Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage
Abstract
1. Introduction
2. Results
2.1. Clinical and Morphometric Features
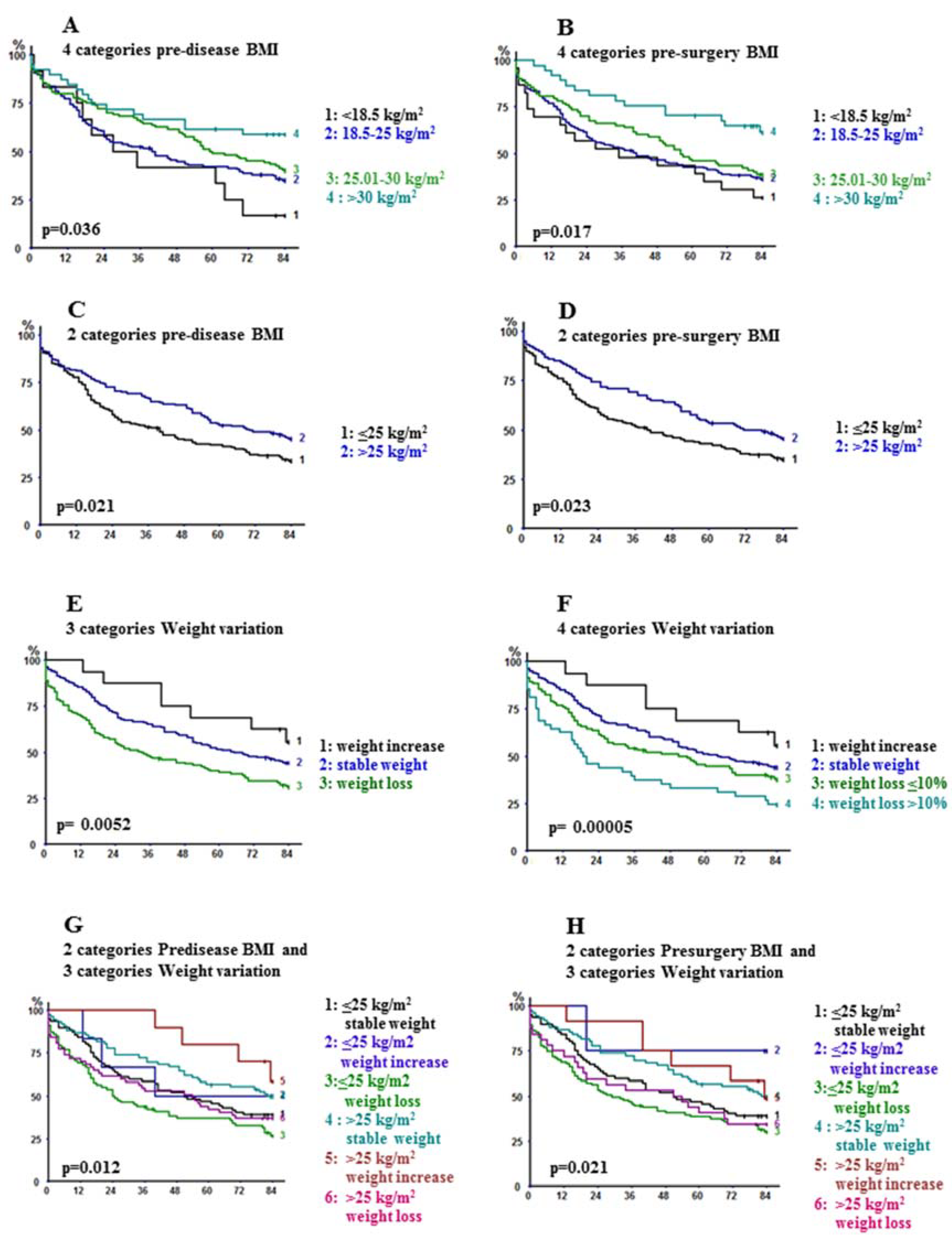
2.2. Long-Term Outcome
2.3. Multivariable Analysis
3. Discussion
3.1. The Obesity Paradox Is a Reality in Resectable NSCLC
3.2. Lower BMI, Weight Loss and Low Muscle Mass Negatively Impact the Long-Term Outcome
3.3. The Difficulties of Standard Assessment of Low Muscle Mass
3.4. Understanding Why Pre-Operative Nutritional Status Impacts on Survival
4. Materials and Methods
4.1. Collected Data and Measurement of Low Muscle Mass
4.2. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Park, Y.; Peterson, L.L.; Colditz, G.A. The Plausibility of Obesity Paradox in Cancer—Point. Cancer Res. 2018, 78, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Majumder, K.; Arora, N.; Mayo, H.G.; Singh, P.P.; Beg, M.S.; Hughes, R.; Singh, S.; Johnson, D.H. Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer 2016, 102, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Huang, J.; Fan, J.; Du, H.; Liu, L.; Che, G. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: Does the ‘obesity paradox’ really exist? Eur. J. Cardio-Thoracic Surg. 2016, 51, 817–828. [Google Scholar] [CrossRef]
- Alifano, M.; Mansuet-Lupo, A.; Lococo, F.; Roche, N.; Bobbio, A.; Canny, E.; Schussler, O.; Dermine, H.; Regnard, J.-F.; Burroni, B.; et al. Systemic Inflammation, Nutritional Status and Tumor Immune Microenvironment Determine Outcome of Resected Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e106914. [Google Scholar] [CrossRef]
- Miller, J.A.; Harris, K.; Roche, C.; Dhillon, S.; Battoo, A.; Demmy, T.; Nwogu, C.E.; Dexter, E.U.; Hennon, M.; Picone, A.; et al. Sarcopenia is a predictor of outcomes after lobectomy. J. Thorac. Dis. 2018, 10, 432–440. [Google Scholar] [CrossRef]
- Ferguson, M.K.; Im, H.K.; Watson, S.; Johnson, E.; Wigfield, C.H.; Vigneswaran, W.T. Association of body mass index and outcomes after major lung resection†. Eur. J. Cardio-Thoracic Surg. 2014, 45, e94–e99. [Google Scholar] [CrossRef]
- Thomas, P.A.; Berbis, J.; Falcoz, P.-E.; Le Pimpec-Barthes, F.; Bernard, A.; Jougon, J.; Porte, H.; Alifano, M.; Dahan, M.; Alauzen, M.; et al. National perioperative outcomes of pulmonary lobectomy for cancer: The influence of nutritional status. Eur. J. Cardio-Thorac Surg. 2014, 45, 652–659. [Google Scholar] [CrossRef]
- Hervochon, R.; Bobbio, A.; Guinet, C.; Mansuet-Lupo, A.; Rabbat, A.; Régnard, J.-F.; Roche, N.; Damotte, D.; Iannelli, A.; Alifano, M. Body Mass Index and Total Psoas Area Affect Outcomes in Patients Undergoing Pneumonectomy for Cancer. Ann. Thorac. Surg. 2017, 103, 287–295. [Google Scholar] [CrossRef]
