Effects of Germline CYP2W1*6 and CYP2B6*6 Single Nucleotide Polymorphisms on Mitotane Treatment in Adrenocortical Carcinoma: A Multicenter ENSAT Study
Abstract
:1. Introduction
2. Results
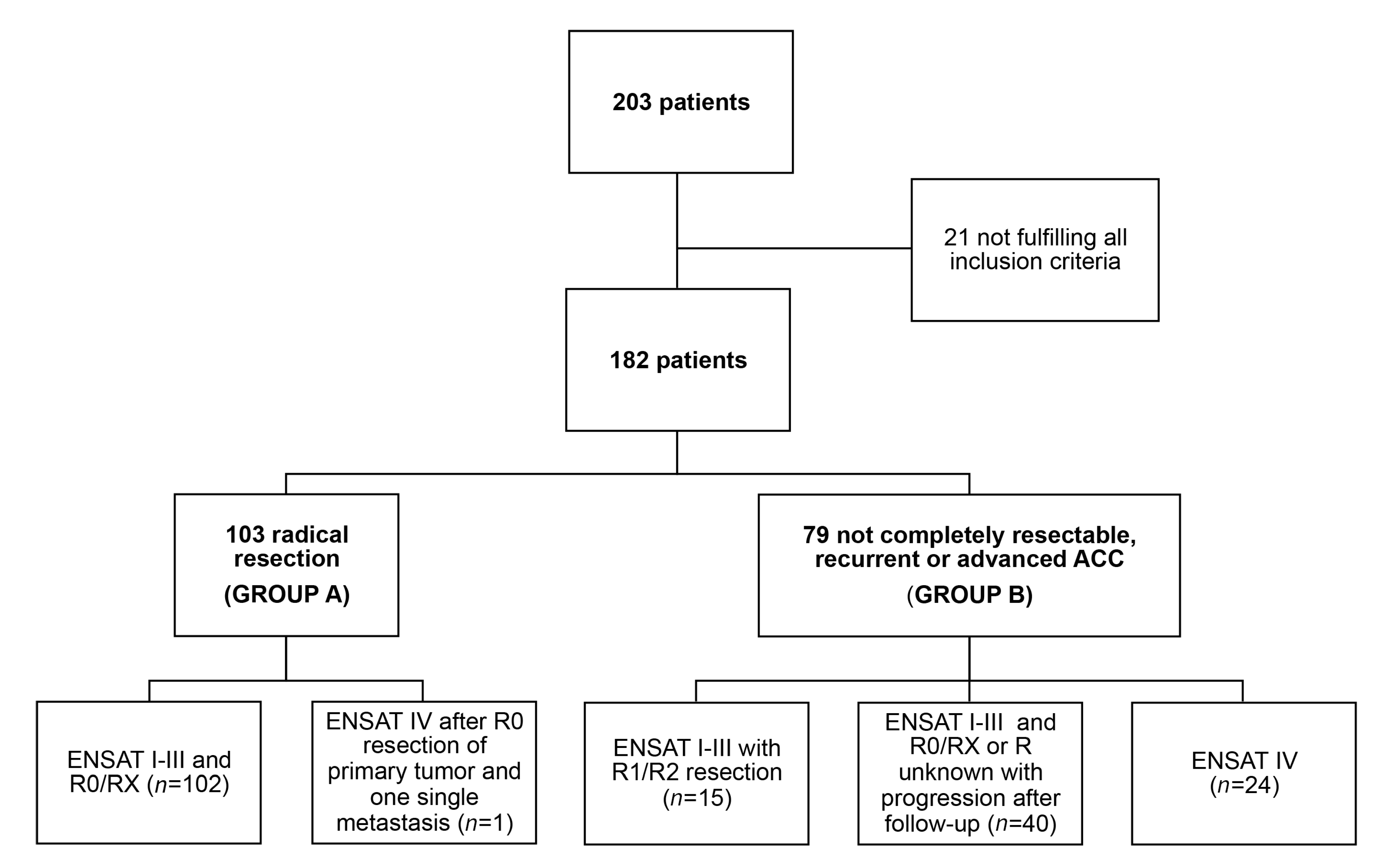
2.1. Patient Characteristics and Mitotane Treatment Details
2.2. CYP2W1 and CYP2B6 Allele Frequency
2.3. Achievement of Mitotane Therapeutic Levels
2.4. Response to Mitotane Treatment
2.4.1. Group A: Patients with Completely Resected Tumor
2.4.2. Group B: Patients with Not Completely Resectable, Recurrent or Advanced ACC
2.5. Adverse Events Associated to Mitotane Treatment
2.6. Mitotane Metabolites and Correlation with CYP2W1*6 and CYP2B6*6 SNPs
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Clinical Data, Mitotane Treatment and Endpoints Assessment
4.3. Genotyping and Sequencing of CYP2W1 and CYP2B6 Polymorphisms
4.4. Evaluation of Mitotane Related Adverse Events
4.5. Measurement of Mitotane Metabolites
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B.; et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a Revised TNM Classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Johanssen, S.; Hahner, S.; Saeger, W.; Quinkler, M.; Beuschlein, F.; Dralle, H.; Haaf, M.; Kroiss, M.; Jurowich, C.; Langer, P.; et al. Deficits in the management of patients with adrenocortical carcinoma in Germany. Dtsch. Arztebl. Int. 2010, 107, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libe, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Libe, R.; Borget, I.; Ronchi, C.L.; Zaggia, B.; Kroiss, M.; Kerkhofs, T.; Bertherat, J.; Volante, M.; Quinkler, M.; Chabre, O.; et al. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): An European Network for the Study of Adrenal Tumor (ENSAT) study. Ann. Oncol. 2015, 26, 2119–2125. [Google Scholar] [CrossRef]
- Lippert, J.; Appenzeller, S.; Liang, R.; Sbiera, S.; Kircher, S.; Altieri, B.; Nanda, I.; Weigand, I.; Gehrig, A.; Steinhauer, S.; et al. Targeted Molecular Analysis in Adrenocortical Carcinomas: A Strategy Toward Improved Personalized Prognostication. J. Clin. Endocrinol. Metab. 2018, 103, 4511–4523. [Google Scholar] [CrossRef]
- Else, T.; Williams, A.R.; Sabolch, A.; Jolly, S.; Miller, B.S.; Hammer, G.D. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Terzolo, M.; Angeli, A.; Fassnacht, M.; Daffara, F.; Tauchmanova, L.; Conton, P.A.; Rossetto, R.; Buci, L.; Sperone, P.; Grossrubatscher, E.; et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N. Engl. J. Med. 2007, 356, 2372–2380. [Google Scholar] [CrossRef] [Green Version]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Schteingart, D.E.; Doherty, G.M.; Gauger, P.G.; Giordano, T.J.; Hammer, G.D.; Korobkin, M.; Worden, F.P. Management of patients with adrenal cancer: Recommendations of an international consensus conference. Endocr. Relat. Cancer 2005, 12, 667–680. [Google Scholar] [CrossRef]
