutive Eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Singh, A.D.; Aronow, M.B. Faculty Opinions recommendation of Mutations in GNA11 in uveal melanoma. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2011, 363, 2191–2199. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nat. Cell Biol. 2009, 457, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Johansson, A.; Aoude, L.G.; Wadt, K.; Glasson, W.J.; Warrier, S.K.; Hewitt, A.W.; Kiilgaard, J.F.; Heegaard, S.; Isaacs, T.; Franchina, M.; et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget 2016, 7, 4624–4631. [Google Scholar] [CrossRef] [Green Version]
- Wedegaertner, P.; Moore, A.R.; Ceraudo, E.; Sher, J.J.; Guan, Y.; Shoushtari, A.N.; Chang, M.T.; Zhang, J.Q.; Walczak, E.G.; Kazmi, M.A.; et al. Faculty Opinions recommendation of Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2016, 48, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholes, A.G.M.; Damato, B.; Nunn, J.; Hiscott, P.; Grierson, I.; Field, J.K. Monosomy 3 in uveal melanoma: Correlation with clinical and histologic predictors of survival. Investig. Opthalmol. Vis. Sci. 2003, 44, 1008–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornfeld, N.; Prescher, G.; Becher, R.; Hirche, H.; Jöckel, K.-H.; Horsthemke, B. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef]
- Sisley, K.; Rennie, I.G.; Parsons, M.A.; Jacques, R.; Hammond, D.W.; Bell, S.M.; Potter, A.M.; Rees, R.C. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosom. Cancer 1997, 19, 22–28. [Google Scholar] [CrossRef]
- Furney, S.J.; Pedersen, M.; Gentien, D.; Dumont, A.G.; Rapinat, A.; Desjardins, L.; Turajlic, S.; Piperno-Neumann, S.; De La Grange, P.; Roman-Roman, S.; et al. SF3B1 Mutations Are Associated with Alternative Splicing in Uveal Melanoma. Cancer Discov. 2013, 3, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef]
- White, A.; Chambers, V.; Courtright, J.D.; Chang, P.D.; Horsman, W.Y.D. Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer 1998, 83, 354–359. [Google Scholar] [CrossRef]
- Shields, C.L.; Ganguly, A.; Bianciotto, C.G.; Turaka, K.; Tavallali, A.; Shields, J.A. Prognosis of Uveal Melanoma in 500 Cases Using Genetic Testing of Fine-Needle Aspiration Biopsy Specimens. Ophthalmology 2011, 118, 396–401. [Google Scholar] [CrossRef]
- Martin, M.; Maßhöfer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; Van De Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Dueñas-Gonzalez, A.; Delgado-Pelayo, S. Clinical presentation and management of uveal melanoma. Mol. Clin. Oncol. 2016, 5, 675–677. [Google Scholar] [CrossRef] [Green Version]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973–2013). Ocul. Oncol. Pathol. 2017, 4, 145–151. [Google Scholar] [CrossRef]
- Szalai, E.; Jiang, Y.; Van Poppelen, N.M.; Jager, M.J.; De Klein, A.; Kiliç, E.; Grossniklaus, H.E. Association of Uveal Melanoma Metastatic Rate With Stochastic Mutation Rate and Type of Mutation. JAMA Ophthalmol. 2018, 136, 1115–1120. [Google Scholar] [CrossRef]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; van Bodegom, A.; Paridaens, D.; Kilic, E.; Klein, A. Faculty Opinions recommendation of Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2018, 123, 1118–1128. [Google Scholar] [CrossRef]
- Kalirai, H.; Dodson, A.; Faqir, S.; Damato, B.E.; Coupland, S.E. Faculty Opinions recommendation of Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2016, 111, 1373–1380. [Google Scholar] [CrossRef]
- Lorigan, J.G.; Wallace, S.; Mavligit, G.M. The prevalence and location of metastases from ocular melanoma: Imaging study in 110 patients. Am. J. Roentgenol. 1991, 157, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients after Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, F.; Grosso, M.; Picasso, V.; Tornari, E.; Pesce, M.; Queirolo, P. Treatment of metastatic uveal melanoma with intravenous fotemustine. Melanoma Res. 2013, 23, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Papadopoulos, N.; Plager, C.; Eton, O.; Ring, S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003, 13, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Nir, I.; Hendler, K.; Lotem, M.; Eid, A.; Jurim, O.; Pe’Er, J. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br. J. Ophthalmol. 2009, 93, 1042–1046. [Google Scholar] [CrossRef]