- Liu, X.; Sepesi, B.; Gold, K.A.; Correa, A.M.; Heymach, J.V.; Vaporciyan, A.A.; Roszik, J.; Dmitrovsky, E. Abstract 5734: The influence of body mass index on overall survival following surgical resection of non-small cell lung cancer. Clin. Trials 2017, 77, 5734. [Google Scholar] [CrossRef]
- Mytelka, D.S.; Li, L.; Benoit, K. Post-diagnosis weight loss as a prognostic factor in non-small cell lung cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Braun, W.; Enderle, J.; Bosy-Westphal, A. Beyond BMI: Conceptual Issues Related to Overweight and Obese Patients. Obes. Facts 2016, 9, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Collins, J.; Noble, S.; Chester, J.; Coles, B.; Byrne, A. The assessment and impact of sarcopenia in lung cancer: A systematic literature review. BMJ Open 2014, 4, e003697. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kazemi-Bajestani, S.M.R.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Boil. 2016, 54, 2–10. [Google Scholar] [CrossRef]
- Al-Gindan, Y.Y.; Hankey, C.R.; Leslie, W.; Govan, L.; Lean, M.E. Predicting muscle mass from anthropometry using magnetic resonance imaging as reference: A systematic review. Nutr. Rev. 2014, 72, 113–126. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; McManus, C.; Smith, J.; Stevens, V.; Nixon, D.W. Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. Am. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef]
- De Koning, F.; Binkhorst, R.; Kauer, J.; Thijssen, H. Accuracy of an Anthropometric Estimate of the Muscle and Bone Area in a Transversal Cross-Section of the Arm. Int. J. Sports Med. 1986, 7, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Nau, K.L.; Dick, A.R.; Peters, K.; Schloerb, P.R. Relative validity of clinical techniques for measuring the body composition of persons with amyotrophic lateral sclerosis. J. Neurol. Sci. 1997, 152, s36–s42. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Boisseau, N.; Moraine, J.-J.; Moreno-Reyes, R.; Goldman, S. Estimation of total-body skeletal muscle mass in children and adolescents. Med. Sci. Sports Exerc. 2005, 37, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Spenst, L.F.; Drinkwater, D.T.; Clarys, J.P. Anthropometric estimation of muscle mass in men. Med. Sci. Sports Exerc. 1990, 22, 729–733. [Google Scholar] [CrossRef]
- Baracos, V.; Reiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body composition in patients with non−small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010, 91, 1133S–1137S. [Google Scholar] [CrossRef]