- Megerle, F.; Herrmann, W.; Schloetelburg, W.; Ronchi, C.L.; Pulzer, A.; Quinkler, M.; Beuschlein, F.; Hahner, S.; Kroiss, M.; Fassnacht, M.; et al. Mitotane Monotherapy in Patients With Advanced Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, I.G.; Fassnacht, M.; Terzolo, M.; Houterman, S.; den Hartigh, J.; Leboulleux, S.; Daffara, F.; Berruti, A.; Chadarevian, R.; Schlumberger, M.; et al. Plasma concentrations of o,p′DDD, o,p′DDA, and o,p′DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: Results of a retrospective ENS@T multicenter study. J. Clin. Endocrinol. Metab. 2011, 96, 1844–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglisi, S.; Calabrese, A.; Basile, V.; Ceccato, F.; Scaroni, C.; Simeoli, C.; Torlontano, M.; Cannavo, S.; Arnaldi, G.; Stigliano, A.; et al. Mitotane Concentrations Influence the Risk of Recurrence in Adrenocortical Carcinoma Patients on Adjuvant Treatment. J. Clin. Med. 2019, 8, 1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volante, M.; Terzolo, M.; Fassnacht, M.; Rapa, I.; Germano, A.; Sbiera, S.; Daffara, F.; Sperone, P.; Scagliotti, G.; Allolio, B.; et al. Ribonucleotide reductase large subunit (RRM1) gene expression may predict efficacy of adjuvant mitotane in adrenocortical cancer. Clin. Cancer Res. 2012, 18, 3452–3461. [Google Scholar] [CrossRef] [Green Version]
- Ronchi, C.L.; Sbiera, S.; Volante, M.; Steinhauer, S.; Scott-Wild, V.; Altieri, B.; Kroiss, M.; Bala, M.; Papotti, M.; Deutschbein, T.; et al. CYP2W1 is highly expressed in adrenal glands and is positively associated with the response to mitotane in adrenocortical carcinoma. PLoS ONE 2014, 9, e105855. [Google Scholar] [CrossRef]
- van Koetsveld, P.M.; Creemers, S.G.; Dogan, F.; Franssen, G.J.H.; de Herder, W.W.; Feelders, R.A.; Hofland, L.J. The Efficacy of Mitotane in Human Primary Adrenocortical Carcinoma Cultures. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef] [Green Version]
- D’Avolio, A.; De Francia, S.; Basile, V.; Cusato, J.; De Martino, F.; Pirro, E.; Piccione, F.; Ardito, A.; Zaggia, B.; Volante, M.; et al. Influence of the CYP2B6 polymorphism on the pharmacokinetics of mitotane. Pharmacogenet. Genom. 2013, 23, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Bozina, N.; Bradamante, V.; Lovric, M. Genetic polymorphism of metabolic enzymes P450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arch. Ind. Hyg. Toxicol. 2009, 60, 217–242. [Google Scholar] [CrossRef]
- Travica, S.; Pors, K.; Loadman, P.M.; Shnyder, S.D.; Johansson, I.; Alandas, M.N.; Sheldrake, H.M.; Mkrtchian, S.; Patterson, L.H.; Ingelman-Sundberg, M. Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clin. Cancer Res. 2013, 19, 2952–2961. [Google Scholar] [CrossRef] [Green Version]