- Sato, T.; Eschelman, D.J.; Gonsalves, C.F.; Terai, M.; Chervoneva, I.; McCue, P.A.; Shields, J.A.; Shields, C.L.; Yamamoto, A.; Berd, D.; et al. Immunoembolization of Malignant Liver Tumors, Including Uveal Melanoma, Using Granulocyte-Macrophage Colony-Stimulating Factor. J. Clin. Oncol. 2008, 26, 5436–5442. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, C.F.; Eschelman, D.J.; Sullivan, K.L.; Anne, P.R.; Doyle, L.; Sato, T. Radioembolization as Salvage Therapy for Hepatic Metastasis of Uveal Melanoma: A Single-Institution Experience. Am. J. Roentgenol. 2011, 196, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, C.F.; Sato, T.; Eschelman, D.J. Transhepatic Therapies for Metastatic Uveal Melanoma. Semin. Interv. Radiol. 2013, 30, 039–048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, R.; Eschelman, D.J.; Gonsalves, C.F.; Adamo, R.D.; Orloff, M.; Amjad, A.; Sharpe-Mills, E.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; et al. An Outcome Assessment of a Single Institution’s Longitudinal Experience with Uveal Melanoma Patients with Liver Metastasis. Cancers 2020, 12, 117. [Google Scholar] [CrossRef] [Green Version]
- Steeb, T.; Wessely, A.; Ruzicka, T.; Heppt, M.V.; Berking, C. How to MEK the best of uveal melanoma: A systematic review on the efficacy and safety of MEK inhibitors in metastatic or unresectable uveal melanoma. Eur. J. Cancer 2018, 103, 41–51. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Falchook, G.S.; Lewis, K.D.; Infante, J.R.; Gordon, M.S.; Vogelzang, N.J.; DeMarini, D.J.; Sun, P.; Moy, C.; Szabo, S.A.; Roadcap, L.T.; et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Pagliara, M.M.; Orteschi, D.; Dosa, T.; Sammarco, M.G.; Caputo, C.G.; Petrone, G.; Rindi, G.; Zollino, M.; Blasi, M.A.; et al. Pembrolizumab as first-line treatment for metastatic uveal melanoma. Cancer Immunol. Immunother. 2019, 68, 1179–1185. [Google Scholar] [CrossRef] [Green Version]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kämpgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef]
- Chandran, S.S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- Damato, B.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljunggren, H.G.; Karre, K. In search of the ’missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M.; Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Ocular Immune Privilege and Ocular Melanoma: Parallel Universes or Immunological Plagiarism? Front. Immunol. 2012, 3, 148. [Google Scholar] [CrossRef] [Green Version]
- Niederkorn, J.Y.; Chiang, E.Y.; Ungchusri, T.; Stroynowski, I. Expression of a Nonclassical Mhc Class Ib Molecule In The Eye1. Transplantation 1999, 68, 1790–1799. [Google Scholar] [CrossRef]
- Cousins, S.W.; McCabe, M.M.; Danielpour, D.; Streilein, J.W. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2201–2211. [Google Scholar]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming growth factor-beta in human aqueous humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef]
- Viel, S.; Marçais, A.; Guimaraes, F.S.F.; Loftus, R.; Rabilloud, J.; Grau, M.; Bienvenu, J. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal 2016, 9, ra19. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Niederkorn, J.Y. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology 1995, 86, 263–269. [Google Scholar] [PubMed]
- Apte, R.S.; Niederkorn, J.Y. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J. Immunol. 1996, 156, 2667–2673. [Google Scholar] [PubMed]
- Apte, R.S.; Sinha, D.; Mayhew, E.; Wistow, G.J.; Niederkorn, J.Y. Cutting edge: Role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J. Immunol. 1998, 160, 5693–5696. [Google Scholar]
- Repp, A.C.; Mayhew, E.S.; Apte, S.; Niederkorn, J.Y. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J. Immunol. 2000, 165, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, H.; Howard, K.; Mellon, J.; Mayhew, E.; Rusciano, D.; Niederkorn, J.Y. Reduction of liver metastasis of intraocular melanoma by interferon-beta gene transfer. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3042–3051. [Google Scholar] [CrossRef] [Green Version]
- Apte, R.S.; Mayhew, E.; Niederkorn, J.Y. Local inhibition of natural killer cell activity promotes the progressive growth of intraocular tumors. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1277–1282. [Google Scholar] [CrossRef]
- Berus, T.; Halon, A.; Markiewicz, A.; Orlowska-Heitzman, J.; Romanowska-Dixon, B.; Donizy, P. Clinical, Histopathological and Cytogenetic Prognosticators in Uveal Melanoma–A Comprehensive Review. Anticancer. Res. 2017, 37, 6541–6549. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.; Jager, M.J. Uveal Melanoma: The Inflammatory Microenvironment. J. Innate Immun. 2012, 4, 454–462. [Google Scholar] [CrossRef]
- Meecham, W.J.; Char, D.H.; Kaleta-Michaels, S. Infiltrating Lymphocytes and Antigen Expression in Uveal Melanoma. Ophthalmic Res. 1992, 24, 20–26. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Johansson, C.C.; Trocme, E.; Economou, M.A.; Larsson, O.; Seregard, S.; Kiessling, R.; All-Ericsson, C. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer 2010, 116, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Lagouros, E.; Salomao, D.; Thorland, E.; Hodge, D.O.; Vile, R.; Pulido, J.S. Infiltrative T Regulatory Cells in Enucleated Uveal Melanomas. Trans. Am. Ophthalmol. Soc. 2009, 107, 223–228. [Google Scholar] [PubMed]