- Morel, H.; Raynard, B.; D’Arlhac, M.; Hauss, P.-A.; Lecuyer, E.; Oliviero, G.; Marty, C.; Gury, J.-P.; Asselain, B.; Grivaux, M.; et al. Prediagnosis weight loss, a stronger factor than BMI, to predict survival in patients with lung cancer. Lung Cancer 2018, 126, 55–63. [Google Scholar] [CrossRef]
- Dewi, N.U.; Boshuizen, H.C.; Johansson, M.; Vineis, P.; Kampman, E.; Steffen, A.; Tjønneland, A.; Halkjær, J.; Overvad, K.; Severi, G.; et al. Anthropometry and the Risk of Lung Cancer in EPIC. Am. J. Epidemiol. 2016, 184, 129–139. [Google Scholar] [CrossRef]
- Godschalk, R.W.L.; Feldker, D.E.M.; A Borm, P.J.; Wouters, E.F.M.; Van Schooten, F.-J. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 790–793. [Google Scholar]
- Nakagawa, T.; Toyazaki, T.; Chiba, N.; Ueda, Y.; Gotoh, M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 560–566. [Google Scholar] [CrossRef]
- Nakamura, R.; Inage, Y.; Tobita, R.; Yoneyama, S.; Numata, T.; Ota, K.; Yanai, H.; Endo, T.; Inadome, Y.; Sakashita, S.; et al. Sarcopenia in Resected NSCLC: Effect on Postoperative Outcomes. J. Thorac. Oncol. 2018, 13, 895–903. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Takamori, S.; Toyokawa, G.; Okamoto, T.; Shimokawa, M.; Kinoshita, F.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takada, K.; et al. Clinical Impact and Risk Factors for Skeletal Muscle Loss After Complete Resection of Early Non-small Cell Lung Cancer. Ann. Surg. Oncol. 2018, 25, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Amini, B.; Boyle, S.P.; Boutin, R.D.; Lenchik, L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography (CT): A Systematic Review. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1671–1678. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fieding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. The authors reply: Letter on: “Pitfalls in the measurement of muscle mass: A need for a reference standard” by Clark et al. J. Cachexia Sarcopenia Muscle 2018, 9, 1272–1274. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; López-Soriano, F.J. Cancer cachexia, a clinical challenge. Curr. Opin. Oncol. 2019, 31, 286–290. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.-L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Cahill, G. Starvation in man. Clin. Endocrinol. Metab. 1976, 5, 397–415. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.-M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updates 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Cruz-Bermúdez, A.; Vicente-Blanco, R.J.; Laza-Briviesca, R.; García-Grande, A.; Laine-Menéndez, S.; Gutiérrez, L.; Calvo, V.; Romero, A.; Martín-Acosta, P.; García, J.M.; et al. PGC-1alpha levels correlate with survival in patients with stage III NSCLC and may define a new biomarker to metabolism-targeted therapy. Sci. Rep. 2017, 7, 16661. [Google Scholar] [CrossRef] [PubMed]