- Karlgren, M.; Gomez, A.; Stark, K.; Svard, J.; Rodriguez-Antona, C.; Oliw, E.; Bernal, M.L.; Ramon y Cajal, S.; Johansson, I.; Ingelman-Sundberg, M. Tumor-specific expression of the novel cytochrome P450 enzyme, CYP2W1. Biochem. Biophys. Res. Commun. 2006, 341, 451–458. [Google Scholar] [CrossRef]
- Stenstedt, K.; Hallstrom, M.; Johansson, I.; Ingelman-Sundberg, M.; Ragnhammar, P.; Edler, D. The expression of CYP2W1: A prognostic marker in colon cancer. Anticancer Res. 2012, 32, 3869–3874. [Google Scholar]
- Zhang, K.; Jiang, L.; He, R.; Li, B.L.; Jia, Z.; Huang, R.H.; Mu, Y. Prognostic value of CYP2W1 expression in patients with human hepatocellular carcinoma. Tumour Biol. 2014, 35, 7669–7673. [Google Scholar] [CrossRef] [PubMed]
- Stenstedt, K.; Travica, S.; Guo, J.; Barragan, I.; Pors, K.; Patterson, L.; Edler, D.; Mkrtchian, S.; Johansson, I.; Ingelman-Sundberg, M. CYP2W1 polymorphism: Functional aspects and relation to risk for colorectal cancer. Pharmacogenomics 2013, 14, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, S.; Shimizu, Y.; Shiraga, Y.; Yoshida, M.; Sugihara, K.; Ohta, S. Reductive metabolism of p,p′-DDT and o,p′-DDT by rat liver cytochrome P450. Drug Metab. Dispos. 2002, 30, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.H.; Blievernicht, J.K.; Klein, K.; Saussele, T.; Schaeffeler, E.; Schwab, M.; Zanger, U.M. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J. Pharmacol. Exp. Ther. 2008, 325, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Armstrong, L.L.; Bradford, Y.; Carlson, C.S.; Crawford, D.C.; Crenshaw, A.T.; de Andrade, M.; Doheny, K.F.; Haines, J.L.; Hayes, G.; et al. Quality control procedures for genome-wide association studies. Curr. Protoc. Hum. Genet. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Nolan, D.; Phillips, E.; Mallal, S. Efavirenz and CYP2B6 polymorphism: Implications for drug toxicity and resistance. Clin. Infect. Dis. 2006, 42, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Murtha, T.D.; Brown, T.C.; Rubinstein, J.C.; Haglund, F.; Juhlin, C.C.; Larsson, C.; Korah, R.; Carling, T. Overexpression of cytochrome P450 2A6 in adrenocortical carcinoma. Surgery 2017, 161, 1667–1674. [Google Scholar] [CrossRef]
- Hescot, S.; Paci, A.; Seck, A.; Slama, A.; Viengchareun, S.; Trabado, S.; Brailly-Tabard, S.; Al Ghuzlan, A.; Young, J.; Baudin, E.; et al. The lack of antitumor effects of o,p’DDA excludes its role as an active metabolite of mitotane for adrenocortical carcinoma treatment. Horm. Cancer 2014, 5, 312–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germano, A.; Rapa, I.; Volante, M.; De Francia, S.; Migliore, C.; Berruti, A.; Papotti, M.; Terzolo, M. RRM1 modulates mitotane activity in adrenal cancer cells interfering with its metabolization. Mol. Cell Endocrinol. 