- Grossniklaus, H.E.; Herwig, M. Faculty Opinions recommendation of Detection of M2-macrophages in uveal melanoma and relation with survival. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2011, 52, 643–650. [Google Scholar] [CrossRef]
- Ksander, B.R.; Rubsamen, P.E.; Olsen, K.R.; Cousins, S.W.; Streilein, J.W. Studies of tumor-infiltrating lymphocytes from a human choroidal melanoma. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3198–3208. [Google Scholar]
- Gezgin, G.; Dogrusöz, M.; Van Essen, T.H.; Kroes, W.G.M.; Luyten, G.P.M.; Van Der Velden, P.A.; Walter, V.; Verdijk, R.M.; Van Hall, T.; Van Der Burg, S.H.; et al. Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment. Cancer Immunol. Immunother. 2017, 66, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Maat, W.; Ly, L.V.; Jordanova, E.S.; De Wolff-Rouendaal, D.; Schalij-Delfos, N.E.; Jager, M.J. Monosomy of Chromosome 3 and an Inflammatory Phenotype Occur Together in Uveal Melanoma. Investig. Opthalmology Vis. Sci. 2008, 49, 505–510. [Google Scholar] [CrossRef]
- McKenna, K.C.; Previte, D.M. Influence of CD8+ T regulatory cells on intraocular tumor development. Front. Immunol. 2012, 3, 303. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, C.R.; Kalirai, H.; Sacco, J.J.; Azevedo, R.A.; Duckworth, A.; Slupsky, J.R.; Coulson, J.M.; Coupland, S.E. Loss of BAP1 expression is associated with an immunosuppressive microenvironment in uveal melanoma, with implications for immunotherapy development. J. Pathol. 2020, 250, 420–439. [Google Scholar] [CrossRef] [Green Version]
- Souri, Z.; Wierenga, A.P.A.; Van Weeghel, C.; Van Der Velden, P.A.; Kroes, W.G.M.; Luyten, G.P.M.; Van Der Burg, S.H.; Jochemsen, A.G.; Jager, M.J. Loss of BAP1 Is Associated with Upregulation of the NFkB Pathway and Increased HLA Class I Expression in Uveal Melanoma. Cancers 2019, 11, 1102. [Google Scholar] [CrossRef] [Green Version]
- Roufas, C.; Chasiotis, D.; Makris, A.; Efstathiades, C.; Dimopoulos, C.; Zaravinos, A. The Expression and Prognostic Impact of Immune Cytolytic Activity-Related Markers in Human Malignancies: A Comprehensive Meta-analysis. Front. Oncol. 2018, 8, 27. [Google Scholar] [CrossRef]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Stålhammar, G.; Seregard, S.; Grossniklaus, H.E. Expression of immune checkpoint receptors Indoleamine 2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus nonmetastatic choroidal melanoma. Cancer Med. 2019, 8, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Ménard, C.; Martin, F.; Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: Relevance during tumor progression. Immunol. Rev. 2006, 214, 229–238. [Google Scholar] [CrossRef]
- Saika, S. TGFbeta pathobiology in the eye. Lab. Investig. 2006, 86, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Esser, P.; Grisanti, S.; Bartz-Schmidt, K. TGF-beta in uveal melanoma. Microsc. Res. Tech. 2001, 52, 396–400. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, X.; Li, J.; Zheng, S.; Li, L.; Zhao, H. TGF-beta secreted by tumor-associated macrophages promotes proliferation and invasion of colorectal cancer via miR-34a-VEGF axis. Cell Cycle 2018, 17, 2766–2778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Che, D.; Yang, F.; Chi, C.; Meng, H.; Shen, J.; Qi, L.; Liu, F.; Lv, L.; Li, Y.; et al. Tumor-associated macrophages promote tumor metastasis via the TGF-beta/SOX9 axis in non-small cell lung cancer. Oncotarget 2017, 8, 99801–99815. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-G.; Mayhew, E.; Mellon, J.; Niederkorn, J.Y. Expression and Possible Function of IL-2 and IL-15 Receptors on Human Uveal Melanoma Cells. Investig. Opthalmology Vis. Sci. 2004, 45, 4240–4246. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Jun, I.H.; Kim, T.-I.; Byeon, S.H.; Koh, H.J.; Lee, S.C. Expression of 12 cytokines in aqueous humour of uveal melanoma before and after combined Ruthenium-106 brachytherapy and transpupillary thermotherapy. Acta Ophthalmol. 2012, 90, e314–e320. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti-Gude, N.; Bronkhorst, I.H.G.; Van Duinen, S.G.; Luyten, G.P.M.; Jager, M.J. Cytokines and Chemokines in the Vitreous Fluid of Eyes with Uveal Melanoma. Investig. Opthalmology Vis. Sci. 2012, 53, 6748–6755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, M.R. The Interferon-Gamma Paradox in Cancer. J. Interf. Cytokine Res. 2019, 39, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Saga, Y.; Mizukami, H.; Sato, N.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; Ozawa, K.; et al. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int. J. Oncol. 2012, 40, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Oyer, J.L.; Gitto, S.B.; Altomare, D.A.; Copik, A.J. PD-L1 blockade enhances anti-tumor efficacy of NK cells. OncoImmunology 2018, 7, e1509819. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Wick, W.; Eynde, B.J.V.D. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012, 72, 5435–5440. [Google Scholar] [CrossRef] [Green Version]