- Riester, M.; Xu, Q.; Moreira, A.; Zheng, J.; Michor, F.; Downey, R. The Warburg effect: Persistence of stem-cell metabolism in cancers as a failure of differentiation. Ann. Oncol. 2018, 29, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Karp, J.E.; Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017, 19, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; Van Der Windt, G.J.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Icard, P.; Iannelli, A.; Lincet, H.; Alifano, M. Sarcopenia in resected non-small cell lung cancer: Let’s move to patient-directed strategies. J. Thorac. Dis. 2018, 10, S3138–S3142. [Google Scholar] [CrossRef]
- Dong, M.; Lin, J.; Lim, W.; Jin, W.; Lee, H.J. Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front. Med. 2018, 12, 130–138. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Schussler, O.; Alifano, M.; Dermine, H.; Strano, S.; Casetta, A.; Sepúlveda, S.; Chafik, A.; Coignard, S.; Rabbat, A.; Regnard, J.-F. Postoperative Pneumonia after Major Lung Resection. Am. J. Respir. Crit. Care Med. 2006, 173, 1161–1169. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Pamoukdjian, F.; Bouillet, T.; Lévy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef]
- Lee, R.C.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef]
| Variable | N (%) or Mean ± SD or Median [IQR] |
|---|---|
| Men | 254 (83.5%) |
| Age (range) | 63 yrs (54–70 yrs) |
| Smoking | |
| Current and former smoking | 286 (94.1%) |
| Smoking cessation for at least 2 months | 177 (58.1%) |
| Cumulative smoking: Pack/Year index | 40 (30–60) |
| Comorbid illnesses and Respiratory status | |
| Alcohol abuse | 82 (26.3%) |
| Diabetes mellitus | 41 (13.5%) |
| Ischaemic heart disease | 43 (14.1%) |
| Stroke | 16 (5.3%) |
| Lower limb atheroma | 60 (18%) |
| Chronic bronchitis | 198 (65.1%) |
| COPD | 127 (41.8%) |
| FEV1 (% predicted) | 80 (70–93.5) |
| FEV1/FVC (%) | 72 (65–79) |
| ASA I–II/III/IV | 6 (2%)/192 (63%)/103 (34%)/3 (1.0%) |
| Surgical procedures | |
| Lobectomy/bilobectomy | 233 (76.6%) |
| Pneumonectomy | 71 (23.4%) |
| Histological type | |
| Adenocarcinoma | 131 (43%) |
| Non-adenocarcinoma | 173 (57%) |
| Pathological stage and Tumor characteristics | |
| I | 113 (37.2%) |
| II | 87 (28.6%) |
| III | 96 (31.6%) |
| IV | 8 (2.7%) |
| Mean tumoral diameter | 35 (25–50) |