2015, 401, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kasperlik-Zaluska, A.A.; Cichocki, A. Clinical role of determination of plasma mitotane and its metabolites levels in patients with adrenal cancer: Results of a long-term follow-up. J. Exp. Ther. Oncol. 2005, 5, 125–132. [Google Scholar] [PubMed]
- Zanger, U.M.; Klein, K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): Advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 2013, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanbrabant, T.; Fassnacht, M.; Assie, G.; Dekkers, O.M. Influence of hormonal functional status on survival in adrenocortical carcinoma: Systematic review and meta-analysis. Eur. J. Endocrinol. 2018, 179, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Kerkhofs, T.M.; Baudin, E.; Terzolo, M.; Allolio, B.; Chadarevian, R.; Mueller, H.H.; Skogseid, B.; Leboulleux, S.; Mantero, F.; Haak, H.R.; et al. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 4759–4767. [Google Scholar] [CrossRef] [Green Version]
- Geiger, J.; Both, S.; Kircher, S.; Neumann, M.; Rosenwald, A.; Jahns, R. Hospital-integrated Biobanking as a Service–The Interdisciplinary Bank of Biomaterials and Data Wuerzburg (ibdw). Open J. Bioresour. 2018, 5. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [Green Version]
- Common Terminology Criteria for Adverse Events (CTCAE) v4. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 1 November 2019).
- De Francia, S.; Pirro, E.; Zappia, F.; De Martino, F.; Sprio, A.E.; Daffara, F.; Terzolo, M.; Berruti, A.; Di Carlo, F.; Ghezzo, F. A new simple HPLC method for measuring mitotane and its two principal metabolites Tests in animals and mitotane-treated patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 837, 69–75. [Google Scholar] [CrossRef]
- Sbiera, S.; Sbiera, I.; Ruggiero, C.; Doghman-Bouguerra, M.; Korpershoek, E.; de Krijger, R.R.; Ettaieb, H.; Haak, H.; Volante, M.; Papotti, M.; et al. Assessment of VAV2 Expression Refines Prognostic Prediction in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 3491–3498. [Google Scholar] [CrossRef] [Green Version]
- Berruti, A.; Fassnacht, M.; Haak, H.; Else, T.; Baudin, E.; Sperone, P.; Kroiss, M.; Kerkhofs, T.; Williams, A.R.; Ardito, A.; et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur. Urol. 2014, 65, 832–838. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics of the Patients | |||||
|---|---|---|---|---|---|
| Parameter | All Patients | Group A | Group B | p | Chi-Square |
| n | 182 | 103 | 79 | - | - |
| Sex | |||||
| F | 121 | 68 (66%) | 53 (67.1%) | 0.88 | 0.023 |
| M | 61 | 35 (34%) | 26 (32.9%) | ||