- Terai, M.; Londin, E.R.; Rochani, A.K.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405. [Google Scholar] [CrossRef] [Green Version]
- Hornyák, L.; Dobos, N.; Koncz, G.; Karányi, Z.; Páll, D.; Szabó, Z.; Halmos, G.; Székvölgyi, L. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Yu, C.-P.; Song, Y.-L.; Zhu, Z.-M.; Huang, B.; Xiao, Y.-Q.; Luo, D. Targeting TDO in cancer immunotherapy. Med. Oncol. 2017, 34, 73. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.W.; Mellon, J.K.; Mayhew, E.; Wang, S.; He, Y.G.; Hogan, N.; Niederkorn, J.Y. Uveal melanoma expression of indoleamine 2,3-deoxygenase: Establishment of an immune privileged environment by tryptophan depletion. Exp. Eye Res. 2007, 85, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma Cells Inhibit Natural Killer Cell Function by Modulating the Expression of Activating Receptors and Cytolytic Activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietra, G.; Vitale, M.; Moretta, L.; Mingari, M.C. How melanoma cells inactivate NK cells. OncoImmunology 2012, 1, 974–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorburn, J. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Pathway Signaling. J. Thorac. Oncol. 2007, 2, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.H.; Mayhew, E.; Hay, C.; Li, H.; Alizadeh, H.; Niederkorn, J.Y. Uveal melanoma expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors and susceptibility to TRAIL-induced apoptosis. Investig. Opthalmol. Vis. Sci. 2004, 45, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Niederkorn, J.Y.; Neelam, S.; Alizadeh, H. Resistance and Susceptibility of Human Uveal Melanoma Cells to TRAIL-Induced Apoptosis. Arch. Ophthalmol. 2005, 123, 654. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Niederkorn, J.Y.; Neelam, S.; Alizadeh, H. Downregulation of survivin expression enhances sensitivity of cultured uveal melanoma cells to cisplatin treatment. Exp. Eye Res. 2006, 83, 176–182. [Google Scholar] [CrossRef]
- Wöll, E.; Bedikian, A.; Legha, S.S. Uveal melanoma: Natural history and treatment options for metastatic disease. Melanoma Res. 1999, 9, 575–581. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.; Vu, T.H.K.; Jordanova, E.S.; Luyten, G.P.M.; Burg, S.H.V.D.; Jager, M.J. Different Subsets of Tumor-Infiltrating Lymphocytes Correlate with Macrophage Influx and Monosomy 3 in Uveal Melanoma. Investig. Opthalmol. Vis. Sci. 2012, 53, 5370–5378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Luyten, G.P.; Luider, T.M.; Niederkorn, J.Y. Relationship between natural killer cell susceptibility and metastasis of human uveal melanoma cells in a murine model. Investig. Ophthalmol. Vis. Sci. 1995, 36, 435–441. [Google Scholar]
- Maat, W.; Van Der Slik, A.; Verhoeven, D.H.J.; Alizadeh, B.Z.; Ly, L.V.; Verduijn, W.; Luyten, G.P.M.; Mulder, A.; Van Hall, T.; Koning, F.; et al. Evidence for Natural Killer Cell–Mediated Protection from Metastasis Formation in Uveal Melanoma Patients. Investig. Opthalmol. Vis. Sci. 2009, 50, 2888–2895. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Luyten, G.P.; Mooy, C.; Kerkvliet, S.; Zwinderman, A.H.; Jager, M.J. Human leukocyte antigen class I expression. Marker of poor prognosis in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1865–1872. [Google Scholar]
- Ericsson, C.; Seregard, S.; Bartolazzi, A.; Levitskaya, E.; Ferrone, S.; Kiessling, R.; Larsson, O. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2153–2156. [Google Scholar]
- Natali, P.G.; Bigotti, A.; Nicotra, M.R.; Nardi, R.M.; Delovu, A.; Segatto, O.; Ferrone, S. Analysis of the antigenic profile of uveal melanoma lesions with anti-cutaneous melanoma-associated antigen and anti-HLA monoclonal antibodies. Cancer Res. 1989, 49, 1269–1274. [Google Scholar]
- Hurks, H.M.; Metzelaar-Blok, J.A.; Mulder, A.; Claas, F.H.; Jager, M.J. High frequency of allele-specific down-regulation of HLA class I expression in uveal melanoma cell lines. Int. J. Cancer 2000, 85, 697–702. [Google Scholar] [CrossRef]
- Van Essen, T.; Van Pelt, S.I.; Bronkhorst, I.H.G.; Versluis, M.; Némati, F.; Laurent, C.; Luyten, G.P.M.; Van Hall, T.; Elsen, P.J.V.D.; Van Der Velden, P.A.; et al. Upregulation of HLA Expression in Primary Uveal Melanoma by Infiltrating Leukocytes. PLoS ONE 2016, 11, e0164292. [Google Scholar] [CrossRef]