| Vascular and lymphatic emboli | 193 (63.5%) |
| Pleural invasion | 160 (52.5%) |
| Morphomics and nutrional parameter | |
| Height | 170 cm (165–175) |
| Pre-disease weight | 72.5 Kg (63–82) |
| Pre-surgery weight | 70 Kg (60.3–80) |
| % body weight variation | 0% (0–6.75%) |
| stable body weight (n = 153) | 0% |
| increase body weight (n = 16) | 4% (2.25–8.75%) |
| decrease body weight (n = 135) | 7% (4–12%) |
| Pre-disease body mass index (BMI) | 24.9 Kg/m2 (22.5–27.3%) |
| Pre-surgery body mass index (BMI) | 24.3 Kg/m2 (21.4–26.8%) |
| Mid-arm circumference (MAC) | 26.9 cm +3.17 |
| Tricipital skin-fold thickness (TSF) | 11 mm (8–16) |
| Mid-arm muscular perimeter (MAMP) | 23.1 cm + 3.2 |
| Mid-arm muscular area (MAMA) | 43.2 cm2 + 11.4 |
| Sex-corrected MAMA (scMAMA) | 33.75 cm2 + 10.8 |
| Total muscular mass (TMM) | 21.2 Kg ± 5.8 |
| Indexed (on height) total muscular mass (iTMM) | 7.3 Kg/m2 ± 1.7 |
| (n = 147) | 7.21 median (6–8.4); 6.49 (33rd percentile) |
| Variable | Pre-Disease BMI ≤25 kg/m2 | Pre-Disease BMI >25 kg/m2 | p | Weight Increase | Stable Weight | Weight Decrease | p | Pre-Surgery BMI ≤25 kg/m2 | Pre-Surgery BMI >25 kg/m2 | p | ITTM ≤6.49 kg/m2 | ITTM >6.49 kg/m2 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | 75.8 | 92.3 | 0.0001 | 75 | 88.8 | 78.5 | 0.038 | 77.7 | 22.5 | 0.00068 | 68 | 90.5 | 0.0008 |
| Age (years) | 59.8 ± 11.0 | 64.4 ± 9.5 | 0.0003 | 60.5 (50–66) | 62 (53–69) | 63 (55–71) | 0.14 | 61.0 ± 11.0 | 63.5 ± 9.5 | 0.069 | 59.4 ± 10.8 | 63.1 ± 9.8 | 0.052 |
| Smokers | 91.9 | 96.5 | 0.09 | 100 | 95.4 | 91.8 | 0.5 | 92.9 | 95.8 | 0.30 | 93.6 | 94.7 | 0.91 |
| Pack/year | 40 (30–59) | 40 (30–60) | 0.75 | 50 (30–67) | 40 (30–50) | 40 (30–60) | 0.6 | 40 (30–60) | 40 (30–60) | 0.82 | 40 (30–60) | 43.5 (35–60) | 0.55 |
| Alcool abuse | 30.4 | 23.1 | 0.15 | 37.5 | 24.8 | 28.1 | 0.63 | 28.3 | 25 | 0.53 | 29.8 | 22.1 | 0.32 |
| Diabetes | 11 | 16.3 | 0.19 | 18.7 | 11.9 | 14.7 | 0.96 | 14.3 | 12.4 | 0.65 | 8.9 | 18.1 | 0.16 |
| Ischemic heart disease | 10.1 | 18.4 | 0.09 | 13.3 | 18.5 | 94.7 | 1.0 | 19.0 | 20.9 | 0.72 | 8.7 | 12.9 | 0.47 |
| Stroke | 2.7 | 8.2 | 0.08 | 0 | 51.5 | 52.6 | 0.81 | 4.0 | 7.4 | 0.45 | 0 | 7.1 | 0.16 |
| Lower limb atheroma | 17.9 | 21.9 | 0.45 | 6.6 | 22.4 | 18.9 | 0.42 | 19.0 | 20.9 | 0.72 | 8.7 | 12.9 | 0.47 |
| Chronic bronchitis | 65.8 | 64.3 | 0.78 | 56.2 | 65.3 | 65.9 | 0.44 | 65.0 | 65.2 | 0.97 | 57.4 | 62.1 | 0.59 |
| COPD | 41.3 | 42.4 | 0.98 | 25 | 58.3 | 44.9 | 0.28 | 42.0 | 41.5 | 0.96 | 40 | 40.2 | 0.78 |
| FEV1 (% predicted) | 81.1 ± 18.1 | 88.7 ± 19.0 | 0.79 | 82.2 ± 23.2 | 80.7 ± 18.5 | 82.0 ± 18.3 | 0.97 | 81.2 ± 17.7 | 81.6 ± 20.0 | 0.83 | 78.2 ± 16.6 | 82.1 ± 21.7 | 0.25 |
