Prospects for NK Cell Therapy of Sarcoma
Simple Summary
Abstract
1. Introduction
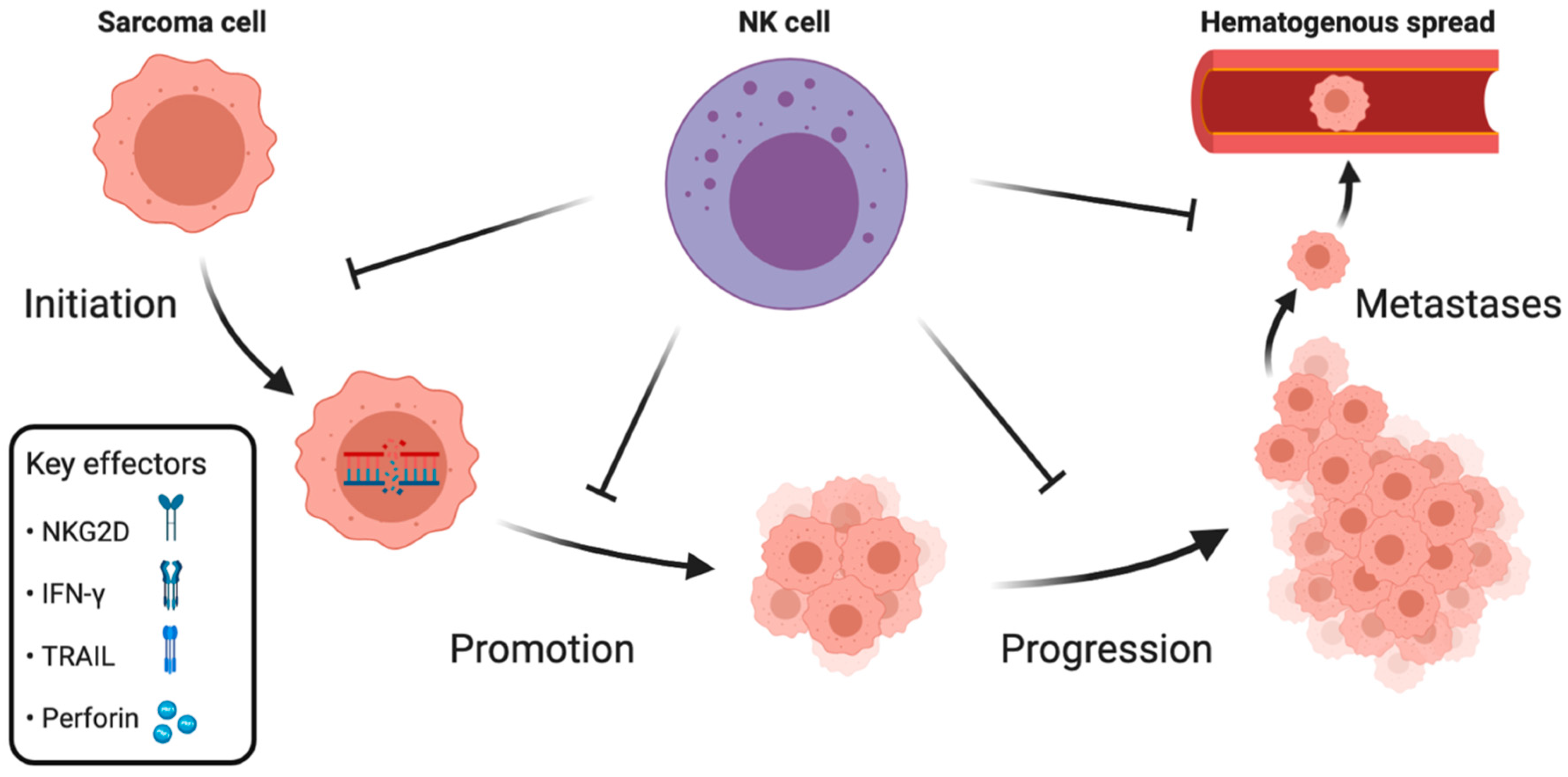
2. NK Cell Immune Surveillance during Distinct Phases of Sarcoma Development
2.1. Initiation and Promotion
2.2. Progression
2.3. Metastases
3. NK Cell Dysfunction in Sarcomas
3.1. Tumor-Infiltrating Immunosuppressive Cells
3.2. Cytokine-Dependent Inhibition
3.3. MHC-Dependent Inhibition
3.4. MICA Shedding
3.5. Apoptosis Resistance
3.6. Immune Checkpoints
3.7. Altered Oxygen Metabolism
3.8. HIV-KS-NK Cell Axis
3.9. Iatrogenic NK Cell Suppression
4. NK Cell-Based Therapies in Sarcomas
4.1. Hematopoietic Stem Cell Transplantation (HSCT)
4.2. Cytokines
4.3. Monoclonal Antibodies
4.4. Immunomodulation
4.5. Adoptive NK Cell Therapies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiessling, R.; Klein, E.; Pross, H.; Wigzell, H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1975, 5, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef]
- Martinet, L.; Smyth, M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Nausch, N.; Cerwenka, A. NKG2D ligands in tumor immunity. Oncogene 2008, 27, 5944–5958. [Google Scholar] [CrossRef]
- Bottino, C.; Biassoni, R.; Millo, R.; Moretta, L.; Moretta, A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum. Immunol. 2000, 61, 1–6. [Google Scholar] [CrossRef]
- Vitale, M.; Falco, M.; Castriconi, R.; Parolini, S.; Zambello, R.; Semenzato, G.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Identification of NKp80, a novel triggering molecule expressed by human NK cells. Eur. J. Immunol. 2001, 31, 233–242. [Google Scholar] [CrossRef]
- Welte, S.; Kuttruff, S.; Waldhauer, I.; Steinle, A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat. Immunol. 2006, 7, 1334–1342. [Google Scholar] [CrossRef]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Parolini, S.; Falco, M.; Marcenaro, E.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. 2B4 functions as a co-receptor in human NK cell activation. Eur. J. Immunol. 2000, 30, 787–793. [Google Scholar] [CrossRef]
- Lanier, L.L.; Le, A.M.; Civin, C.; Loken, M.; Phillips, J. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol. 1986, 136, 4480–4486. [Google Scholar] [PubMed]
- Vilches, C.; Parham, P. KIR: Diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 2002, 20, 217–251. [Google Scholar] [CrossRef]
- Anfossi, N.; André, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Jacobs, B.; Saetersmoen, M.L.; Clement, D.; Hammer, Q.; Clancy, T.; Skarpen, E.; Brech, A.; Landskron, J.; Grimm, C.; et al. Remodeling of secretory lysosomes during education tunes functional potential in NK cells. Nat. Commun. 2019, 10, 514. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Önfelt, B.; Malmberg, K.-J. Newtonian cell interactions shape natural killer cell education. Immunol. Rev. 2015, 267, 197–213. [Google Scholar] [CrossRef]
- Liu, L.L.; Pfefferle, A.; Yi Sheng, V.O.; Björklund, A.T.; Béziat, V.; Goodridge, J.P.; Malmberg, K.-J. Harnessing adaptive natural killer cells in cancer immunotherapy. Mol. Oncol. 2015, 9, 1904–1917. [Google Scholar] [CrossRef]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.A.; Yagita, H.; Takeda, K.; Dommelen, S.L.H.v.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Sedelies, K.A.; Ciccone, A.; Clarke, C.J.P.; Oliaro, J.; Sutton, V.R.; Scott, F.L.; Silke, J.; Susanto, O.; Green, D.R.; Johnstone, R.W.; et al. Blocking granule-mediated death by primary human NK cells requires both protection of mitochondria and inhibition of caspase activity. Cell Death Differ. 2008, 15, 708–717. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.-G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.-J.; Carlsten, M.; Björklund, A.; Sohlberg, E.; Bryceson, Y.T.; Ljunggren, H.-G. Natural killer cell-mediated immunosurveillance of human cancer. Semin. Immunol. 2017, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.-J.; Sohlberg, E.; Goodridge, J.P.; Ljunggren, H.-G. Immune selection during tumor checkpoint inhibition therapy paves way for NK-cell “missing self” recognition. Immunogenetics 2017, 69, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Saetersmoen, M.L.; Hammer, Q.; Valamehr, B.; Kaufman, D.S.; Malmberg, K.J. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin. Immunopathol. 2019, 41, 59–68. [Google Scholar] [CrossRef]
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.S.; Klingemann, H.G.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 485–492. [Google Scholar] [CrossRef]
- Ljunggren, H.-G.; Malmberg, K.-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339. [Google Scholar] [CrossRef]
- Domagala, J.; Lachota, M.; Klopotowska, M.; Graczyk-Jarzynka, A.; Domagala, A.; Zhylko, A.; Soroczynska, K.; Winiarska, M. The Tumor Microenvironment-A Metabolic Obstacle to NK Cells’ Activity. Cancers 2020, 12, 3542. [Google Scholar] [CrossRef]
- Skubitz, K.M.; D’Adamo, D.R. Sarcoma. Mayo Clin. Proc. 2007, 82, 1409–1432. [Google Scholar] [CrossRef]
- Cho, D.; Shook, D.R.; Shimasaki, N.; Chang, Y.H.; Fujisaki, H.; Campana, D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin. Cancer Res. 2010, 16, 3901–3909. [Google Scholar] [CrossRef]
- Tarek, N.; Lee, D.A. Natural killer cells for osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Tullius, B.P.; Setty, B.A.; Lee, D.A. Natural Killer Cell Immunotherapy for Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1257, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Ratan, R.; Patel, S.R. Chemotherapy for soft tissue sarcoma. Cancer 2016, 122, 2952–2960. [Google Scholar] [CrossRef]
- Digesu, C.S.; Wiesel, O.; Vaporciyan, A.A.; Colson, Y.L. Management of Sarcoma Metastases to the Lung. Surg. Oncol. Clin. N. Am. 2016, 25, 721–733. [Google Scholar] [CrossRef]
- Smyth, M.J.; Thia, K.Y.T.; Cretney, E.; Kelly, J.M.; Snook, M.B.; Forbes, C.A.; Scalzo, A.A. Perforin Is a Major Contributor to NK Cell Control of Tumor Metastasis. J. Immunol. 1999, 162, 6658–6662. [Google Scholar]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; Cretney, E.; Trapani, J.A.; Taniguchi, M.; Kawano, T.; Pelikan, S.B.; Crowe, N.Y.; Godfrey, D.I. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 2000, 191, 661–668. [Google Scholar] [CrossRef]
- Street, S.E.; Cretney, E.; Smyth, M.J. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001, 97, 192–197. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Xiao, P.; Xue, L.; Che, L.H.; Peng, J.J.; Wu, H.X.; Li, Y.; Qiao, H. Expression and roles of MICA in human osteosarcoma. Histopathology 2008, 52, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Xiao, P.; Xue, L.; Che, L.H.; Yang, P.; Li, Y.; Qiao, H. Prevalent expression of MHC class I chain-related molecule A in human osteosarcoma. Neoplasma 2008, 55, 266–272. [Google Scholar] [PubMed]