- Guzman, L.G.M.; Keating, N.; Nicholson, S.E. Natural Killer Cells: Tumor Surveillance and Signaling. Cancers 2020, 12, 952. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.S.; Purdy, A.K. Structure/function of human killer cell immunoglobulin-like receptors: Lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 2011, 132, 315–325. [Google Scholar] [CrossRef]
- Wu, J.; Song, Y.; Bakker, A.B.H.; Bauer, S.; Spies, T.; Lanier, L.L.; Phillips, J.H. An Activating Immunoreceptor Complex Formed by NKG2D and DAP10. Science 1999, 285, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Cosman, D.; Müllberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Hecht, M.-L.; Rosental, B.; Horlacher, T.; Hershkovitz, O.; De Paz, J.L.; Noti, C.; Schauer, S.; Porgador, A.; Seeberger, P.H. Natural Cytotoxicity Receptors NKp30, NKp44 and NKp46 Bind to Different Heparan Sulfate/Heparin Sequences. J. Proteome Res. 2009, 8, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Bottino, C.; Castriconi, R.; Cantoni, C.; Marcenaro, S.; Rivera, P.; Spaggiari, G.M.; Dondero, A.; Carnemolla, B.; Reymond, N.; et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: Involvement in tumor cell lysis. Mol. Immunol. 2005, 42, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Curti, A.; Ruggeri, L.; D’Addio, A.; Bontadini, A.; Dan, E.; Motta, M.R.; Trabanelli, S.; Giudice, V.; Urbani, E.; Martinelli, G.; et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011, 118, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Hurks, H.M.; Valter, M.M.; Wilson, L.; Hilgert, I.; Elsen, P.J.V.D.; Jager, M.J. Uveal melanoma: No expression of HLA-G. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3081–3084. [Google Scholar]
- Braud, V.; Allan, D.S.J.; O’Callaghan, C.A.; Söderström, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nat. Cell Biol. 1998, 391, 795–799. [Google Scholar] [CrossRef]
- Damato, B.; Dopierala, J.; Klaasen, A.; Van Dijk, M.; Sibbring, J.; Coupland, S.E. Multiplex Ligation-Dependent Probe Amplification of Uveal Melanoma: Correlation with Metastatic Death. Investig. Opthalmology Vis. Sci. 2009, 50, 3048–3055. [Google Scholar] [CrossRef] [Green Version]
- Dogrusöz, M.; Jager, M.J. Genetic prognostication in uveal melanoma. Acta Ophthalmol. 2017, 96, 331–347. [Google Scholar] [CrossRef]
- Höglund, M.; Gisselsson, D.; Hansen, G.B.; White, V.A.; Säll, T.; Mitelman, F.; Horsman, D. Dissecting karyotypic patterns in malignant melanomas: Temporal clustering of losses and gains in melanoma karyotypic evolution. Int. J. Cancer 2003, 108, 57–65. [Google Scholar] [CrossRef]
- Metzelaar-Blok, J.A.; Jager, M.J.; Moghaddam, P.H.; Van Der Slik, A.; Giphart, M.J. Frequent loss of heterozygosity on chromosome 6p in uveal melanoma. Hum. Immunol. 1999, 60, 962–969. [Google Scholar] [CrossRef]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of Metastatic Disease After Enrollment in the COMS Trials for Treatment of Choroidal Melanoma. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar] [CrossRef]
- Bakalian, S.; Marshall, J.-C.; Logan, P.; Faingold, D.; Maloney, S.; Di Cesare, S.; Martins, C.; Fernandes, B.F.; Burnier, M.N. Molecular Pathways Mediating Liver Metastasis in Patients with Uveal Melanoma. Clin. Cancer Res. 2008, 14, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskelin, S. Tumor doubling times in metastatic malignant melanoma of the uvea Tumor progression before and after treatment. Ophthalmol. 2000, 107, 1443–1449. [Google Scholar] [CrossRef]
- Singh, A.D. Uveal melanoma: Implications of tumor doubling time. Ophthalmology 2001, 108, 829–831. [Google Scholar] [CrossRef]
- Borthwick, N.J.; Thombs, J.; Polak, M.E.; Gabriel, F.G.; Hungerford, J.L.; Damato, B.; Rennie, I.G.; Jager, M.J.; Cree, I.A. The biology of micrometastases from uveal melanoma. J. Clin. Pathol. 2011, 64, 666–671. [Google Scholar] [CrossRef]
- Nichols, E.E.; Richmond, A.; Daniels, A.B. Micrometastatic Dormancy in Uveal Melanoma: A Comprehensive Review of the Evidence, Mechanisms, and Implications for Future Adjuvant Therapies. Int. Ophthalmol. Clin. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Logan, P.; Fernandes, B.F.; Di Cesare, S.; Marshall, J.-C.A.; Maloney, S.C.; Burnier, M.N., Jr. Single-cell tumor dormancy model of uveal melanoma. Clin. Exp. Metastasis 2008, 25, 509–516. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Zhang, Q.; You, S.; McCarthy, C.; Heegaard, S.; Coupland, S.E. Metastatic ocular melanoma to the liver exhibits infiltrative and nodular growth patterns. Hum. Pathol. 2016, 57, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Grossniklaus, H.E. Progression of ocular melanoma metastasis to the liver: The 2012 Zimmerman lecture. JAMA Ophthalmol. 