| ASA class I | 2.5 | 1.4 | 0.7 | 0 | 2.6 | 1.5 | 0.68 | 2.2 | 1.7 | 0.8 | 0 | 3.2 | 0.18 |
| ASA class II | 64.6 | 60.8 | 75 | 63.6 | 60.4 | 63 | 62.5 | 76.6 | 60 | ||||
| ASA class III | 32.3 | 36.4 | 42.5 | 33.1 | 36.6 | 34.2 | 34.2 | 23.4 | 35.5 | ||||
| ASA class IV | 0.6 | 1.4 | 0 | 0.7 | 1.5 | 0.5 | 1.7 | 0 | 1 | ||||
| Lobectomy and bilobectomy | 79.4 | 73.4 | 0.22 | 87.5 | 81.6 | 69.6 | 0.23 | 75.4 | 78.3 | 0.56 | 76.1 | 74.8 | 0.86 |
| Squamous Non-Squamous | 58.8 | 52.5 | 0.045 | 46.6 | 55.4 | 46.4 | 0.38 | 56.9 | 53.4 | 0.018 | 56.8 | 54.3 | 0.3 |
| Mean tumor diameter (mm) | 40 (25–53) | 35 (25–50) | 0.82 | 37.0 ± 11.5 | 37.7 ± 18.1 | 44.1 ± 21.9 | 0.20 | 40 (26–55) | 35 (25–50) | 0.30 | 35 (25–51) | 35 (25–50) | 0.96 |
| Pathologic stage I | 31.4 | 43.7 | 0.063 | 37.5 | 44.4 | 29.1 | 0.07 | 46.2 | 31.2 | 0.039 | 30.4 | 38.9 | 0.058 |
| Pathologic stage II | 33.3 | 23.2 | 12.5 | 25.2 | 34.3 | 21 | 33.5 | 34.8 | 25.2 | ||||
| Pathologic stage III | 31.4 | 31.7 | 37.5 | 28.5 | 34.3 | 30.2 | 32.4 | 26.1 | 34.7 | ||||
| Pathologic stage IV | 3.8 | 1.4 | 12.5 | 2 | 2.2 | 2.5 | 2.7 | 8.7 | 1 | ||||
| Emboli | 66.9 | 59.8 | 0.22 | 73.3 | 63.6 | 62.3 | 0.4 | 65.1 | 61.3 | 0.52 | 63.4 | 64.4 | 0.91 |
| Pleural invasion | 59.3 | 45 | 0.014 | 40 | 48.9 | 58.0 | 0.18 | 60.1 | 41.0 | 0.0013 | 53.3 | 50 | 0.71 |
| Variable | 5-Year Survival Rate (95% CI) | 7-Year Survival Rate (95% CI) | p Value |
|---|---|---|---|
| Sex | |||
| Men | 45.4 (39.4–55.5) | 37.7 (31.9–43.8) | |
| Women | 51.9 (38.7–64.9) | 42.3 (29.9–55.8) | 0.056 |
| Age | |||
| ≤65 years | 52.2 (44.9–59.4) | 42.5 (35.4–49.8) | |
| >65 years | 38.7 (30.7–47.2) | 32.9 (25.4–41.5) | 0.017 |
| Smoking | |||
| Never or former smoker | 49.4 (28.3–70.7) | 49.4 (28.3–70.7) | |
| Current Smoker | 46.7 (41.0–52.4) | 38.1 (32.6–43.8) | 0.57 |
| Alcool | |||
| Abuse | 43.9 (33.7–54.7) | 38.6 (28.7–49.5) | |
| No abuse | 47.2 (40.8–53.7) | 38.5 (42.3–45.0) | 0.87 |
| Diabetes mellitus | |||
| Yes | 41.7 (27.4–57.5) | 38.9 (25.0–54.9) | |
| No | 46.5 (40.4–52.7) | 38.9 (33.0–45.2) | 0.92 |
| Angor | |||
| Yes | 44.8 (28.4–62.5) | 41.4 (25.5–59.3) | |
| No | 46.7 (39.6–54.0) | 39.3 (32.5–46.6) | 0.97 |
| Stroke | |||
| Yes | 36.4 (15.2–64.6) | 27.3 (9.7–56.6) | |
| No | 47.0 (40.2–53.9) | 40.3 (33.7–47.2) | 0.17 |
| Lower limb atheroma | |||
| Yes | 41.3 (28.3–55.7) | 37.0 (24.5–51.4) | |
| No | 48.3 (41.2–55.5) | 39.3 (32.5–46.6) | 0.47 |
| Chronic bronchitis | |||
| Yes | 43 (36.0–50.3) | 36.0 (29.3–43.3) | |
| No | 52.2 (43.6–60.7) | 42.4 (34.1–51.2) | 0.15 |
