Establishment of a Mouse Ovarian Cancer and Peritoneal Metastasis Model to Study Intraperitoneal Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
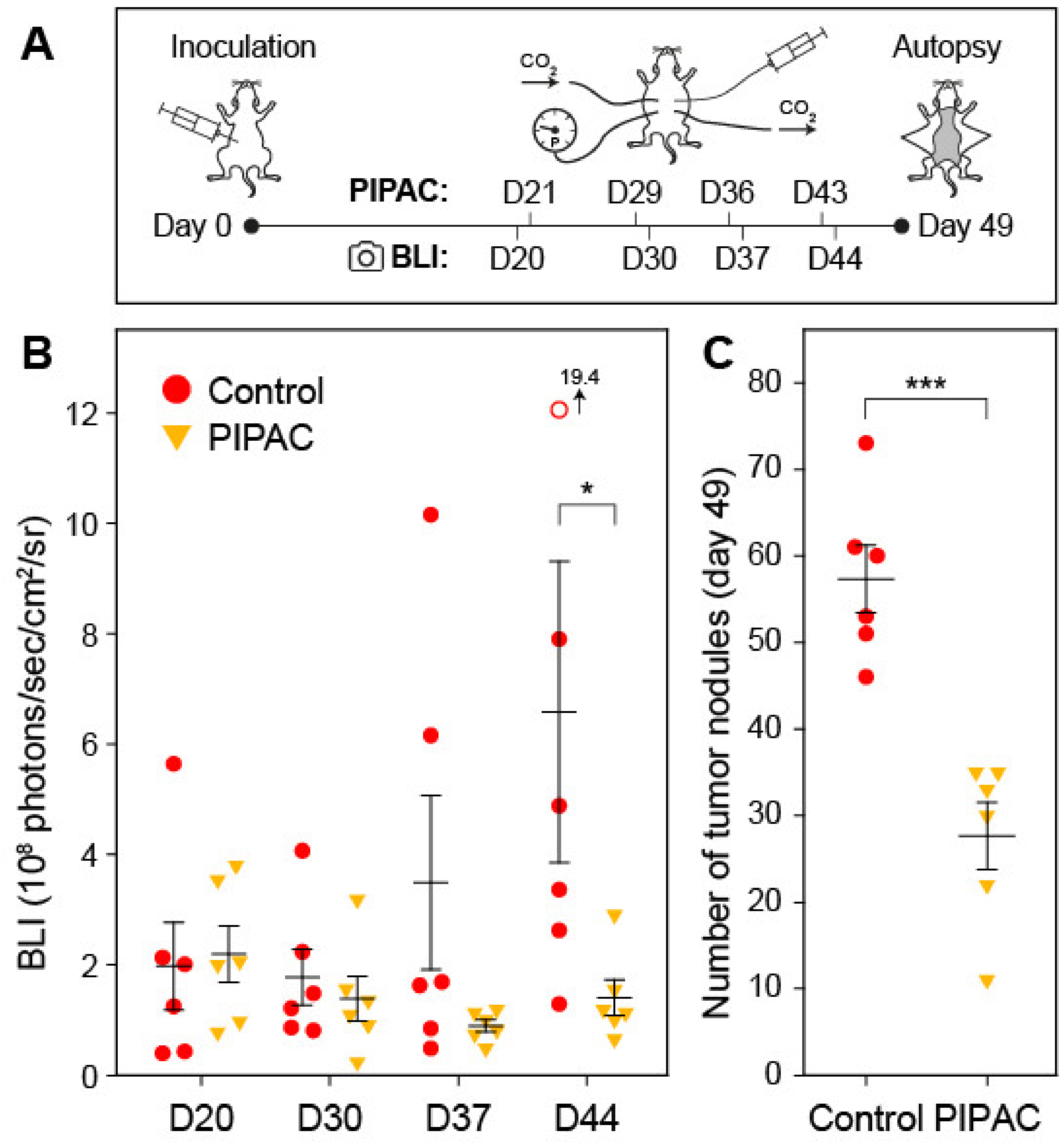
2. Results
2.1. Mouse Ovarian PM Model
2.2. Micro-Nozzle for Aerosolized Delivery into a Capnoperitoneum
2.3. Intraperitoneal Distribution of Doxorubicin
2.4. PIPAC Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ovarian Cancer Peritoneal Metastasis Mouse Model
4.3. Positron Emission Tomography (PET) and Bioluminescence Imaging (BLI)
4.4. Necroscopy and Histology
4.5. Granulometric Analysis
4.6. Characterization of Spatial Drug Distribution in Mice
4.7. PIPAC in Mice
4.8. Data Handling and Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 2019, 19, 984. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A. Real-world evidence in the treatment of ovarian cancer. Ann. Oncol. 2017, 28, viii61–viii65. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Van Baal, J.O.A.M.; van Noorden, C.J.F.; Nieuwland, R.; van de Vijver, K.K.; Sturk, A.; van Driel, W.J.; Kenter, G.G.; Lok, C.A.R. Development of Peritoneal Carcinomatosis in Epithelial Ovarian Cancer: A Review. J. Histochem. Cytochem. 2018, 66, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Archid, R.; Solass, W.; Tempfer, C.; Königsrainer, A.; Adolph, M.; Reymond, M.A.; Wilson, R.B. Cachexia Anorexia Syndrome and Associated Metabolic Dysfunction in Peritoneal Metastasis. Int. J. Mol. Sci. 2019, 20, 5444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugarbaker, P.H. Intraperitoneal delivery of chemotherapeutic agents for the treatment of peritoneal metastases: Current challenges and how to overcome them. Expert Opin. Drug Deliv. 2019, 16, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Walker, J.L. Role of Intraperitoneal Therapy in the Initial Management of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2416–2419. [Google Scholar] [CrossRef]
- Guardiola, E.; Delroeux, D.; Heyd, B.; Combe, M.; Lorgis, V.; Demarchi, M.; Stein, U.; Royer, B.; Chauffert, B.; Pivot, X. Intra-operative intra-peritoneal chemotherapy with cisplatin in patients with peritoneal carcinomatosis of ovarian cancer. World J. Surg. Oncol. 2009, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Cianci, S.; Riemma, G.; Ronsini, C.; de Franciscis, P.; Torella, M.; Schiattarella, A.; La Verde, M.; Colacurci, N. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer recurrence: Systematic review and meta-analysis. Gland Surg. 2020, 9, 1140–1148. [Google Scholar] [CrossRef]
- Huo, Y.R.; Richards, A.; Liauw, W.; Morris, D.L. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2015, 41, 1578–1589. [Google Scholar] [CrossRef]
- Tempfer, C.; Giger-Pabst, U.; Hilal, Z.; Dogan, A.; Rezniczek, G.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: Systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch. Gynecol. Obstet. 2018, 298, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Van de Sande, L.; Willaert, W.; Cosyns, S.; de Clercq, K.; Shariati, M.; Remaut, K.; Ceelen, W. Establishment of a rat ovarian peritoneal metastasis model to study pressurized intraperitoneal aerosol chemotherapy (PIPAC). BMC Cancer 2019, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M.; Lollo, G.; Matha, K.; Descamps, B.; Vanhove, C.; van de Sande, L.; Willaert, W.; Balcaen, L.; Vanhaecke, F.; Benoit, J.-P.; et al. Synergy between Intraperitoneal Aerosolization (PIPAC) and Cancer Nanomedicine: Cisplatin-Loaded Polyarginine-Hyaluronic Acid Nanocarriers Efficiently Eradicate Peritoneal Metastasis of Advanced Human Ovarian Cancer. ACS Appl. Mater. Interfaces 2020, 12, 29024–29036. [Google Scholar] [CrossRef] [PubMed]
- Century, T.J. A New Intrapulmonary Aerosol Delivery Device. In Respiratory Drug Delivery VII: Biological, Pharmaceutical, Clinical and Regulatory Issues Relating to Optimized Drug Delivery by Aeroso; Serentec Press: Raleigh, NC, USA, 2000; pp. 459–462. ISBN 9781930114166. [Google Scholar]
- Göhler, D.; Khosrawipour, V.; Khosrawipour, T.; Diaz-Carballo, D.; Falkenstein, T.A.; Zieren, J.; Stintz, M.; Giger-Pabst, U. Technical description of the microinjection pump (MIP®) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg. Endosc. 2017, 31, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- ISO 9276-1:1998 Representation of results of particle size analysis—Part 1: Graphical representation. Available online: https://www.iso.org (accessed on 15 December 2020).
- Curwen, J.O.; Wedge, S.R. The use and refinement of rodent models in anti-cancer drug discovery: A review. Altern. Lab. Anim. 2009, 37, 173–180. [Google Scholar] [CrossRef]
- Maillet, A.; Guilleminault, L.; Lemarié, E.; Lerondel, S.; Azzopardi, N.; Montharu, J.; Congy-Jolivet, N.; Reverdiau, P.; Legrain, B.; Parent, C.; et al. The airways, a novel route for delivering monoclonal antibodies to treat lung tumors. Pharm. Res. 2011, 28, 2147–2156. [Google Scholar] [CrossRef]
- Solaß, W.; Hetzel, A.; Nadiradze, G.; Sagynaliev, E.; Reymond, M.A. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg. Endosc. 2012, 26, 1849–1855. [Google Scholar] [CrossRef]
- Bellendorf, A.; Khosrawipour, V.; Khosrawipour, T.; Siebigteroth, S.; Cohnen, J.; Diaz-Carballo, D.; Bockisch, A.; Zieren, J.; Giger-Pabst, U. Scintigraphic peritoneography reveals a non-uniform 99mTc-Pertechnetat aerosol distribution pattern for Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) in a swine model. Surg. Endosc. 2018, 32, 166–174. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Lerondel, S.; Sobilo, J.; Launay, A.; Le Pape, A. (CNRS UPS44, CIPA, PHENOMIN-TAAM, Orléans, France). 2020; Unpublished work. [Google Scholar]
- ISO 13320:2009 Particle size analysis—Laser diffraction methods. Available online: https://www.iso.org (accessed on 15 December 2020).
- Heuer, M.; Leschonski, K. Results Obtained with a New Instrument for the Measurement of particle size distributions from diffraction patterns. Part. Part. Syst. Charact. 1985, 2, 7–13. [Google Scholar] [CrossRef]
- Göhler, D.; Große, S.; Bellendorf, A.; Falkenstein, T.A.; Ouaissi, M.; Zieren, J.; Stintz, M.; Giger-Pabst, U. Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an improved approach for pressurised intraperitoneal aerosol chemotherapy (PIPAC): Technical description, experimental validation and first proof of concept. Beilstein J. Nanotechnol. 2017, 8, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Giger-Pabst, U.; Tempfer, C.B. How to Perform Safe and Technically Optimized Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Experience After a Consecutive Series of 1200 Procedures. J. Gastrointest. Surg. 2018, 22, 2187–2193. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezniczek, G.A.; Buggisch, J.; Sobilo, J.; Launay, A.; Lerondel, S.; Le Pape, A.; Ouaissi, M.; Göhler, D.; Senkal, M.; Giger-Pabst, U.; et al. Establishment of a Mouse Ovarian Cancer and Peritoneal Metastasis Model to Study Intraperitoneal Chemotherapy. Cancers 2020, 12, 3818. https://doi.org/10.3390/cancers12123818
Rezniczek GA, Buggisch J, Sobilo J, Launay A, Lerondel S, Le Pape A, Ouaissi M, Göhler D, Senkal M, Giger-Pabst U, et al. Establishment of a Mouse Ovarian Cancer and Peritoneal Metastasis Model to Study Intraperitoneal Chemotherapy. Cancers. 2020; 12(12):3818. https://doi.org/10.3390/cancers12123818
Chicago/Turabian StyleRezniczek, Günther A., Jonathan Buggisch, Julien Sobilo, Alexandre Launay, Stéphanie Lerondel, Alain Le Pape, Mehdi Ouaissi, Daniel Göhler, Metin Senkal, Urs Giger-Pabst, and et al. 2020. "Establishment of a Mouse Ovarian Cancer and Peritoneal Metastasis Model to Study Intraperitoneal Chemotherapy" Cancers 12, no. 12: 3818. https://doi.org/10.3390/cancers12123818