| Age yrs | 49 (16–80) | 47 (18–75) | 51 (16–80) | 0.027 | - |
| Tumor size cm | 10 (2–24) | 10 (2–24) | 11 (3–24) | 0.05 | - |
| Hormone secretion | 0.007 | 16.02 | |||
| Cortisol (alone or with other steroids) | 80 (44.0%) | 37 (35.9%) | 43 (54.4%) | ||
| Androgens | 15 (8.2%) | 14 (13.6%) | 1 (1.3%) | ||
| Aldosterone | 4 (2.2%) | 4 (3.9%) | 0 (0%) | ||
| Estrogens | 2 (1.1%) | 1 (1.0%) | 1 (1.3%) | ||
| Inactive | 53 (29.1%) | 33 (32.0%) | 20 (25.3%) | ||
| Unknown | 28 (15.4%) | 14 (13.6%) | 14 (17.7%) | ||
| ENSAT Tumor Stage at diagnosis | <0.001 | 35.45 | |||
| I–II | 108 (59.3%) | 75 (72.8%) | 33 (41.8%) | ||
| III | 49 (27.0%) | 27 (26.2%) | 22 (27.8%) | ||
| IV | 25 (13.7%) | 1 (1.0%) | 24 (30.4%) | ||
| Resection Status | <0.001 | 56.60 | |||
| R0 | 114 (62.6%) | 83 (80.6%) | 31 (39.2%) | ||
| RX | 34 (18.7%) | 20 (19.4%) | 14 (17.7%) | ||
| R1/R2 | 28 (15.4%) | 0 (0%) | 28 (15.4%) | ||
| unknown | 6 (3.3%) | 0 (0%) | 6 (7.6%) | ||
| Ki67% proliferation index a | 17 (1–90) | 20 (1–90) | 10 (1–60) | 0.076 | - |
| Weiss score b | 6 (2–9) | 6 (2–9) | 6 (3–9) | 0.19 | - |
| Group A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | TTP | DSS | ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | |
| Mitotane therapeutic range | 0.024 | 1.92 (1.09–3.37) | 0.019 | 2.04 (1.13–3.71) | 0.032 | 2.38 (1.08–5.26) | 0.046 | 2.25 (1.02–4.96) |
| ENSAT stage at diagnosis | 0.13 | 1.57 (0.88–2.79) | n.a | - | 0.77 | 1.44 (0.48–2.96) | n.a | - |
| Ki67 index | <0.001 | 3.46 (1.76–6.78) | 0.001 | 3.06 (1.55–6.05) | 0.026 | 2.68 (1.13–6.35) | 0.043 | 2.46 (1.03–5.87) |
| Hormonal secretion | 0.055 | 1.8 (0.99–3.30) | n.a. | - | 0.08 | 2.22 (0.92–5.39) | n.a. | - |
| Age at diagnosis | 0.93 | 1.03 (0.59–1.78) | n.a. | - | 0.51 | 0.79 (0.21–1.31) | n.a. | - |
| CYP2W1*2 | 0.15 | 0.54 (0.23–1.26) | n.a. | - | 0.12 | 0.21 (0.03–1.53) | n.a. | - |
| CYP2W1*6 | 0.39 | 1.30 (0.71–2.34) | n.a. | - | 0.79 | 1.12 (0.49–2.56) | n.a. | - |
| CYP2B6*6 | 0.77 | 0.92 (0.52–1.61) | n.a. | - | 0.63 | 1.21 (0.56–2.58) | n.a. | - |
| CYP2B6 intronic variant | 0.07 | 0.61 (0.35–1.05) | n.a. | - | 0.29 | 0.66 (0.31–1.42) | n.a. | - |
| Group A (n = 98) * | |||||||
|---|---|---|---|---|---|---|---|
| SNPs | n | TTP | DSS | ||||
| Median TTP Months | HR (95% CI) | p | Median DSS Months | HR (95% CI) | p | ||
| CYP2W1*6 | |||||||
| target levels | 15 | Not reached | Not reached | ||||
| mitotane levels <14 | 12 | 10.5 | 3.45 (1.45–10.42) | 0.03 | 60.0 | 2.72 (0.65–11.3) | 0.17 |
| CYP2W1*6 WT | |||||||
| target levels | 36 | 66.0 | Not reached | ||||
| mitotane levels <14 | 35 | 22.0 | 1.61 (0.81–0.65) | 0.18 | Not reached | 2.40 (0.93–6.21) | 0.07 |
| CYP2B6*6 | |||||||
| target levels | 23 | 66.0 | Not reached | ||||