2013, 131, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Dithmar, S.; Rusciano, D.; Lynn, M.J.; Lawson, D.H.; Armstrong, C.A.; Grossniklaus, H.E. Neoadjuvant Interferon alfa-2b Treatment in a Murine Model for Metastatic Ocular MelanomaA Preliminary Study. Arch. Ophthalmol. 2000, 118, 1085–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunda, M.J.; Rosenbaum, D.; Stern, L. Inhibition of experimentally-induced murine metastases by recombinant alpha interferon: Correlation between the modulatory effect of interferon treatment on natural killer cell activity and inhibition of metastases. Int. J. Cancer 1984, 34, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dithmar, S.; Grossniklaus, H.E. Interferon alpha 2b decreases hepatic micrometastasis in a murine model of ocular melanoma by activation of intrinsic hepatic natural killer cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2056–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Brackett, C.M.; Morales-Tirado, V.M.; Li, Z.; Zhang, Q.; Wilson, M.W.; Benjamin, C.; Harris, W.; Waller, E.K.; Gudkov, A.V.; et al. The Toll-like receptor 5 agonist entolimod suppresses hepatic metastases in a murine model of ocular melanoma via an NK cell-dependent mechanism. Oncotarget 2015, 7, 2936–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdelya, L.G.; Brackett, C.M.; Kojouharov, B.; Gitlin, I.I.; Leonova, K.I.; Gleiberman, A.S.; Aygun-Sunar, S.; Veith, J.; Johnson, C.; Haderski, G.J.; et al. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Proc. Natl. Acad. Sci. USA 2013, 110, E1857–E1866. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.M.; Yang, H.; Zhang, Q.; Morales-Tirado, V.M.; Grossniklaus, H.E. Natural killer cells and pigment epithelial-derived factor control the infiltrative and nodular growth of hepatic metastases in an Orthotopic murine model of ocular melanoma. BMC Cancer 2019, 19, 484. [Google Scholar] [CrossRef]
- Salmon, P.; Le Cotonnec, J.Y.; Galazka, A.; Abdul-Ahad, A.; Darragh, A. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J. Interferon. Cytokine Res. 1996, 16, 759–764. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E.; Fagone, P.; Longo, A.; Russo, A.; Fallico, M.; Bonfiglio, V.; Nicoletti, F.; Avitabile, T.; Reibaldi, M. Immunobiology of Uveal Melanoma: State of the Art and Therapeutic Targets. Front. Oncol. 2019, 9, 1145. [Google Scholar] [CrossRef] [Green Version]
- Krishna, Y.; McCarthy, C.; Kalirai, H.; Coupland, S.E. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum. Pathol. 2017, 66, 159–166. [Google Scholar] [CrossRef]
- Qin, Y.; De Macedo, M.P.; Reuben, A.; Forget, M.-A.; Haymaker, C.; Bernatchez, C.; Spencer, C.N.; Gopalakrishnan, V.; Reddy, S.; Cooper, Z.A.; et al. Parallel profiling of immune infiltrate subsets in uveal melanoma versus cutaneous melanoma unveils similarities and differences: A pilot study. OncoImmunology 2017, 6, e1321187. [Google Scholar] [CrossRef] [Green Version]
- Rothermel, L.D.; Sabesan, A.C.; Stephens, D.J.; Chandran, S.S.; Paria, B.C.; Srivastava, A.K.; Somerville, R.; Wunderlich, J.R.; Lee, C.-C.R.; Xi, L.; et al. Identification of an Immunogenic Subset of Metastatic Uveal Melanoma. Clin. Cancer Res. 2016, 22, 2237–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, A.; Arguello, D.; Johnston, C.; Gatalica, Z.; Terai, M.; Weight, R.M.; Orloff, M.; Mastrangelo, M.J.; Sato, T. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Ophthalmology 2017, 9, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blom, D.J.R.; Schurmans, L.R.H.M.; De Waard-Siebinga, I.; De Wolff-Rouendaa, D.; Keunen, J.E.; Jager, M.J. HLA expression in a primary uveal melanoma, its cell line, and four of its metastases. Br. J. Ophthalmol. 1997, 81, 989–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagle, R.A.; Trowsdale, J. Promiscuity and the single receptor: NKG2D. Nat. Rev. Immunol. 2007, 7, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Vetter, C.S.; Lieb, W.; Bröcker, E.-B.; Becker, J.C. Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br. J. Cancer 2004, 91, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Easom, N.J.W.; Stegmann, K.A.; Swadling, L.; Pallett, L.J.; Burton, A.R.; Odera, D.; Schmidt, N.; Huang, W.-C.; Fusai, G.; Davidson, B.; et al. IL-15 Overcomes Hepatocellular Carcinoma-Induced NK Cell Dysfunction. Front. Immunol. 2018, 9, 1009. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, C. The Roles of Liver-Resident Lymphocytes in Liver Diseases. Front. Immunol. 2019, 10, 1582. [Google Scholar] [CrossRef]
- Doherty, D.G.; O’Farrelly, C. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 2000, 174, 5–20. [Google Scholar] [CrossRef]
- Cunningham, E.C.; Sharland, A.F.; Bishop, G.A. Liver Transplant Tolerance and Its Application to the Clinic: Can We Exploit the High Dose Effect? Clin. Dev. Immunol. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Opelz, G.; Wujciak, T.; Döhler, B.; Scherer, S.; Mytilineos, J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev. Immunogenet. 1999, 1, 334–342. [Google Scholar] [PubMed]
- Knoll, P.; Schlaak, J.; Uhrig, A.; Kempf, P.; Büschenfelde, K.-H.M.Z.; Gerken, G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J. Hepatol. 1995, 22, 226–229. [Google Scholar] [CrossRef]
- Horst, A.K.; Neumann, K.; Diehl, L.; Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell. Mol. Immunol. 2016, 13, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Carambia, A.; Freund, B.; Schwinge, D.; Heine, M.; Laschtowitz, A.; Huber, S.; Wraith, D.C.; Korn, T.; Schramm, C.; Lohse, A.W.; et al. TGF-beta-dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J. Hepatol. 2014, 61, 594–599. [Google Scholar] [CrossRef]
- Karrar, A.; Broome, U.; Uzunel, M.; Qureshi, A.R.; Sumitran-Holgersson, S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: A role in tolerance induction. Gut 2007, 56, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Schon, H.T.; Weiskirchen, R. Immunomodulatory effects of transforming growth factor-beta in the liver. Hepatobiliary Surg. Nutr. 2014, 3, 386–406. [Google Scholar]
- Khatib, N. Tumor-infiltrating Foxp3+ Regulatory T Cells in Posterior Uveal Melanoma: Clinicopathologic Correlation ARVO Annual Meeting Abstract. Investig. Ophthalmol. Vis. Sci. 2011, 52, 14. [Google Scholar]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Tian, Z. Diversity of tissue-resident NK cells. Semin. Immunol. 2017, 31, 3–10. [Google Scholar] [CrossRef]
- Gaynor, L.M.; Colucci, F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front. Immunol. 2017, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Simonetta, F.; Pradier, A.; Roosnek, E. T-bet and Eomesodermin in NK Cell Development, Maturation, and Function. Front. Immunol. 2016, 7, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Male, V. Liver-Resident NK Cells: The Human Factor. Trends Immunol. 2017, 38, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.; Robinson, M.W.; Fahey, R.; Whelan, S.; Houlihan, D.D.; Geoghegan, J.; O’Farrelly, C. Tissue-resident Eomes hi T-bet lo CD56 bright NK cells with reduced proinflammatory potential are enriched in the adult human liver. Eur. J. Immunol. 2016, 46, 2111–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.; Sun, R. Liver-resident NK cells and their potential functions. Cell. Mol. Immunol. 2017, 14, 890–894. [Google Scholar] [CrossRef]
- Stegmann, K.A.; Robertson, F.; Hansi, N.; Gill, U.; Pallant, C.; Christophides, T.; Male, V. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci. Rep. 2016, 6, 26157. [Google Scholar] [CrossRef]
- Cuff, A.O.; Robertson, F.P.; Stegmann, K.A.; Pallett, L.J.; Maini, M.K.; Davidson, B.R.; Male, V. EomeshiNK Cells in Human Liver Are Long-Lived and Do Not Recirculate but Can Be Replenished from the Circulation. J. Immunol. 2016, 197, 4283–4291. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, N.; Béziat, V.; Nyström, S.; Hengst, J.; Ivarsson, M.A.; Kekäläinen, E.; Johansson, H.; Mjösberg, J.; Westgren, M.; Lankisch, T.O.; et al. Cutting Edge: Identification and Characterization of Human Intrahepatic CD49a+ NK Cells. J. Immunol. 2015, 194, 2467–2471. [Google Scholar] [CrossRef] [Green Version]
- Lunemann, S.; Martrus, G.; Goebels, H.; Kautz, T.; Langeneckert, A.; Salzberger, W.; Koch, M.; Bunders, M.J.; Nashan, B.; Van Gisbergen, K.P.J.M.; et al. Hobit expression by a subset of human liver-resident CD56bright Natural Killer cells. Sci. Rep. 2017, 7, 6676. [Google Scholar] [CrossRef] [Green Version]
- Pugh, S.; Harrison, R.J.; Primrose, J.N.; Khakoo, S.I. T cells but not NK cells are associated with a favourable outcome for resected colorectal liver metastases. BMC Cancer 2014, 14, 180. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kuang, D.-M.; Pan, W.-D.; Wan, Y.-L.; Lao, X.-M.; Wang, D.; Li, X.-F.; Zheng, L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013, 57, 1107–1116. [Google Scholar] [CrossRef]
- Sun, H.; Liu, L.; Huang, Q.; Liu, H.; Huang, M.; Wang, J.; Wen, H.; Lin, R.; Qu, K.; Li, K.; et al. Accumulation of Tumor-Infiltrating CD49a+ NK Cells Correlates with Poor Prognosis for Human Hepatocellular Carcinoma. Cancer Immunol. Res. 2019, 7, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Maini, M.K.; Peppa, D. NK Cells: A Double-Edged Sword in Chronic Hepatitis B Virus Infection. Front. Immunol. 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Peng, H.; Li, K.; Qu, K.; Wang, B.; Wu, Y.; Ye, L.; Dong, Z.; Wei, H.; Sun, R.; et al. Liver-Resident NK Cells Control Antiviral Activity of Hepatic T Cells via the PD-1-PD-L1 Axis. Immunity 2019, 50, 403–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.-B.; Lu, F.-T.; Ma, H.-D.; Wang, Y.-H.; Yang, W.; Long, J.; Miao, Q.; Zhang, W.; Tian, Z.; Ridgway, W.M.; et al. Liver-resident NK cells suppress autoimmune cholangitis and limit the proliferation of CD4+ T cells. Cell. Mol. Immunol. 2019, 17, 178–189. [Google Scholar] [CrossRef]