- Buddingh, E.P.; Schilham, M.W.; Ruslan, S.E.; Berghuis, D.; Szuhai, K.; Suurmond, J.; Taminiau, A.H.; Gelderblom, H.; Egeler, R.M.; Serra, M.; et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol. Immunother. 2011, 60, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Yamanegi, K.; Ohyama, H.; Hata, M.; Nakasho, K.; Futani, H.; Okamura, H.; Terada, N. Hypoxia downregulates the expression of cell surface MICA without increasing soluble MICA in osteosarcoma cells in a HIF-1alpha-dependent manner. Int. J. Oncol. 2012, 41, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Sayitoglu, E.C.; Chrobok, M.; Georgoudaki, A.-M.; Josey, B.J.; Hartman, M.; Vallabhaneni, E.; Herekar, R.; Bergeron, S.; Krueger, R.; Sutlu, T.; et al. Abstract A55: Natural killer cells genetically modified to overexpress DNAM-1 exert enhanced antitumor responses against CD112/CD155+ sarcomas and other malignancies. Cancer Immunol. Res. 2020, 8, A55. [Google Scholar] [CrossRef]
- de Hooge, A.S.; Berghuis, D.; Santos, S.J.; Mooiman, E.; Romeo, S.; Kummer, J.A.; Egeler, R.M.; van Tol, M.J.; Melief, C.J.; Hogendoorn, P.C.; et al. Expression of cellular FLICE inhibitory protein, caspase-8, and protease inhibitor-9 in Ewing sarcoma and implications for susceptibility to cytotoxic pathways. Clin. Cancer Res. 2007, 13, 206–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fischer, K.; Tognarelli, S.; Roesler, S.; Boedicker, C.; Schubert, R.; Steinle, A.; Klingebiel, T.; Bader, P.; Fulda, S.; Ullrich, E. The Smac Mimetic BV6 Improves NK Cell-Mediated Killing of Rhabdomyosarcoma Cells by Simultaneously Targeting Tumor and Effector Cells. Front. Immunol. 2017, 8, 202. [Google Scholar] [CrossRef]
- Somanchi, S.S.; McCulley, K.J.; Somanchi, A.; Chan, L.L.; Lee, D.A. A Novel Method for Assessment of Natural Killer Cell Cytotoxicity Using Image Cytometry. PLoS ONE 2015, 10, e0141074. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, B.; Shi, J. Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget 2016, 7, 47163. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Markiewicz, K.; Zeman, K.; Kozar, A.; Golebiowska-Wawrzyniak, M.; Wozniak, W. [Evaluation of selected parameters of cellular immunity in children with osteosarcoma at diagnosis]. Med. Wieku Rozwoj. 2012, 16, 212–221. [Google Scholar] [PubMed]
- Balch, C.M.; Riley, L.B.; Bae, Y.J.; Salmeron, M.A.; Platsoucas, C.D.; von Eschenbach, A.; Itoh, K. Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Arch. Surg. 1990, 125, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, S.W.; Kilvaer, T.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T. Prognostic impact of lymphocytes in soft tissue sarcomas. PLoS ONE 2011, 6, e14611. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef]
- Koirala, P.; Roth, M.E.; Gill, J.; Piperdi, S.; Chinai, J.M.; Geller, D.S.; Hoang, B.H.; Park, A.; Fremed, M.A.; Zang, X.; et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016, 6, 30093. [Google Scholar] [CrossRef]
- Stahl, D.; Gentles, A.J.; Thiele, R.; Gutgemann, I. Prognostic profiling of the immune cell microenvironment in Ewing s Sarcoma Family of Tumors. Oncoimmunology 2019, 8, e1674113. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Xu, P. NK cell and macrophages confer prognosis and reflect immune status in osteosarcoma. J. Cell Biochem. 2018. [Google Scholar] [CrossRef]
- Rusakiewicz, S.; Semeraro, M.; Sarabi, M.; Desbois, M.; Locher, C.; Mendez, R.; Vimond, N.; Concha, A.; Garrido, F.; Isambert, N.; et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013, 73, 3499–3510. [Google Scholar] [CrossRef]
- Lee, L.; Fei, L.; Pope, J.; Wagner, L.M. Early Lymphocyte Recovery and Outcome in Osteosarcoma. J. Pediatr. Hematol. Oncol. 2017, 39, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Eslin, D.; Levy, A.; Roberson, J.; Giusti, V.; Sutphin, R. Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatr. Blood Cancer 2010, 55, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Luksch, R.; Perotti, D.; Cefalo, G.; Passerini, C.G.; Massimino, M.; Spreafico, F.; Casanova, M.; Ferrari, A.; Terenziani, M.; Polastri, D.; et al. Immunomodulation in a Treatment Program Including Pre- and Post-Operative Interleukin-2 and Chemotherapy for Childhood Osteosarcoma. Tumori J. 2003, 89, 263–268. [Google Scholar] [CrossRef]
- Delahaye, N.F.; Rusakiewicz, S.; Martins, I.; Menard, C.; Roux, S.; Lyonnet, L.; Paul, P.; Sarabi, M.; Chaput, N.; Semeraro, M.; et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat. Med. 2011, 17, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Nicoletti, G.; Landuzzi, L.; Croci, S.; Murgo, A.; Palladini, A.; Antognoli, A.; Ianzano, M.L.; Stivani, V.; Grosso, V.; et al. High metastatic efficiency of human sarcoma cells in Rag2/gammac double knockout mice provides a powerful test system for antimetastatic targeted therapy. Eur. J. Cancer 2010, 46, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.; Huang, G.; Worth, L.L. The role of interferon gamma and NK cells in the eradication of pulmonary osteosarcoma metastases by IL-12. Cancer Res. 2005, 65, 1413. [Google Scholar]
- Masuyama, K.; Ochiai, H.; Ishizawa, S.; Tazawa, K.; Niwayama, S.; Fujimaki, M. Relation of H-2 expression on murine RCT(+) sarcoma cells to lung colonization and sensitivity to NK cells. J. Cancer Res. Clin. Oncol. 1988, 114, 487–492. [Google Scholar] [CrossRef]
- Harris, M.A.; Shekhar, T.M.; Coupland, L.A.; Miles, M.A.; Hawkins, C.J. Transient NK Cell Depletion Facilitates Pulmonary Osteosarcoma Metastases After Intravenous Inoculation in Athymic Mice. J. Adolesc. Young Adult Oncol. 2020. [Google Scholar] [CrossRef]
- Smyth, M.J. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int. Immunol. 2008, 20, 631. [Google Scholar] [CrossRef]
- Smyth, M.J.; Swann, J.; Cretney, E.; Zerafa, N.; Yokoyama, W.M.; Hayakawa, Y. NKG2D function protects the host from tumor initiation. J. Exp. Med. 2005, 202, 583–588. [Google Scholar] [CrossRef]
- Elboim, M.; Gazit, R.; Gur, C.; Ghadially, H.; Betser-Cohen, G.; Mandelboim, O. Tumor immunoediting by NKp46. J. Immunol. 2010, 184, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Sceneay, J.; Paget, C.; Wong, C.S.; Duret, H.; Tschopp, J.; Moller, A.; Smyth, M.J. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012, 72, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front. Immunol. 2020, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, M.C.; Vincenzi, L.; Topino, S.; Giovannetti, A.; Mazzetta, F.; Libi, F.; Scaramuzzi, D.; Andreoni, M.; Pinter, E.; Baccarini, S.; et al. NK cell activity controls human herpesvirus 8 latent infection and is restored upon highly active antiretroviral therapy in AIDS patients with regressing Kaposi’s sarcoma. Eur. J. Immunol. 2002, 32, 2711–2720. [Google Scholar] [CrossRef]