| mitotane levels <14 | 14 | 5.0 | 3.58 (1.22–10.49) | 0.02 | 60.0 | 7.10 (1.97–25.60) | 0.003 |
| CYP2B6*6 WT | |||||||
| target levels | 28 | 53.0 | Not reached | ||||
| mitotane levels <14 | 33 | 19.0 | 1.57 (0.77–3.20) | 0.22 | Not reached | 1.30 (0.47–3.61) | 0.32 |
| Group B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | TTP | DSS | ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | |
| Mitotane therapeutic range | 0.003 | 2.11 (1.84–3.47) | 0.002 | 2.29 (1.37–3.85) | 0.77 | 1.69 (0.94–3.04) | n.a. | - |
| PreM-TTP | 0.012 | 0.67 (0.49–0.92) | 0.004 | 0.64 (0.47–0.87) | 0.004 | 1.65 (1.17–2.32) | 0.13 | 0.68 (0.42–1.12) |
| Ki67 index | 0.057 | 1.63 (0.99–2.69) | n.a. | - | <0.001 | 3.07 (1.67–5.65) | 0.037 | 2.07 (1.05–4.10) |
| R status | 0.24 | 1.35 (0.81–2.25) | n.a. | - | <0.001 | 3.34 (1.80–6.20) | 0.09 | 1.92 (0.88–4.16) |
| Hormonal secretion | 0.21 | 0.70 (0.40–1.22) | n.a. | - | 0.19 | 0.65 (0.35–1.23) | n.a. | - |
| Age at diagnosis | 0.97 | 0.99 (0.61–1.59) | n.a. | - | 0.78 | 0.93 (0.53–1.61) | n.a. | - |
| CYP2W1*2 | 0.97 | 1.01 (0.52–1.98) | n.a. | - | 0.65 | 1.18 (0.57–2.44) | n.a. | - |
| CYP2W1*6 | 0.028 | 1.78 (1.06–2.99) | 0.10 | 1.54 (0.91–2.61) | 0.36 | 1.32 (0.72–2.43) | n.a. | - |
| CYP2B6*6 | 0.54 | 1.01 (0.52–1.98) | n.a. | - | 0.80 | 1.08 (0.62–1.88) | n.a. | - |
| CYP2B6 intronic variant | 0.49 | 1.18 (0.73–190) | n.a. | - | 0.88 | 1.04 (0.59–1.83) | n.a. | - |
| Best Response | CYP2W1*6 WT (n = 58) | CYP2W1*6 (n = 21) | ||
|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | |
| Complete response | 4 | 6.9% (1.9–16.7) | 1 | 4.8% (0.1–23.8) |
| Partial response | 7 | 12.1% (5.0–23.3) | 1 | 4.8% (0.1–23.8) |
| Stable disease | 25 | 43.1% (30.2–56.8) | 4 | 19.0% (5.4–41.9) |
| Progressive disease | 22 | 37.9% (25.5–51.6) | 15 | 71.4% (47.8–51.6) |
| Group B (n = 79) | |||||||
|---|---|---|---|---|---|---|---|
| SNPs | n | TTP | DSS | ||||
| Median TTP Months | HR (95% CI) | p | Median DSS Months | HR (95% CI) | p | ||
| CYP2W1*6 | |||||||
| target levels | 5 | 18.0 | 115.0 | ||||
| mitotane levels <14 | 16 | 3.0 | 3.52 (1.16–10.65) | 0.03 | 36.0 | 1.67 (0.52–5.39) | 0.39 |
| CYP2W1*6 WT | |||||||
| target levels | 28 | 13.0 | 75 | ||||
| mitotane levels <14 | 30 | 5.0 | 1.95 (1.07–3.55) | 0.03 | 40 | 1.45 (0.73–2.86) | 0.23 |
| CYP2B6*6 | |||||||
| target levels | 22 | 15.0 | 75.0 | ||||
| mitotane levels <14 | 18 | 4.0 | 5.59 (2.40–13.00) | <0.0001 | 25.0 | 3.89 (1.61–9.43) | 0.003 |
| CYP2B6*6 WT | |||||||
| target levels | 11 | 13.0 | 50.5 | ||||
| mitotane levels <14 | 28 | 4.5 | 1.37 (0.34–2.91) | 0.42 | 58.0 | 0.99 (0.39–2.43) | 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altieri, B.; Sbiera, S.; Herterich, S.; De Francia, S.; Della Casa, S.; Calabrese, A.; Pontecorvi, A.; Quinkler, M.; Kienitz, T.; