Role of Hypoxia and the Adenosine System in Immune Evasion and Prognosis of Patients with Brain Metastases of Melanoma: A Multiplex Whole Slide Immunofluorescence Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
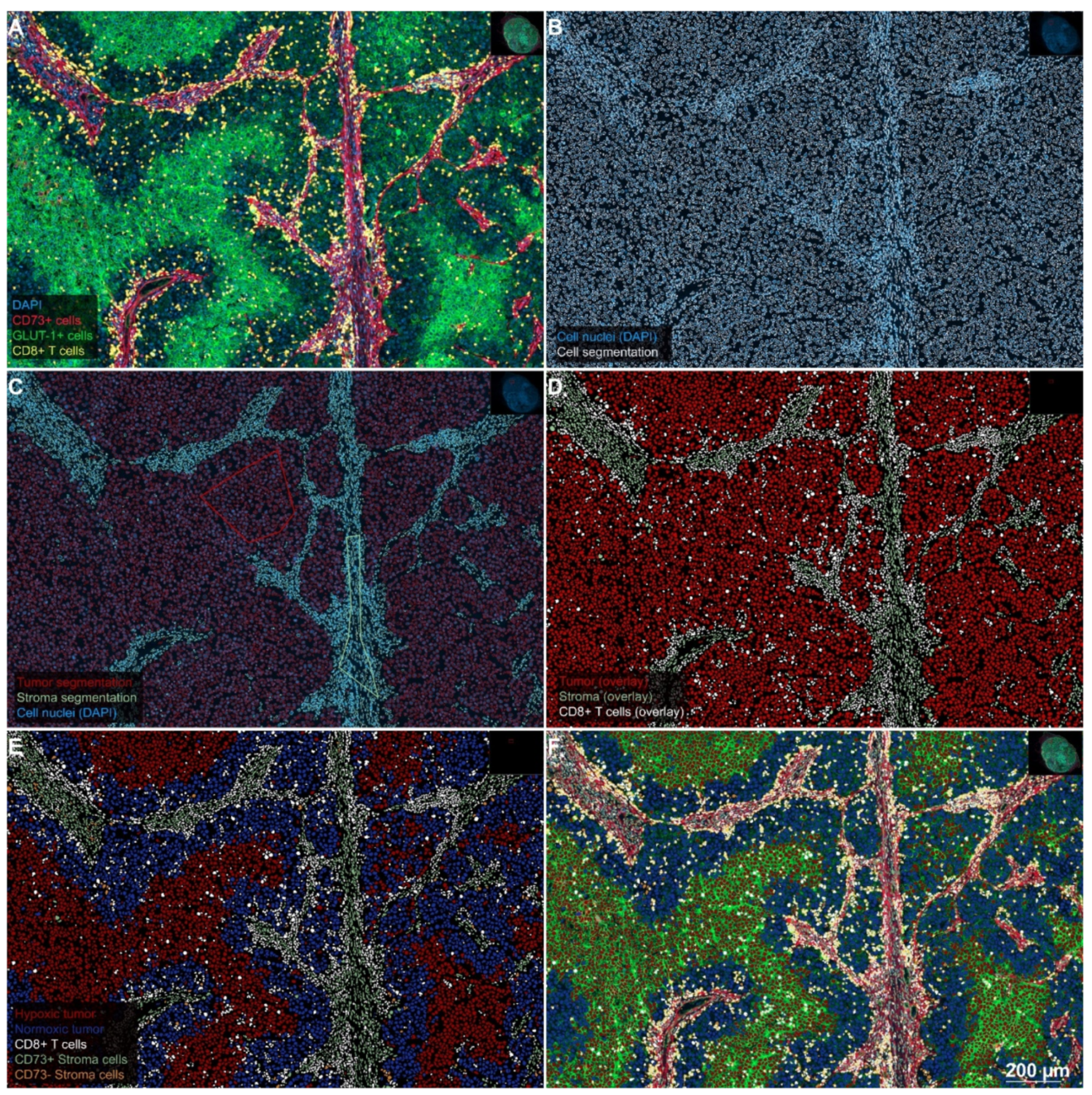
2.1. Separating Tumor, Stroma, and CTL in Preparation for the Analysis
2.2. The Occurrence of Hypoxic Areas in the Examined Tumor Sections
2.3. Expression of the ADO-Generating Enzymes CD73 and CD39 and Associated Findings
2.4. Density and Distribution of CTL
2.5. Hypoxia-Induced Immune Evasion in Spatial and Statistical Analysis
2.6. Prognostic Significance of the Investigated Markers
3. Discussion
4. Patients and Methods
4.1. Patient Cohort
4.2. Immunofluorescence Staining
4.3. Quantitative Analyses
4.4. Spatial Analyses
4.5. Statistical Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Consent for Publications
Abbreviations
| A2AR | A2A-receptor |
| ADO | Extracellular adenosine |
| CA-IX | Carbonic anhydrase IX |
| CCR | CC chemokine receptors |
| CNS | Central nervous system |
| CTL | Cytotoxic T-lymphocyte |
| CTLA-4 | Cytotoxic-T-lymphocyte protein 4 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GLUT | Glucose transporter |
| H&E | Hematoxylin and eosin |
| HIF | Hypoxia-inducible factor |
| HRP | Horseradish peroxidase |
| ICD | Immunogenic cell death |
| ICI | Immune checkpoint inhibitor |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| IPI | Ipilimumab |
| LAG3 | Lymphocyte-activation gene 3 |
| LN | Lymph node |
| mAb | Monoclonal antibody |
| MBM | Melanoma brain metastases |
| OS | Overall survival |
| PD-1 | Programmed cell death protein 1 |
| ROI | Region of interest |
| RT | Radiotherapy |
| STX | Stereotactic brain radiotherapy |
| TIL | Tumor-infiltrating lymphocytes |
| TME | Tumor microenvironment |
| WBRT | Whole-brain radiotherapy |
References
- Buchsbaum, J.C.; Suh, J.H.; Lee, S.Y.; Chidel, M.A.; Greskovich, J.F.; Barnett, G.H. Survival by radiation therapy oncology group recursive partitioning analysis class and treatment modality in patients with brain metastases from malignant melanoma: A retrospective study. Cancer 2002, 94, 2265–2272. [Google Scholar] [CrossRef]
- Staudt, M.; Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Bamberg, M.; Tatagiba, M.; Brossart, P.; Garbe, C. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br. J. Cancer 2010, 102, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.A.; Liu, P.; McIntyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.J.; Hwu, P.; Bedikian, A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011, 117, 1687–1696. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidberger, H.; Rapp, M.; Ebersberger, A.; Hey-Koch, S.; Loquai, C.; Grabbe, S.; Mayer, A. Long-term survival of patients after ipilimumab and hypofractionated brain radiotherapy for brain metastases of malignant melanoma: Sequence matters. Strahlenther. Onkol. 2018, 194, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J. Clin. Oncol. 2019, 37, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Cortes, A.; Niemierko, A.; Oh, K.S.; Flaherty, K.T.; Lawrence, D.P.; Sullivan, R.J.; Shih, H.A. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: Intracranial progression, survival and toxicity. J. Neurooncol. 2018, 138, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.P.; Yu, J.B.; Flanigan, J.; Sznol, M.; Kluger, H.M.; Chiang, V.L. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012, 117, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.; Tampouri, I.; Eigentler, T.; Keim, U.; Klumpp, B.; Heinrich, V.; Zips, D.; Paulsen, F.; Gepfner-Tuma, I.; Skardelly, M.; et al. Immunotherapy plus surgery/radiosurgery is associated with favorable survival in patients with melanoma brain metastasis. Immunotherapy 2019, 11, 297–309. [Google Scholar] [CrossRef]
- Vosoughi, E.; Lee, J.M.; Miller, J.R.; Nosrati, M.; Minor, D.R.; Abendroth, R.; Lee, J.W.; Andrews, B.T.; Leng, L.Z.; Wu, M.; et al. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer 2018, 18, 490. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, A.; Tucci, M.; Mannavola, F.; Felici, C.; Silvestris, F. The metabolic milieu in melanoma: Role of immune suppression by CD73/adenosine. Tumour Biol. 2019, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, Y.G.; Menk, A.V.; Sander, C.; Rao, U.; Karunamurthy, A.; Bhatia, R.; Zhai, S.; Kirkwood, J.M.; Delgoffe, G.M. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.; Livingstone, E.; Roesch, A.; Schilling, B.; Schadendorf, D. Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. 2017, 43, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, I.; Hellstrom, K.E.; Pierce, G.E.; Yang, J.P. Cellular and humoral immunity to different types of human neoplasms. Nature 1968, 220, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.; Higham, E.; Eisen, H.N.; Wittrup, K.D.; Chen, J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc. Natl. Acad. Sci. USA 2008, 105, 13003–13008. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Tilden, A.B.; Balch, C.M. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986, 46, 3011–3017. [Google Scholar]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia-Driven Adenosine Accumulation: A Crucial Microenvironmental Factor Promoting Tumor Progression. Adv. Exp. Med. Biol. 2016, 876, 177–183. [Google Scholar] [CrossRef]
- Li, Y.; Patel, S.P.; Roszik, J.; Qin, Y. Hypoxia-Driven Immunosuppressive Metabolites in the Tumor Microenvironment: New Approaches for Combinational Immunotherapy. Front. Immunol. 2018, 9, 1591. [Google Scholar] [CrossRef] [Green Version]
- Lartigau, E.; Randrianarivelo, H.; Avril, M.F.; Margulis, A.; Spatz, A.; Eschwege, F.; Guichard, M. Intratumoral oxygen tension in metastatic melanoma. Melanoma Res. 1997, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayan, D.; Young, A.; Teng, M.W.L.; Smyth, M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 2017, 17, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Morandi, B.; Horenstein, A.L.; Chillemi, A.; Quarona, V.; Zaccarello, G.; Carrega, P.; Ferlazzo, G.; Mingari, M.C.; Moretta, L.; et al. A non-canonical adenosinergic pathway led by CD38 in human melanoma cells induces suppression of T cell proliferation. Oncotarget 2015, 6, 25602–25618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umansky, V.; Shevchenko, I.; Bazhin, A.V.; Utikal, J. Extracellular adenosine metabolism in immune cells in melanoma. Cancer Immunol. Immunother. 2014, 63, 1073–1080. [Google Scholar] [CrossRef]
- Allard, B.; Beavis, P.A.; Darcy, P.K.; Stagg, J. Immunosuppressive activities of adenosine in cancer. Curr. Opin. Pharmacol. 2016, 29, 7–16. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, I.; Vigano, S.; Faouzi, M.; Treilleux, I.; Michielin, O.; Menetrier-Caux, C.; Caux, C.; Romero, P.; de Leval, L. CD73 expression and clinical significance in human metastatic melanoma. Oncotarget 2018, 9, 26659–26669. [Google Scholar] [CrossRef] [Green Version]
- Bastid, J.; Regairaz, A.; Bonnefoy, N.; Dejou, C.; Giustiniani, J.; Laheurte, C.; Cochaud, S.; Laprevotte, E.; Funck-Brentano, E.; Hemon, P.; et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol. Res. 2015, 3, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Investig. 2002, 110, 993–1002. [Google Scholar] [CrossRef]
- Ladanyi, A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigment Cell Melanoma Res. 2015, 28, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Kaanders, J.H.; Wijffels, K.I.; Marres, H.A.; Ljungkvist, A.S.; Pop, L.A.; van den Hoogen, F.J.; de Wilde, P.C.; Bussink, J.; Raleigh, J.A.; van der Kogel, A.J. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002, 62, 7066–7074. [Google Scholar] [PubMed]
- Lyng, H.; Sundfor, K.; Rofstad, E.K. Oxygen tension in human tumours measured with polarographic needle electrodes and its relationship to vascular density, necrosis and hypoxia. Radiother. Oncol. 1997, 44, 163–169. [Google Scholar] [CrossRef]
- Vaupel, P.; Hockel, M.; Mayer, A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Signal. 2007, 9, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Gaustad, J.V.; Simonsen, T.G.; Leinaas, M.N.; Rofstad, E.K. Sunitinib treatment does not improve blood supply but induces hypoxia in human melanoma xenografts. BMC Cancer 2012, 12, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihic-Probst, D.; Ikenberg, K.; Tinguely, M.; Schraml, P.; Behnke, S.; Seifert, B.; Civenni, G.; Sommer, L.; Moch, H.; Dummer, R. Tumor cell plasticity and angiogenesis in human melanomas. PLoS ONE 2012, 7, e33571. [Google Scholar] [CrossRef] [Green Version]