- Matthews, N.C.; Goodier, M.R.; Robey, R.C.; Bower, M.; Gotch, F.M. Killing of Kaposi’s sarcoma-associated herpesvirus-infected fibroblasts during latent infection by activated natural killer cells. Eur. J. Immunol. 2011, 41, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Martin, M.P.; Vitale, F.; Lauria, C.; Whitby, D.; Qi, Y.; Gao, X.; Carrington, M. Risk of Classic Kaposi Sarcoma With Combinations of Killer Immunoglobulin-Like Receptor and Human Leukocyte Antigen Loci: A Population-Based Case-control Study. J. Infect. Dis. 2016, 213, 432–438. [Google Scholar] [CrossRef]
- Stebbing, J.; Gazzard, B.; Flore, O.; Thomas, C.; Benlahrech, A.; Mandalia, S.; Bower, M.; Gotch, F.; Patterson, S. Natural killer cells are not infected by Kaposi’s sarcoma-associated herpesvirus in vivo, and natural killer cell counts do not correlate with the risk of developing Kaposi’s sarcoma. AIDS 2003, 17, 1988–2000. [Google Scholar] [CrossRef]
- Karnbach, C.; Daws, M.R.; Niemi, E.C.; Nakamura, M.C. Immune rejection of a large sarcoma following cyclophosphamide and IL-12 treatment requires both NK and NK T cells and is associated with the induction of a novel NK T cell population. J. Immunol. 2001, 167, 2569–2576. [Google Scholar] [CrossRef]
- Takeda, K.; Smyth, M.J.; Cretney, E.; Hayakawa, Y.; Yamaguchi, N.; Yagita, H.; Okumura, K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell. Immunol. 2001, 214, 194–200. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sato-Matsushita, M.; Takeda, K.; Iwakura, Y.; Tahara, H.; Irimura, T. Early activation and interferon-gamma production of tumor-infiltrating mature CD27 high natural killer cells. Cancer Sci. 2011, 102, 1967–1971. [Google Scholar] [CrossRef]
- Aquino-López, A.; Senyukov, V.V.; Vlasic, Z.; Kleinerman, E.S.; Lee, D.A. Interferon Gamma Induces Changes in Natural Killer (NK) Cell Ligand Expression and Alters NK Cell-Mediated Lysis of Pediatric Cancer Cell Lines. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Bui, J.D.; Carayannopoulos, L.N.; Lanier, L.L.; Yokoyama, W.M.; Schreiber, R.D. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J. Immunol. 2006, 176, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; Saddawi-Konefka, R.; Gross, E.; Tran, M.; Mayfield, S.P.; Ikeda, H.; Bui, J.D. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 2014, 7, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Nolibe, D.; Poupon, M.F. Enhancement of pulmonary metastases induced by decreased lung natural killer cell activity. J. Natl. Cancer Inst. 1986, 77, 99–103. [Google Scholar] [PubMed]
- Hafner, M.; Orosz, P.; Kruger, A.; Mannel, D.N. TNF promotes metastasis by impairing natural killer cell activity. Int. J. Cancer 1996, 66, 388–392. [Google Scholar] [CrossRef]
- Lopez-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef]
- Hanamatsu, Y.; Saigo, C.; Kito, Y.; Takeuchi, T. An obstructive role of NK cells on metastatic growth of clear-cell sarcoma cells in a xenoplant murine model. Mol. Clin. Oncol. 2021, 14, 9. [Google Scholar] [CrossRef]
- Gopas, J.; Rager-Zisman, B.; Har-Vardi, I.; Hammerling, G.J.; Bar-Eli, M.; Segal, S. NK sensitivity, H-2 expression and metastatic potential: Analysis of H-2Dk gene transfected fibrosarcoma cells. J. Immunogenet. 1989, 16, 305–313. [Google Scholar] [CrossRef]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Korner, C.; Ben-Baruch, A. Tumor-Stroma-Inflammation Networks Promote Pro-metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 757. [Google Scholar] [CrossRef]
- Ben-Baruch, A. Partners in crime: TNFalpha-based networks promoting cancer progression. Cancer Immunol. Immunother. 2020, 69, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Molgora, M.; Bonavita, E.; Ponzetta, A.; Riva, F.; Barbagallo, M.; Jaillon, S.; Popović, B.; Bernardini, G.; Magrini, E.; Gianni, F.; et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 2017, 551, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.A.; Hashem, H.; Eid, S.; Allen, F.; Kingsley, D.; Huang, A.Y. Adoptive natural killer cell therapy is effective in reducing pulmonary metastasis of Ewing sarcoma. Oncoimmunology 2017, 6, e1303586. [Google Scholar] [CrossRef] [PubMed]
- Esartia, P.T.; Deichman, G.I.; Kluchareva, T.E.; Matveeva, V.A.; Uvarova, E.N.; Trapesnikov, N.N. Allogenic bone-marrow transfusion suppresses development of lung metastases in osteogenic sarcoma patients after radical surgery. Int. J. Cancer 1993, 54, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Feuchtinger, T.; Nitschke-Gerard, C.; Seidel, U.J.; Lang, A.M.; Kyzirakos, C.; Teltschik, H.M.; Ebinger, M.; Schumm, M.; Koscielniak, E.; et al. Favorable NK cell activity after haploidentical hematopoietic stem cell transplantation in stage IV relapsed Ewing’s sarcoma patients. Bone Marrow Transplant. 2015, 50 (Suppl. 2), S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.; Varesio, L.; Herberman, R.B. Systemic and in situ natural killer and suppressor cell activities in mice bearing progressively growing murine sarcoma-virus-induced tumors. Int. J. Cancer 1981, 27, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, D.H.; de Hooge, A.S.; Mooiman, E.C.; Santos, S.J.; ten Dam, M.M.; Gelderblom, H.; Melief, C.J.; Hogendoorn, P.C.; Egeler, R.M.; van Tol, M.J.; et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol. Immunol. 2008, 45, 3917–3925. [Google Scholar] [CrossRef]
- Bucklein, V.; Adunka, T.; Mendler, A.N.; Issels, R.; Subklewe, M.; Schmollinger, J.C.; Noessner, E. Progressive natural killer cell dysfunction associated with alterations in subset proportions and receptor expression in soft-tissue sarcoma patients. Oncoimmunology 2016, 5, e1178421. [Google Scholar] [CrossRef][Green Version]
- Sayitoglu, E.C.; Georgoudaki, A.M.; Chrobok, M.; Ozkazanc, D.; Josey, B.J.; Arif, M.; Kusser, K.; Hartman, M.; Chinn, T.M.; Potens, R.; et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Pahl, J.H.; Ruslan, S.E.; Kwappenberg, K.M.; van Ostaijen-Ten Dam, M.M.; van Tol, M.J.; Lankester, A.C.; Schilham, M.W. Antibody-dependent cell lysis by NK cells is preserved after sarcoma-induced inhibition of NK cell cytotoxicity. Cancer Immunol. Immunother. 2013, 62, 1235–1247. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Ruslan, S.E.; Berghuis, D.; Gelderblom, H.; Anninga, J.K.; Hogendoorn, P.C.; Egeler, R.M.; Schilham, M.W.; Lankester, A.C. Intact interferon signaling in peripheral blood leukocytes of high-grade osteosarcoma patients. Cancer Immunol. Immunother. 2012, 61, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Johann, P.D.; Vaegler, M.; Gieseke, F.; Mang, P.; Armeanu-Ebinger, S.; Kluba, T.; Handgretinger, R.; Muller, I. Tumour stromal cells derived from paediatric malignancies display MSC-like properties and impair NK cell cytotoxicity. BMC Cancer 2010, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Menard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Lian, J.; Yang, H.; Li, F.; Zhao, S.; Qi, Y.; Zhang, Y.; Huang, L. TNF-alpha-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J. Transl. Med. 2019, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Tarozzi, A.; Meneghetti, A.; Cattini, L.; Facchini, A. TNF-alpha but not IL-1 and IL-6 modifies the susceptibility of human osteosarcoma cells to NK lysis. Int. J. Oncol. 1998, 13, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Tarozzi, A.; Meneghetti, A.; Cattini, L.; Facchini, A. Human osteosarcoma cell susceptibility to natural killer cell lysis depends on CD54 and increases after TNFα incubation. FEBS Lett. 1997, 406, 83–88. [Google Scholar] [CrossRef]