- Harmon, C.; Jameson, G.; Almuaili, D.; Houlihan, D.D.; Hoti, E.; Geoghegan, J.; Robinson, M.W.; O’Farrelly, C. Liver-Derived TGF-beta Maintains the Eomes(hi)Tbet(lo) Phenotype of Liver Resident Natural Killer Cells. Front. Immunol. 2019, 10, 1502. [Google Scholar] [CrossRef]
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56bright/CD16neg NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Anfossi, N.; Andre, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.J.; Yang, L.; Yokoyama, W.M. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005, 436, 709–713. [Google Scholar] [CrossRef]
- Orr, M.T.; Lanier, L.L. Natural Killer Cell Education and Tolerance. Cell 2010, 142, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Burt, B.M.; Plitas, G.; Zhao, Z.; Bamboat, Z.M.; Nguyen, H.M.; Dupont, B.; DeMatteo, R.P. The Lytic Potential of Human Liver NK Cells is Restricted by Their Limited Expression of Inhibitory Killer Ig-Like Receptors. J. Immunol. 2009, 183, 1789–1796. [Google Scholar] [CrossRef]
- Lopes, J.E.; Fisher, J.L.; Flick, H.L.; Wang, C.; Sun, L.; Ernstoff, M.S.; Alvarez, J.C.; Losey, H.C. ALKS 4230: A novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000673. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, J.; Bensussan, A.; Marie-Cardine, A. Identification and Characterization of a Transmembrane Isoform of CD160 (CD160-TM), a Unique Activating Receptor Selectively Expressed upon Human NK Cell Activation. J. Immunol. 2009, 182, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Liver-Directed Therapy | Systemic Therapy |
|---|---|
| Embolization: chemotherapy, immunotherapy, radio-embolization, bland embolization | Consideration for clinical trial |
| Ablative procedures | Immune checkpoint inhibitors |
| External beam radiation therapy | Cytotoxic chemotherapy |
| Surgical metastasectomy (in select cases) | Targeted therapy |
| Common Human NK Inhibitory Receptors (iKIRs) and Their Corresponding Ligands | Common Human NK Activating Receptors and Their Corresponding Ligands | NK Ligands Expressed in Uveal Melanoma and Their Corresponding NK Receptors |
|---|---|---|
| KIR2DL1 (HLA-C2) KIR2DL2–3 (HLA-C1) KIR3DL1 (HLA-HLA-Bw4) KIR3DL2 (HLA-A*03, A*011) CD94/NKG2A/B (HLA-E) [110] | NKG2D (MIC A/B, ULBP) DNAM-1 (CD112, CD155) NCRs: NKp46, NKp44, NKp30 (Heparan Sulfate Glycosaminoglycans and others) [111,112,113,114] | HLA-A/B/C (iKIRs) HLA-E (NKG2A) ULBP1–3 (NKG2D) MIC-A/B (NKG2D) CD155, CD112 (DNAM-1) [103,116] |
| Clinical Trials.Gov ID | Study Drug(s) | Mechanism of Study Agent Utilizing NK Cell Anti-Tumor Effect |
|---|---|---|
| NCT03841110 Phase I Advanced malignancy (Including melanoma) | FT500 Pembrolizumab Atezolizumab Nivolumab IL-2 | FT500 is an allogeneic, off the shelf, NK cell product derived from induced pluripotent stem cell (FT500 administered either as monotherapy, in combination with check point inhibitor, or in combination with check-point inhibitor and IL-2). Study drugs include fludarabine and cyclophosphamide as lympho-conditioning agents |
| NCT04592653 Phase II Advanced malignancy, including cutaneous melanoma | ALKS 4230 Pembrolizumab | ALKS 4230 is an engineered fusion protein comprised of modified IL-2 designed to selectively expand anti-tumor T cells and NK cells while avoiding activation of immunosuppressive cells [181]. |
| NCT04477876 Cutaneous melanoma | Anti-CD160-TM agonist antibody | Transmembrane isoform of CD160 (CD160-TM) is expressed on activated NK cells. Binding of agonist antibody to CD160-TM can promote NK cell dependent anti-tumor effect [182] |
| NCT03420963 Phase I Advanced malignancy, including cutaneous melanoma | Ex-vivo expanded allogeneic NK cells | Cord Blood-derived Expanded Allogeneic NK cells are cord-blood derived, expanded ex-vivo and administered to patients after pre-treatment with etoposide and cyclophosphamide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, A.; Milhem, M. Role of Natural Killer Cells in Uveal Melanoma. Cancers 2020, 12, 3694. https://doi.org/10.3390/cancers12123694
Javed A, Milhem M. Role of Natural Killer Cells in Uveal Melanoma. Cancers. 2020; 12(12):3694. https://doi.org/10.3390/cancers12123694
Chicago/Turabian StyleJaved, Asad, and Mohammed Milhem. 2020. "Role of Natural Killer Cells in Uveal Melanoma" Cancers 12, no. 12: 3694. https://doi.org/10.3390/cancers12123694
APA StyleJaved, A., & Milhem, M. (2020). Role of Natural Killer Cells in Uveal Melanoma. Cancers, 12(12), 3694. https://doi.org/10.3390/cancers12123694