- Dura, M.; Nemejcova, K.; Jaksa, R.; Bartu, M.; Kodet, O.; Ticha, I.; Michalkova, R.; Dundr, P. Expression of Glut-1 in Malignant Melanoma and Melanocytic Nevi: An Immunohistochemical Study of 400 Cases. Pathol. Oncol. Res. 2019, 25, 361–368. [Google Scholar] [CrossRef]
- Mayer, A.; Hockel, M.; Wree, A.; Leo, C.; Horn, L.C.; Vaupel, P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008, 68, 4719–4726. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Lang, S.A.; Wild, P.J.; Gantner, S.; Mahli, A.; Spanier, G.; Berneburg, M.; Muller, M.; Bosserhoff, A.K.; Hellerbrand, C. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget 2015, 6, 32748–32760. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.M.; Menezes, A.N.; Ibrahim, M.; Archer, L.; Lal, N.; Bagnall, C.J.; von Zeidler, S.V.; Valentine, H.R.; Spruce, R.J.; Batis, N.; et al. Development and Validation of a Combined Hypoxia and Immune Prognostic Classifier for Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 5315–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, K.; Kastenberger, M.; Gottfried, E.; Hammerschmied, C.G.; Buttner, M.; Aigner, M.; Seliger, B.; Walter, B.; Schlosser, H.; Hartmann, A.; et al. Warburg phenotype in renal cell carcinoma: High expression of glucose-transporter 1 (GLUT-1) correlates with low CD8(+) T-cell infiltration in the tumor. Int. J. Cancer 2011, 128, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015, 7, 277ra230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilla, L.; Alberti, A.; Di Mauro, P.; Gemelli, M.; Cogliati, V.; Cazzaniga, M.E.; Bidoli, P.; Maccalli, C. Molecular and Immune Biomarkers for Cutaneous Melanoma: Current Status and Future Prospects. Cancers 2020, 12, 3456. [Google Scholar] [CrossRef]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef]
- Maccalli, C.; Giannarelli, D.; Capocefalo, F.; Pilla, L.; Fonsatti, E.; Di Giacomo, A.M.; Parmiani, G.; Maio, M. Immunological markers and clinical outcome of advanced melanoma patients receiving ipilimumab plus fotemustine in the NIBIT-M1 study. Oncoimmunology 2016, 5, e1071007. [Google Scholar] [CrossRef] [Green Version]
- Valpione, S.; Galvani, E.; Tweedy, J.; Mundra, P.A.; Banyard, A.; Middlehurst, P.; Barry, J.; Mills, S.; Salih, Z.; Weightman, J.; et al. Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat. Cancer 2020, 1, 210–221. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Mayer, A.; Steimel, M.; Wree, A.; Kelleher, D.; Vaupel, P. Solid tumours arising from differently pre-oxygenated cells: Comparable growth rates despite dissimilar tissue oxygenation. Int. J. Radiat. Biol. 2009, 85, 981–988. [Google Scholar] [CrossRef]