- Meneghetti, A.; Mariani, E.; Santi, S.; Riccio, M.; Cattini, L.; Paoletti, S.; Facchini, A. NK binding capacity and lytic activity depend on the expression of ICAM-1 on target bone tumours. Int. J. Oncol. 1999, 15, 909–914. [Google Scholar] [CrossRef]
- Zamai, L.; Zauli, G.; Bavelloni, A.; Marmiroli, S.; Cataldi, A.; Weber, G.; Vitale, M. Tiazofurin Induces a Down-Modulation of ICAM-1 Expression on K562 Target Cells Impairing NK Adhesion and Killing. Cell. Immunol. 1995, 164, 100–104. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; He, J.; Jiang, L.; Li, X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol. Lett. 2019, 17, 2592–2598. [Google Scholar] [CrossRef]
- Noda, M.; Yoon, K.; Prince, C.W.; Butler, W.T.; Rodan, G.A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J. Biol. Chem. 1988, 263, 13916–13921. [Google Scholar]
- Wang, D.; Saga, Y.; Mizukami, H.; Sato, N.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; Ozawa, K.; et al. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int. J. Oncol. 2012, 40, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Saga, Y.; Mizukami, H.; Wang, D.; Takahashi, S.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; et al. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumor growth by promoting natural killer cell accumulation. Oncol. Rep. 2012, 28, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J.; et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Valkov, A.; Sorbye, S.W.; Kilvaer, T.K.; Donnem, T.; Smeland, E.; Bremnes, R.M.; Busund, L.T. The prognostic impact of TGF-beta1, fascin, NF-kappaB and PKC-zeta expression in soft tissue sarcomas. PLoS ONE 2011, 6, e17507. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef]
- Kailayangiri, S.; Altvater, B.; Spurny, C.; Jamitzky, S.; Schelhaas, S.; Jacobs, A.H.; Wiek, C.; Roellecke, K.; Hanenberg, H.; Hartmann, W.; et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology 2017, 6, e1250050. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kawaguchi, S.; Torigoe, T.; Asanuma, H.; Nakazawa, E.; Shimozawa, K.; Nabeta, Y.; Kimura, S.; Kaya, M.; Nagoya, S.; et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006, 97, 1374–1380. [Google Scholar] [CrossRef]
- Holmes, T.D.; El-Sherbiny, Y.M.; Davison, A.; Clough, S.L.; Blair, G.E.; Cook, G.P. A human NK cell activation/inhibition threshold allows small changes in the target cell surface phenotype to dramatically alter susceptibility to NK cells. J. Immunol. 2011, 186, 1538–1545. [Google Scholar] [CrossRef]
- Delgado, D.; Webster, D.E.; DeSantes, K.B.; Durkin, E.T.; Shaaban, A.F. KIR receptor-ligand incompatibility predicts killing of osteosarcoma cell lines by allogeneic NK cells. Pediatr. Blood Cancer 2010, 55, 1300–1305. [Google Scholar] [CrossRef]
- Oppenheim, D.E.; Roberts, S.J.; Clarke, S.L.; Filler, R.; Lewis, J.M.; Tigelaar, R.E.; Girardi, M.; Hayday, A.C. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005, 6, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, S.; Stieber, P.; Peterfi, A.; Nagel, D.; Steinle, A.; Salih, H.R. Soluble MICA in malignant diseases. Int. J. Cancer 2006, 118, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, X.; Zhang, H.; Deng, L.; Zhang, Y. MMP9 mediates MICA shedding in human osteosarcomas. Cell Biol. Int. 2011, 35, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, T.; Wang, W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine 2018, 97, e13051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X. Association of MMP-2 expression and prognosis in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 14965–14970. [Google Scholar]
- Lafleur, E.A.; Jia, S.F.; Worth, L.L.; Zhou, Z.; Owen-Schaub, L.B.; Kleinerman, E.S. Interleukin (IL)-12 and IL-12 gene transfer up-regulate Fas expression in human osteosarcoma and breast cancer cells. Cancer Res. 2001, 61, 4066–4071. [Google Scholar]
- Gordon, N.; Koshkina, N.V.; Jia, S.F.; Khanna, C.; Mendoza, A.; Worth, L.L.; Kleinerman, E.S. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin. Cancer Res. 2007, 13, 4503–4510. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yoshikawa, H.; Shiiki, K.; Hamada, Y.; Nakajima, Y.; Tasaka, K. Cisplatin (CDDP) sensitizes human osteosarcoma cell to Fas/CD95-mediated apoptosis by down-regulating FLIP-L expression. Int. J. Cancer 2000, 88, 986–991. [Google Scholar] [CrossRef]
- Rao-Bindal, K.; Zhou, Z.; Kleinerman, E.S. MS-275 sensitizes osteosarcoma cells to Fas ligand-induced cell death by increasing the localization of Fas in membrane lipid rafts. Cell Death Dis. 2012, 3, e369. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Rao-Bindal, K.; Kleinerman, E.S. Effect of the histone deacetylase inhibitor SNDX-275 on Fas signaling in osteosarcoma cells and the feasibility of its topical application for the treatment of osteosarcoma lung metastases. Cancer 2011, 117, 3457–3467. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Xu, Y.; Wang, Z.; Du, X.; Li, C.; Peng, J.; Gao, L.; Liang, X.; Ma, C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 2017, 36, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vely, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbe, C.; et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Chen, L.; Li, Y.J.; Kong, D.L. PDL1/PD1 axis serves an important role in natural killer cellinduced cytotoxicity in osteosarcoma. Oncol. Rep. 2019, 42, 2049–2056. [Google Scholar] [CrossRef]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, W.; Shan, B.; Ding, Y.; Zhang, G.; Cao, N.; Liu, L.; Zhang, Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE 2013, 8, e70689. [Google Scholar] [CrossRef]
- Gregorio, A.; Corrias, M.V.; Castriconi, R.; Dondero, A.; Mosconi, M.; Gambini, C.; Moretta, A.; Moretta, L.; Bottino, C. Small round blue cell tumours: Diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008, 53, 73–80. [Google Scholar] [CrossRef]