| COPD | |||
| Yes | 43.0 (34.6–51.8) | 34.7 (26.8–43.4) | |
| No | 49.3 (42.0–56.7) | 40.9 (33.7–48.3) | 0.33 |
| FEV1 (% predicted) | |||
| ≥80 | 45.0 (37.4–52.9) | 38.3 (31.0–46.2) | |
| <80 | 47.5 (39.5–55.6) | 38.0 (30.4–46.2) | 0.95 |
| ASA score | |||
| ASA I–II | 51.2 (44.2–58.1) | 42.2 (35.5–49.3) | |
| ASA III/IV | 38.5 (29.8–48.1) | 32.6 (24.3–42.0) | 0.025 |
| Resection type | |||
| obectomy/bilobectomy | 52.8 (46.4–59.1) | 44.9 (38.6–51.3) | |
| Pneumonectomy | 27.1 (18.3–38.2) | 18.4 (11.1–28.9) | 0.0001 |
| Pathological type | |||
| Adenocarcinoma | 50.8 (42.8–58.6) | 41.8 (34.1–49.8) | |
| Non -adenocarcinoma | 44.1 (36.2–52.3) | 37.5 (29.9–45.7) | 0.17 |
| Pathological stage | |||
| I | 68.4 (59.5–76.2) | 55.6 (46.3–64.5) | |
| II | 43.8 (33.9–54.3) | 34.1 (24.9–44.7) | |
| III–IV | 25.5 (18.2–34.6) | 23.5 (16.4–32.4) | <0.0001 |
| Mean tumoral diameter | |||
| <3 cm | 59.1 (49.0–68.6) | 50.1 (40.1–60.2) | |
| ≥3 cm | 41.1 (34.8–47.8) | 33.4 (27.4–40.0) | 0.0011 |
| Vascular/lymphatic emboli | |||
| Yes | 42 (35.0–49.4) | 36.8 (30.1–44.2) | |
| No | 57.1 (47.6–66.2) | 43.4 (34.2–53.0) | 0.06 |
| Pleural invasion | |||
| Yes | 38.0 (30.8–45.7) | 33.4 (26.5–41.1) | |
| No | 57.0 (48.8–64.9) | 44.6 (36.6–52.9) | 0.0012 |
| Variable | 5–Year Survival Rate (95% CI) | 7–Year Survival Rate (95% CI) | p-Value |
|---|---|---|---|
| Pre-surgery BMI (kg/m2) | |||
| <18.5 | 43.5 (25.6–63.2) | 26.1 (12.5–46.5) | 0.017 |
| 18.5–25 | 42.6 (35.2–50.4) | 36.1 (29.0–43.9) | |
| 25.01–30 | 45.8 (35.5–56.4) | 38.5 (28.7–49.2) | |
| >30 | 70.3 (54.2–82.5) | 61.2 (44.8–75.4) | |
| ≤25 | 42.2 (35.3–49.4) | 34.8 (28.3–42.0) | |
| >25 | 53.3 (44.4–62.0) | 45.5 (36.8–54.5) | 0.023 |
| Pre-disease BMI (kg/m2) | |||
| <18.5 | 41.7 (19.3–68.0) | 16.7 (4.7–44.8) | 0.036 |
| 18.5–25 | 42.0 (34.4–50.1) | 34.9 (27.7–43.0) | |
| 25.01–30 | 49.0 (39.6–58.5) | 40.2 (38.3–49.9) | |
| >30 | 61.5 (45.9–75.1) | 58.9 (43.3–72.8) | |
| ≤25 | 42.0 (34.6–49.8) | 33.5 (26.6–41.2) | |
| >25 | 52.4 (44.3–60.5) | 45.2 (37.2–53.4) | 0.021 |
| Weight variation | |||
| Stable | 51.3 (43.5–59.0) | 44.1 (36.5–51.9) | |
| Increase | 68.8 (44.4–85.8) | 55.6 (32.3–76.6) | |
| Decrease | 39.0 (31.2–47.5) | 31.1 (23.8–39.5) | 0.0052 |
| Pre–surgery height normalized total muscular mass (Kg/m2) | |||
| (n = 147) | 50.7 (39.3–62.0) | 36.5 (26.3–48.2) | 0.11 |
| ≤7.21 (median) | 56.0 (44.4–67.0) | 51.6 (40.1–62.9) | |
| >7.21 | |||
| ≤6.49 (33rd percentile) | 48.9 (35.3–62.8) | 31.6 (20.1–46.0) | |
| >6.49 | 55.6 (45.5–65.2) | 50.1 (40.2–60) | 0.042 |
| Variable | Relative Risk | 95% CI | p Value |
|---|---|---|---|
| Model 1 | |||
| Age (years) | |||
| ≤65 | 1 | ||
| >65 | 1.45 | (1.08–1.94) | 0.0014 |
| Resection type | |||
| Lobectomy/bilobectomy | 1 | ||
| Pneumonectomy | 1.77 | (1.27–2.47) | 0.00083 |
| ASA score | |||
| I–II | 1 | ||
| III/IV | 1.56 | (1.15–2.10) | 0.0038 |
| Stage | |||
| I | 1 | ||
| II | 1.50 | (1.25–1.81) | |
| III/IV | 2.26 | (1.56–3.28) | 0.000018 |
| Pre–disease BMI (Kg/m2) | |||
| <25 | 1 | ||
| >25 | 0.66 | (0.49–0.89) | 0.006 |
| Weight variation | |||
| Increase | 1 | ||
| Stable | 1.36 | (1.06–1.75) | |
| Decrease | 1.86 | (1.13–3.06) | 0.014 |
| Model 2 | |||
| Age (years) | |||
| <65 | 1 | ||
| >65 | 1.42 | (1.06–1.89) | 0.019 |
| Resection type | |||
| Lobectomy/bilobectomy | 1 | ||
| Pneumonectomy | 1.75 | (1.26–2.44) | 0.00084 |
| ASA | |||
| I–II | 1 | ||
| III/IV | 1.54 | (1.14–2.07) | 0.0047 |
| Stage | |||
| I | 1 | ||
| II | 1.51 | (1.25–1.81) | |
| III/IV | 2.27 | (21.57–3.29) | 0.000012 |
| Pre-surgery BMI (Kg/m2) | |||
| <25 | 1 | ||
| >25 | 0.72 | (0.54–0.98) | 0.034 |
| Model 3 | |||
| Age (years) | |||
| <65 | |||
| >65 | n.s. | ||
| Resection type | |||
| Lobectomy/bilobectomy | |||
| Pneumonectomy | n.s. | ||
| ASA | |||
| I–II | 1 | ||
| III/IV | 1.72 | (1.10–2.69) | 0.018 |
| Stage | |||
| I | 1 | ||
| II | 1.57 | (1.22–2.03) | |
| III/IV | 2.47 | (1.49–4.11) | 0.00048 |
| Pre–surgery height normalized total muscular mass (Kg/m2) | |||
| ≤6.49 (33rd percentile) | 1 | ||
| >6.49 | 0.56 | (0.37–0.87) | 0.0091 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Icard, P.; Schussler, O.; Loi, M.; Bobbio, A.; Mansuet Lupo, A.; Wislez, M.; Iannelli, A.; Fournel, L.; Damotte, D.; Alifano, M. Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage. Cancers 2020, 12, 266. https://doi.org/10.3390/cancers12020266
Icard P, Schussler O, Loi M, Bobbio A, Mansuet Lupo A, Wislez M, Iannelli A, Fournel L, Damotte D, Alifano M. Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage. Cancers. 2020; 12(2):266. https://doi.org/10.3390/cancers12020266
Chicago/Turabian StyleIcard, Philippe, Olivier Schussler, Mauro Loi, Antonio Bobbio, Audrey Mansuet Lupo, Marie Wislez, Antonio Iannelli, Ludovic Fournel, Diane Damotte, and Marco Alifano. 2020. "Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage" Cancers 12, no. 2: 266. https://doi.org/10.3390/cancers12020266
APA StyleIcard, P., Schussler, O., Loi, M., Bobbio, A., Mansuet Lupo, A., Wislez, M., Iannelli, A., Fournel, L., Damotte, D., & Alifano, M. (2020). Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage. Cancers, 12(2), 266. https://doi.org/10.3390/cancers12020266