- Inoue, Y.; Yoshimura, K.; Kurabe, N.; Kahyo, T.; Kawase, A.; Tanahashi, M.; Ogawa, H.; Inui, N.; Funai, K.; Shinmura, K.; et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 2017, 8, 8738–8751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordass, T.; Osen, W.; Eichmuller, S.B. Controlling the Immune Suppressor: Transcription Factors and MicroRNAs Regulating CD73/NT5E. Front. Immunol. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.F. Therapeutic potential of adenosine kinase inhibition-Revisited. Pharmacol. Res. Perspect. 2019, 7, e00506. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.; Zahnreich, S.; Brieger, J.; Vaupel, P.; Schmidberger, H. Downregulation of EGFR in hypoxic, diffusion-limited areas of squamous cell carcinomas of the head and neck. Br. J. Cancer 2016, 115, 1351–1358. [Google Scholar] [CrossRef] [Green Version]
- Toth, Z.E.; Mezey, E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J. Histochem. Cytochem. 2007, 55, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [Green Version]
- Gonciarz, K.; Bankhead, P.; Otto, M.; Schmidberger, H.; Sbalzarini, I.F.; Mayer, A. Spatial interaction analysis in QuPath using MOSAICsuite. In Proceedings of the Jahrestagung der GBS, Frankfurt, Germany, 17–19 September 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]



| Clinicopathological Features | N (%) |
|---|---|
| Median age at initiation of RT (range) | 59 (29–83) |
| Gender | |
| Female | 10/33 (30.4%) |
| Male | 23/33 (69.6%) |
| Primary tumor | |
| Melanoma type | |
| Cutaneous | 28/33 (84.8%) |
| Ocular | 1/33 (3.0%) |
| Mucosal | 2/33 (6.1%) |
| Unknown primary site | 2/33 (6.1%) |
| Breslow depth 1 (median) | 2.2 mm |
| Clark-level 1 (median) | IV |
| BRAF mutated | 14/33 (42.4%) |
| Metastatic lesion | |
| Biopsy sites (N = 69) | |
| Lymph node (LN) | 24/69 (34.8%) |
| Skin and subcutaneous tissue | 29/69 (42.0%) |
| Lung | 3/69 (4.3%) |
| Central nervous system (CNS) | 6/69 (8.7%) |
| Liver | 1 (1.4%) |
| Gastrointestinal tract | 3 (4.3%) |
| Other (Bone, Palate mucosae, myocard) | 3 (4.3%) |
| Time between histological resection and IPI initiation (median) | 8 months |
| RPA class (median) | 2 |
| Quantitative data from immunofluorescence analysis | |
| % CD8+ CTL median (range) | 4 (1–37) |
| % GLUT-1high tumor cells median (range) | 42 (17–82) |
| % GLUT-1low tumor cells median (range) | 20 (2–58) |
| % CD73+ tumor cells median (range) | 7 (0–72) |
| Treatments | |
| Previous treatments | |
| Treatment-naïve | 3/33 (9.1%) |
| Previously treated | 30/33 (90.9%) |
| Treatment received | |
| Chemotherapy | 10/33 (30.3%) |
| Radiotherapy | |
| 14/33 (42.4%) 12/33 (36.3%) 7/33 (21.2%) |
| Ipilimumab | 33/33 (100%) |
| Anti-PD1/anti-PD-L1 inhibitors | 5/33 (15.1%) |
| BRAF ± MEK inhibitors | 8/33 (24.2%) |
| IPI prior to RT | 18/33 (54.5%) |
| Time between IPI initiation and RT (median) | 1 month |
| Follow-up | |
| Median survival upon RT (range) | 6 months (0–73 months) |
| Deceased | 29/33 (87.8%) |
| Antigen | Primary AB (Cat.-No., Clone, Species, Dilution, Incubation) | Primary AB Supplier | Fluorochrome (Cat.-No.) | Fluorochrome Supplier | Staining Pattern |
|---|---|---|---|---|---|
| CD73/NT5E | 13160, D7F9A, rabbit (mono), 1:50, for 1 h at 28−30 °C | Cell Signaling Technology, Danvers, MA (USA) | TSA Plus Cyanine 5 (NEL745001KT) | PerkinElmer, Waltham, MA, USA | See main text |
| GLUT-1 | GTX62480, EPR3915, rabbit (mono), 1:500, overnight at 4 °C | GeneTex, Irvine, CA (USA) | Alexa Fluor 488 Tyramide (B40953) | Thermo Fisher Scientific, Waltham, MA, USA | Membranous |
| CD8 | M7103, CD8/144B, mouse (mono), 1:50, for 1 h at 28−30°C | DAKO, Glostrup, Denmark A/S (DK) | TSA Plus Cyanine 3 (NEL744001KT) | PerkinElmer, Waltham, MA, USA | Membranous |
| Na+-K+- ATPase | ab76020, EP1845Y, rabbit (mono), 1:100, for 1 h at 28–30 °C | Abcam, Cambridge (UK) | Alexa Fluor 647 Tyramide (T20951) | Thermo Fisher Scientific, Waltham, MA, USA | Membranous |
| CD34 | ab81289, EPR373Y, rabbit (mono), 1:100, overnight at 4 °C | Abcam, Cambridge (UK) | Alexa Fluor 488 Tyramide (B40953) | Thermo Fisher Scientific, Waltham, MA, USA | Membranous (endothelial cells) |
| CD45 | M0701, 2B11+PD7/26, mouse (mono), 1:50, for 1 h at 28−30 °C | DAKO, Glostrup, Denmark A/S (DK) | TSA Plus Cyanine 3 (NEL744001KT) | PerkinElmer, Waltham, MA, USA | Membranous |
| CD39/ENTP1 | NBP2-45447, OTI2B10, mouse (mono), 1:100, for 1 h at 28–30 °C | Novus Biologicals, Littleton, CO (USA) | TSA Plus Cyanine 5 (NEL745001KT) | PerkinElmer, Waltham, MA, USA | See main text |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, A.; Haist, M.; Loquai, C.; Grabbe, S.; Rapp, M.; Roth, W.; Vaupel, P.; Schmidberger, H. Role of Hypoxia and the Adenosine System in Immune Evasion and Prognosis of Patients with Brain Metastases of Melanoma: A Multiplex Whole Slide Immunofluorescence Study. Cancers 2020, 12, 3753. https://doi.org/10.3390/cancers12123753
Mayer A, Haist M, Loquai C, Grabbe S, Rapp M, Roth W, Vaupel P, Schmidberger H. Role of Hypoxia and the Adenosine System in Immune Evasion and Prognosis of Patients with Brain Metastases of Melanoma: A Multiplex Whole Slide Immunofluorescence Study. Cancers. 2020; 12(12):3753. https://doi.org/10.3390/cancers12123753
Chicago/Turabian StyleMayer, Arnulf, Maximilian Haist, Carmen Loquai, Stephan Grabbe, Matthias Rapp, Wilfried Roth, Peter Vaupel, and Heinz Schmidberger. 2020. "Role of Hypoxia and the Adenosine System in Immune Evasion and Prognosis of Patients with Brain Metastases of Melanoma: A Multiplex Whole Slide Immunofluorescence Study" Cancers 12, no. 12: 3753. https://doi.org/10.3390/cancers12123753