- Loo, D.; Alderson, R.F.; Chen, F.Z.; Huang, L.; Zhang, W.; Gorlatov, S.; Burke, S.; Ciccarone, V.; Li, H.; Yang, Y.; et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer Res. 2012, 18, 3834–3845. [Google Scholar] [CrossRef]
- Pu, F.; Chen, F.; Zhang, Z.; Qing, X.; Lin, H.; Zhao, L.; Xia, P.; Shao, Z. TIM-3 expression and its association with overall survival in primary osteosarcoma. Oncol. Lett. 2019, 18, 5294–5300. [Google Scholar] [CrossRef]
- Lewis, D.M.; Park, K.M.; Tang, V.; Xu, Y.; Pak, K.; Eisinger-Mathason, T.S.; Simon, M.C.; Gerecht, S. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc. Natl. Acad. Sci. USA 2016, 113, 9292–9297. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Y.; Lu, Y.; Liu, L.; Shi, D.; Wen, Y.; Yang, L.; Ma, Q.; Liu, T.; Zhu, X.; et al. The HIF-1alpha/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells. Cancer Lett. 2015, 357, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Manzini, C.; Pietra, G.; Raggi, F.; Blengio, F.; Mingari, M.C.; Varesio, L.; Moretta, L.; Bosco, M.C.; Vitale, M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur. J. Immunol. 2013, 43, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Alsner, J.; Keller, J.; Nielsen, O.S.; Jensen, O.M.; Horsman, M.R.; Overgaard, J. Hypoxia in human soft tissue sarcomas: Adverse impact on survival and no association with p53 mutations. Br. J. Cancer 2001, 84, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Nathan, F.M.; Singh, V.A.; Dhanoa, A.; Palanisamy, U.D. Oxidative stress and antioxidant status in primary bone and soft tissue sarcoma. BMC Cancer 2011, 11, 382. [Google Scholar] [CrossRef]
- Siernicka, M.; Winiarska, M.; Bajor, M.; Firczuk, M.; Muchowicz, A.; Bobrowicz, M.; Fauriat, C.; Golab, J.; Olive, D.; Zagozdzon, R. Adenanthin, a new inhibitor of thiol-dependent antioxidant enzymes, impairs the effector functions of human natural killer cells. Immunology 2015, 146, 173–183. [Google Scholar] [CrossRef]
- Goodier, M.R.; Mela, C.M.; Steel, A.; Gazzard, B.; Bower, M.; Gotch, F. NKG2C+ NK cells are enriched in AIDS patients with advanced-stage Kaposi’s sarcoma. J. Virol. 2007, 81, 430–433. [Google Scholar] [CrossRef]
- Weltfriend, S.; Friedman-Birnbaum, R.; Pollack, S. Decreased natural killer cell function in patients with classical Kaposi’s sarcoma. Dermatologica 1990, 181, 207–210. [Google Scholar] [CrossRef]
- Dupuy, S.; Lambert, M.; Zucman, D.; Choukem, S.P.; Tognarelli, S.; Pages, C.; Lebbe, C.; Caillat-Zucman, S. Human Herpesvirus 8 (HHV8) sequentially shapes the NK cell repertoire during the course of asymptomatic infection and Kaposi sarcoma. PLoS Pathog. 2012, 8, e1002486. [Google Scholar] [CrossRef]
- Holt, D.; Ma, X.; Kundu, N.; Fulton, A. Prostaglandin E(2) (PGE (2)) suppresses natural killer cell function primarily through the PGE(2) receptor EP4. Cancer Immunol. Immunother. 2011, 60, 1577–1586. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Q.; Jiang, Y.; Yu, J.; Hu, Y.; Mou, T.; Chen, G.; Li, G. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology 2016, 5, e1069936. [Google Scholar] [CrossRef]
- Ishido, S.; Choi, J.K.; Lee, B.S.; Wang, C.; DeMaria, M.; Johnson, R.P.; Cohen, G.B.; Jung, J.U. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity 2000, 13, 365–374. [Google Scholar] [CrossRef]
- Thomas, M.; Boname, J.M.; Field, S.; Nejentsev, S.; Salio, M.; Cerundolo, V.; Wills, M.; Lehner, P.J. Down-regulation of NKG2D and NKp80 ligands by Kaposi’s sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2008, 105, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Wills, M.; Lehner, P.J. Natural killer cell evasion by an E3 ubiquitin ligase from Kaposi’s sarcoma-associated herpesvirus. Biochem. Soc. Trans. 2008, 36, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, B.; Olszewski, W.L.; Engeset, A.; Kolstad, P. The effect of surgery and chemotherapy on blood NK cell activity in patients with ovarian cancer. Cancer 1983, 51, 465–469. [Google Scholar] [CrossRef]
- Kubota, Y.; Ohji, H.; Itoh, K.; Sasagawa, I.; Nakada, T. Changes in cellular immunity during chemotherapy for testicular cancer. Int. J. Urol. 2001, 8, 604–608. [Google Scholar] [CrossRef]
- Mantovani, A.; Luini, W.; Peri, G.; Vecchi, A.; Spreafico, F. Effect of chemotherapeutic agents on natural cell-mediated cytotoxicity in mice. J. Natl. Cancer Inst. 1978, 61, 1255–1261. [Google Scholar] [CrossRef]
- Charamella, L.J.; Meyer, C.; Thompson, G.E.; Dimitrov, N.V. Chemotherapeutic agents and modulation of natural killer cell activity in vitro. J. Immunopharmacol. 1985, 7, 53–65. [Google Scholar] [CrossRef]
- Mueller, S.K.; Altvater, B.; Chen, C.; Kailayangiri, S.; Ahlmann, M.; Dirksen, U.; Juergens, H.; Rossig, C. Zoledronic acid negatively affects the expansion of in vitro activated human NK cells and their cytolytic interactions with Ewing sarcoma cells. Oncol. Rep. 2013, 29, 2348–2354. [Google Scholar] [CrossRef][Green Version]
- Marhelava, K.; Pilch, Z.; Bajor, M.; Graczyk-Jarzynka, A.; Zagozdzon, R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers 2019, 11, 1756. [Google Scholar] [CrossRef]
- Burgess, M.; Tawbi, H. Immunotherapeutic approaches to sarcoma. Curr. Treat. Opt. Oncol. 2015, 16, 26. [Google Scholar] [CrossRef]
- Dyson, K.A.; Stover, B.D.; Grippin, A.; Mendez-Gomez, H.R.; Lagmay, J.; Mitchell, D.A.; Sayour, E.J. Emerging trends in immunotherapy for pediatric sarcomas. J. Hematol. Oncol. 2019, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Florou, V.; Rosenberg, A.E.; Wieder, E.; Komanduri, K.V.; Kolonias, D.; Uduman, M.; Castle, J.C.; Buell, J.S.; Trent, J.C.; Wilky, B.A. Angiosarcoma patients treated with immune checkpoint inhibitors: A case series of seven patients from a single institution. J. Immunother. Cancer 2019, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Lewin, J.; Davidson, S.; Anderson, N.D.; Lau, B.Y.; Kelly, J.; Tabori, U.; Salah, S.; Butler, M.O.; Aung, K.L.; Shlien, A.; et al. Response to Immune Checkpoint Inhibition in Two Patients with Alveolar Soft-Part Sarcoma. Cancer Immunol. Res. 2018, 6, 1001–1007. [Google Scholar] [CrossRef]
- Perez-Martinez, A.; de Prada Vicente, I.; Fernandez, L.; Gonzalez-Vicent, M.; Valentin, J.; Martin, R.; Maxwell, H.; Sevilla, J.; Vicario, J.L.; Diaz, M.A. Natural killer cells can exert a graft-vs-tumor effect in haploidentical stem cell transplantation for pediatric solid tumors. Exp. Hematol. 2012, 40, 882–891. [Google Scholar] [CrossRef]
- Lang, P.; Pfeiffer, M.; Muller, I.; Schumm, M.; Ebinger, M.; Koscielniak, E.; Feuchtinger, T.; Foll, J.; Martin, D.; Handgretinger, R. Haploidentical stem cell transplantation in patients with pediatric solid tumors: Preliminary results of a pilot study and analysis of graft versus tumor effects. Klin. Padiatr. 2006, 218, 321–326. [Google Scholar] [CrossRef]
- Perez-Martinez, A.; Leung, W.; Munoz, E.; Iyengar, R.; Ramirez, M.; Vicario, J.L.; Lassaletta, A.; Sevilla, J.; Gonzalez-Vicent, M.; Madero, L.; et al. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr. Blood Cancer 2009, 53, 120–124. [Google Scholar] [CrossRef]
- Thiel, U.; Koscielniak, E.; Blaeschke, F.; Grunewald, T.G.; Badoglio, M.; Diaz, M.A.; Paillard, C.; Prete, A.; Ussowicz, M.; Lang, P.; et al. Allogeneic stem cell transplantation for patients with advanced rhabdomyosarcoma: A retrospective assessment. Br. J. Cancer 2013, 109, 2523–2532. [Google Scholar] [CrossRef]
- Flannery, G.R.; Pelham, J.M.; Gray, J.D.; Baldwin, R.W. Immunomodulation: NK cells activated by interferon-conjugated monoclonal antibody against human osteosarcoma. Eur. J. Cancer Clin. Oncol. 1984, 20, 791–798. [Google Scholar] [CrossRef]
- Mariani, E.; Meneghetti, A.; Tarozzi, A.; Cattini, L.; Facchini, A. Interleukin-12 induces efficient lysis of natural killer-sensitive and natural killer-resistant human osteosarcoma cells: The synergistic effect of interleukin-2. Scand. J. Immunol. 2000, 51, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.S.; Findley, H.W.; Chan, W.C.; Hnath, R.S.; Abdel-Mageed, A.; Pais, R.C.; Kutner, M.H.; Ragab, A.H. Natural killer cells in children with malignant solid tumors. Effect of recombinant interferon-alpha and interleukin-2 on natural killer cell function against tumor cell lines. Cancer 1989, 63, 83–89. [Google Scholar] [CrossRef]
- Reiter, Z.; Ozes, O.N.; Blatt, L.M.; Sturzl, M.; Taylor, M.W. A possible role for interferon-alpha and activated natural killer cells in remission of AIDS-related Kaposi’s sarcoma: In vitro studies. J. Acquir. Immune Defic. Syndr. 1992, 5, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Boerman, G.H.; van Ostaijen-ten Dam, M.M.; Kraal, K.C.; Santos, S.J.; Ball, L.M.; Lankester, A.C.; Schilham, M.W.; Egeler, R.M.; van Tol, M.J. Role of NKG2D, DNAM-1 and natural cytotoxicity receptors in cytotoxicity toward rhabdomyosarcoma cell lines mediated by resting and IL-15-activated human natural killer cells. Cancer Immunol. Immunother. 2015, 64, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Fuller, J.A.; Kremlev, S.G.; Kamdar, S.J.; Evans, R. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. J. Immunol. 1998, 161, 6977–6984. [Google Scholar] [PubMed]
- Evans, R.; Fuller, J.A.; Christianson, G.; Krupke, D.M.; Troutt, A.B. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: The potential role of NK cell subpopulations. Cell Immunol. 1997, 179, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.J.; Tanimoto, T.; Kohno, K.; Ikeda, M.; Kurimoto, M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997, 57, 4557–4563. [Google Scholar]
- Numasaki, M.; Tagawa, M.; Iwata, F.; Suzuki, T.; Nakamura, A.; Okada, M.; Iwakura, Y.; Aiba, S.; Yamaya, M. IL-28 elicits antitumor responses against murine fibrosarcoma. J. Immunol. 2007, 178, 5086–5098. [Google Scholar] [CrossRef]
- Sabel, M.S.; Arora, A.; Su, G.; Mathiowitz, E.; Reineke, J.J.; Chang, A.E. Synergistic effect of intratumoral IL-12 and TNF-alpha microspheres: Systemic anti-tumor immunity is mediated by both CD8+ CTL and NK cells. Surgery 2007, 142, 749–760. [Google Scholar] [CrossRef]
- Bielack, S.S.; Smeland, S.; Whelan, J.S.; Marina, N.; Jovic, G.; Hook, J.M.; Krailo, M.D.; Gebhardt, M.; Pápai, Z.; Meyer, J.; et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 2279–2287. [Google Scholar] [CrossRef]
- Dutcher, J.P.; Schwartzentruber, D.J.; Kaufman, H.L.; Agarwala, S.S.; Tarhini, A.A.; Lowder, J.N.; Atkins, M.B. High dose interleukin-2 (Aldesleukin)—Expert consensus on best management practices-2014. J. Immunother. Cancer 2014, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. CII 2014, 63, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Worth, L.L.; Jia, S.F.; Zhou, Z.; Chen, L.; Kleinerman, E.S. Intranasal therapy with an adenoviral vector containing the murine interleukin-12 gene eradicates osteosarcoma lung metastases. Clin. Cancer Res. 2000, 6, 3713–3718. [Google Scholar] [PubMed]
- Jia, S.F.; Worth, L.L.; Densmore, C.L.; Xu, B.; Zhou, Z.; Kleinerman, E.S. Eradication of osteosarcoma lung metastases following intranasal interleukin-12 gene therapy using a nonviral polyethylenimine vector. Cancer Gene. Ther. 2002, 9, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, C.; de Wilt, J.H.W.; Grünhagen, D.J.; van Geel, A.N.; ten Hagen, T.L.M.; Eggermont, A.M.M. Isolated limb perfusion with melphalan and TNF-alpha in the treatment of extremity sarcoma. Curr. Treat. Opt. Oncol. 2007, 8, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Parton, A.; Lu, L.; Adams, M.; Schafer, P.; Bartlett, J.B. Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: Influence of host immune and tumor markers. Cancer Immunol. Immunother. 2011, 60, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Ko, Y.; Kim, D.H.; Lim, J.S.; Kong, C.B.; Cho, W.H.; Jeon, D.G.; Lee, S.Y.; Koh, J.S. Epidermal growth factor receptor: Is it a feasible target for the treatment of osteosarcoma? Cancer Res. Treat. 2012, 44, 202–209. [Google Scholar] [CrossRef]
- Pahl, J.H.; Ruslan, S.E.; Buddingh, E.P.; Santos, S.J.; Szuhai, K.; Serra, M.; Gelderblom, H.; Hogendoorn, P.C.; Egeler, R.M.; Schilham, M.W.; et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin. Cancer Res. 2012, 18, 432–441. [Google Scholar] [CrossRef]
- Ha, H.T.; Griffith, K.A.; Zalupski, M.M.; Schuetze, S.M.; Thomas, D.G.; Lucas, D.R.; Baker, L.H.; Chugh, R. Phase II trial of cetuximab in patients with metastatic or locally advanced soft tissue or bone sarcoma. Am. J. Clin. Oncol. 2013, 36, 77–82. [Google Scholar] [CrossRef]
- Desantes, K.; Maris, J.M.; McDowell, K.; Mackall, C.; Shankar, S.; Vasselli, J.; Chen, F.; Loo, D.; Moore, P.A.; Wigginton, J.M.; et al. A phase 1, open-label, dose escalation study of enoblituzumab (MGA271) in pediatric patients with B7-H3-expressing relapsed or refractory solid tumors. J. Clin. Oncol. 2017, 35, TPS2596. [Google Scholar] [CrossRef]
- Pappo, A.S.; Patel, S.R.; Crowley, J.; Reinke, D.K.; Kuenkele, K.-P.; Chawla, S.P.; Toner, G.C.; Maki, R.G.; Meyers, P.A.; Chugh, R.; et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II Sarcoma Alliance for Research through Collaboration study. J. Clin. Oncol. 2011, 29, 4541–4547. [Google Scholar] [CrossRef] [PubMed]
- Jamitzky, S.; Krueger, A.C.; Janneschuetz, S.; Piepke, S.; Kailayangiri, S.; Spurny, C.; Rossig, C.; Altvater, B. Insulin-like growth factor-1 receptor (IGF-1R) inhibition promotes expansion of human NK cells which maintain their potent antitumor activity against Ewing sarcoma cells. Pediatr. Blood Cancer 2015, 62, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Yamanegi, K.; Yamane, J.; Kobayashi, K.; Kato-Kogoe, N.; Ohyama, H.; Nakasho, K.; Yamada, N.; Hata, M.; Nishioka, T.; Fukunaga, S.; et al. Sodium valproate, a histone deacetylase inhibitor, augments the expression of cell-surface NKG2D ligands, MICA/B, without increasing their soluble forms to enhance susceptibility of human osteosarcoma cells to NK cell-mediated cytotoxicity. Oncol. Rep. 2010, 24, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Yamanegi, K.; Yamane, J.; Kobayashi, K.; Kato-Kogoe, N.; Ohyama, H.; Nakasho, K.; Yamada, N.; Hata, M.; Fukunaga, S.; Futani, H.; et al. Valproic acid cooperates with hydralazine to augment the susceptibility of human osteosarcoma cells to Fas- and NK cell-mediated cell death. Int. J. Oncol. 2012, 41, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Denman, C.J.; Cobanoglu, Z.S.; Kiany, S.; Lau, C.C.; Gottschalk, S.M.; Hughes, D.P.; Kleinerman, E.S.; Lee, D.A. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm. Res. 2015, 32, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Rao-Bindal, K.; Koshkina, N.V.; Stewart, J.; Kleinerman, E.S. The histone deacetylase inhibitor, MS-275 (entinostat), downregulates c-FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Curr. Cancer Drug Targets 2013, 13, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kiany, S.; Huang, G.; Kleinerman, E.S. Effect of entinostat on NK cell-mediated cytotoxicity against osteosarcoma cells and osteosarcoma lung metastasis. Oncoimmunology 2017, 6, e1333214. [Google Scholar] [CrossRef] [PubMed]
- Idso, J.M.; Lao, S.; Schloemer, N.J.; Knipstein, J.; Burns, R.; Thakar, M.S.; Malarkannan, S. Entinostat augments NK cell functions via epigenetic upregulation of IFIT1-STING-STAT4 pathway. Oncotarget 2020, 11, 1799. [Google Scholar] [CrossRef]
- Ogbomo, H.; Michaelis, M.; Kreuter, J.; Doerr, H.W.; Cinatl, J., Jr. Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett. 2007, 581, 1317–1322. [Google Scholar] [CrossRef]
- Kopp, L.M.; Ray, A.; Denman, C.J.; Senyukov, V.S.; Somanchi, S.S.; Zhu, S.; Lee, D.A. Decitabine has a biphasic effect on natural killer cell viability, phenotype, and function under proliferative conditions. Mol. Immunol. 2013, 54, 296–301. [Google Scholar] [CrossRef]
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Smith, R.C.; Monjazeb, A.M.; Chen, M.; Murphy, W.J. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology 2015, 4, e1036212. [Google Scholar] [CrossRef] [PubMed]
- Cheda, A.; Wrembel-Wargocka, J.; Lisiak, E.; Nowosielska, E.M.; Marciniak, M.; Janiak, M.K. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat. Res. 2004, 161, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Pfannenstiel, V.; Waldmann, A.; Bergs, J.W.J.; Brill, B.; Huenecke, S.; Klingebiel, T.; Rodel, F.; Buchholz, C.J.; Wels, W.S.; et al. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front. Immunol. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.; Berchtold, S.; Schmidt, M.; Beil, J.; Smirnow, I.; Venturelli, S.; Burkard, M.; Handgretinger, R.; Lauer, U.M. Biological treatment of pediatric sarcomas by combined virotherapy and NK cell therapy. BMC Cancer 2019, 19, 1172. [Google Scholar] [CrossRef]
- Routes, J.M.; Ryan, S.; Morris, K.; Takaki, R.; Cerwenka, A.; Lanier, L.L. Adenovirus serotype 5 E1A sensitizes tumor cells to NKG2D-dependent NK cell lysis and tumor rejection. J. Exp. Med. 2005, 202, 1477–1482. [Google Scholar] [CrossRef]
- Cook, J.L.; Wilson, B.A.; Wolf, L.A.; Walker, T.A. E1A oncogene expression level in sarcoma cells: An independent determinant of cytolytic susceptibility and tumor rejection. Oncogene 1993, 8, 625–635. [Google Scholar]
- Kelly, C.M.; Antonescu, C.R.; Bowler, T.; Munhoz, R.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Dholakia, R.; et al. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 402–408. [Google Scholar] [CrossRef]
- Mirandola, P.; Sponzilli, I.; Gobbi, G.; Marmiroli, S.; Rinaldi, L.; Binazzi, R.; Piccari, G.G.; Ramazzotti, G.; Gaboardi, G.C.; Cocco, L.; et al. Anticancer agents sensitize osteosarcoma cells to TNF-related apoptosis-inducing ligand downmodulating IAP family proteins. Int. J. Oncol. 2006, 28, 127–133. [Google Scholar] [CrossRef][Green Version]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Issels, R.D. High-risk soft tissue sarcoma: Clinical trial and hyperthermia combined chemotherapy. Int. J. Hyperth. 2006, 22, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G. Heat shock protein 72 (HSP72), a hyperthermia-inducible immunogenic determinant on leukemic K562 and Ewing’s sarcoma cells. Int. J. Hyperth. 1997, 13, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kubista, B.; Trieb, K.; Blahovec, H.; Kotz, R.; Micksche, M. Hyperthermia increases the susceptibility of chondro- and osteosarcoma cells to natural killer cell-mediated lysis. Anticancer. Res. 2002, 22, 789–792. [Google Scholar] [PubMed]
- Begovic, M.; Herberman, R.; Gorelik, E. Increase in immunogenicity and sensitivity to natural cell-mediated cytotoxicity following in vitro exposure of MCA105 tumor cells to ultraviolet radiation. Cancer Res. 1991, 51, 5153–5159. [Google Scholar] [PubMed]
- Fernandez, L.; Valentin, J.; Zalacain, M.; Leung, W.; Patino-Garcia, A.; Perez-Martinez, A. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. 2015, 368, 54–63. [Google Scholar] [CrossRef]
- Algarra, I.; Perez, M.; Gaforio, J.J.; Gasca, F.; Garrido, F. In vivo activation of NK cells induces inhibition of lung colonization of H-2 positive and H-2 negative fibrosarcoma tumor clones. Clin. Exp. Metastasis 1994, 12, 31–36. [Google Scholar] [CrossRef]
- Honorati, M.C.; Neri, S.; Cattini, L.; Facchini, A. IL-17 enhances the susceptibility of U-2 OS osteosarcoma cells to NK cell lysis. Clin. Exp. Immunol. 2003, 133, 344–349. [Google Scholar] [CrossRef]
- Liebau, C.; Merk, H.; Schmidt, S.; Roesel, C.; Karreman, C.; Prisack, J.B.; Bojar, H.; Baltzer, A.W. Interleukin-12 and interleukin-18 change ICAM-I expression, and enhance natural killer cell mediated cytolysis of human osteosarcoma cells. Cytokines Cell Mol. Ther. 2002, 7, 135–142. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Cavnar, M.J.; Zeng, S.; Bamboat, Z.M.; Ocuin, L.M.; Obaid, H.; Sorenson, E.C.; Popow, R.; Ariyan, C.; Rossi, F.; et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011, 17, 1094–1100. [Google Scholar] [CrossRef]
- Billingsley, K.G.; Burt, M.E.; Jara, E.; Ginsberg, R.J.; Woodruff, J.M.; Leung, D.H.; Brennan, M.F. Pulmonary metastases from soft tissue sarcoma: Analysis of patterns of diseases and postmetastasis survival. Ann. Surg. 1999, 229, 602–612. [Google Scholar] [CrossRef]
- Amankwah, E.K.; Conley, A.P.; Reed, D.R. Epidemiology and therapies for metastatic sarcoma. Clin. Epidemiol. 2013, 5, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, A.; Fernandez, L.; Valentin, J.; Martinez-Romera, I.; Corral, M.D.; Ramirez, M.; Abad, L.; Santamaria, S.; Gonzalez-Vicent, M.; Sirvent, S.; et al. A phase I/II trial of interleukin-15–stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy 2015, 17, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A. Cellular therapy: Adoptive immunotherapy with expanded natural killer cells. Immunol. Rev. 2019, 290, 85–99. [Google Scholar] [CrossRef]
- Vahedi, F.; Nham, T.; Poznanski, S.M.; Chew, M.V.; Shenouda, M.M.; Lee, D.; Ashkar, A.A. Ex Vivo Expanded Human NK Cells Survive and Proliferate in Humanized Mice with Autologous Human Immune Cells. Sci. Rep. 2017, 7, 12083. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef]
- Canter, R.J.; Grossenbacher, S.K.; Foltz, J.A.; Sturgill, I.R.; Park, J.S.; Luna, J.I.; Kent, M.S.; Culp, W.T.N.; Chen, M.; Modiano, J.F.; et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunother. Cancer 2017, 5, 98. [Google Scholar] [CrossRef]
- Chu, Y.; Rosenblum, J.; Jeng, E.K.; Alter, S.; Rhode, P.R.; Lee, J.H.; Lee, D.; Wong, H.C.; Cairo, M.S. Efficiently Targeting Metastatic Osteosarcoma, Neuroblastoma and Glioblastoma with Ex-Vivo Expanded Natural Killer Cells Combined with N-803 (ALT-803, IL-15 Superagonist) and TIM-3 Blockage. Biol. Blood Marrow Transplant. 2019, 25. [Google Scholar] [CrossRef]
- Vela, M.; Bueno, D.; González-Navarro, P.; Brito, A.; Fernández, L.; Escudero, A.; Valentín, J.; Mestre-Durán, C.; Arranz-Álvarez, M.; Pérez de Diego, R.; et al. Anti-CXCR4 Antibody Combined With Activated and Expanded Natural Killer Cells for Sarcoma Immunotherapy. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Baird, K.; Delbrook, C.P.; Fleisher, T.A.; Kohler, M.E.; Rampertaap, S.; Lemberg, K.; Hurley, C.K.; Kleiner, D.E.; Merchant, M.S.; et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 2015, 125, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, F.; Alvarez, M.; Negrin, R.S. Natural Killer Cells in Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2017, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A.; Leveson-Gower, D.B.; Gill, S.; Baker, J.; Beilhack, A.; Negrin, R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Poon, V.I.; Roth, M.; Piperdi, S.; Geller, D.; Gill, J.; Rudzinski, E.R.; Hawkins, D.S.; Gorlick, R. Ganglioside GD2 expression is maintained upon recurrence in patients with osteosarcoma. Clin. Sarcoma Res. 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.-K.V. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatric Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Mao, X.; Wang, W.; Chen, Y.; Li, D.; Li, H.; Dou, P. Anti-ganglioside GD2 monoclonal antibody synergizes with cisplatin to induce endoplasmic reticulum-associated apoptosis in osteosarcoma cells. Pharmazie 2018, 73, 80–86. [Google Scholar] [CrossRef]
- Park, H.; Awasthi, A.; Ayello, J.; Chu, Y.; Riddell, S.; Rosenblum, J.; Lee, D.A.; Cairo, M.S. ROR1-Specific Chimeric Antigen Receptor (CAR) NK Cell Immunotherapy for High Risk Neuroblastomas and Sarcomas. Biol. Blood Marrow Transplant. 2017, 23, S136–S137. [Google Scholar] [CrossRef][Green Version]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef]
- Lehner, M.; Gotz, G.; Proff, J.; Schaft, N.; Dorrie, J.; Full, F.; Ensser, A.; Muller, Y.A.; Cerwenka, A.; Abken, H.; et al. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS ONE 2012, 7, e31210. [Google Scholar] [CrossRef]
- Chang, Y.H.; Connolly, J.; Shimasaki, N.; Mimura, K.; Kono, K.; Campana, D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013, 73, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Metais, J.Y.; Escudero, A.; Vela, M.; Valentin, J.; Vallcorba, I.; Leivas, A.; Torres, J.; Valeri, A.; Patino-Garcia, A.; et al. Memory T Cells Expressing an NKG2D-CAR Efficiently Target Osteosarcoma Cells. Clin. Cancer Res. 2017, 23, 5824–5835. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, K.; Ovali, E.; Ozdamarlar, U.; Celen, S.; Karasu, G.; Yesilipek, A.; Hazar, V. NK-92 cellular therapy for pediatric relapsed/refractory Ewing sarcoma. Int. Cancer Conf. J. 2020, 9, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Guma, S.R.; Lee, D.A.; Yu, L.; Gordon, N.; Hughes, D.; Stewart, J.; Wang, W.L.; Kleinerman, E.S. Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis. Pediatr. Blood Cancer 2014, 61, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gordon, N.; Kleinerman, E.S.; Huang, G. Promoting NK cell trafficking to improve therapeutic effect of NK cell therapy on osteosarcoma. J. Immunother. Cancer 2015, 3. [Google Scholar] [CrossRef]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L.; et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti–PD-1 therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef]
- Crowe, N.Y.; Smyth, M.J.; Godfrey, D.I. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 2002, 196, 119–127. [Google Scholar] [CrossRef]


| Receptor | Known Ligands | Molecular Structure | Function |
|---|---|---|---|
| Killer Immunoglobulin-like receptors (KIR) | HLA-A, Bw, C, G | Immunoglobulin Superfamily | Stimulatory (short cytoplasmic tail) or inhibitory (long cytoplasmic tail) |
| CD16 (FcγRIII) | Fc portion of IgG | Immunoglobulin Superfamily | Stimulatory |
| CD2 receptor family | Immunoglobulin Superfamily | ||
| 2B4 (CD244) | CD48 | Stimulatory | |
| DNAM-1 (CD226) | PVR (CD155) and Nectin-2 (CD112) | Stimulatory | |
| NTB-A | Homophilic | Stimulatory | |
| CS1 (CRACC) | Homophilic | Stimulatory | |
| NKG2 receptor family | C-type lectins | ||
| NKG2D | MICA/B, ULBPs | Stimulatory | |
| CD94/NKG2A | HLA-E | Inhibitory | |
| CD94/NKG2C | HLA-E | Stimulatory | |
| CD94/NKG2E | HLA-E | Stimulatory | |
| Natural Cytotoxicity Receptors (NCRs) | Immunoglobulin Superfamily | ||
| NKp30a/b | B7-H6, BAG6 | Stimulatory | |
| NKp30c | B7-H6, BAG6 | Inhibitory | |
| NKp44 | PCNA | Stimulatory | |
| NKp46 | Vimentin | Stimulatory | |
| NKp80 | AICL | Stimulatory |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachota, M.; Vincenti, M.; Winiarska, M.; Boye, K.; Zagożdżon, R.; Malmberg, K.-J. Prospects for NK Cell Therapy of Sarcoma. Cancers 2020, 12, 3719. https://doi.org/10.3390/cancers12123719
Lachota M, Vincenti M, Winiarska M, Boye K, Zagożdżon R, Malmberg K-J. Prospects for NK Cell Therapy of Sarcoma. Cancers. 2020; 12(12):3719. https://doi.org/10.3390/cancers12123719
Chicago/Turabian StyleLachota, Mieszko, Marianna Vincenti, Magdalena Winiarska, Kjetil Boye, Radosław Zagożdżon, and Karl-Johan Malmberg. 2020. "Prospects for NK Cell Therapy of Sarcoma" Cancers 12, no. 12: 3719. https://doi.org/10.3390/cancers12123719
APA StyleLachota, M., Vincenti, M., Winiarska, M., Boye, K., Zagożdżon, R., & Malmberg, K.-J. (2020). Prospects for NK Cell Therapy of Sarcoma. Cancers, 12(12), 3719. https://doi.org/10.3390/cancers12123719






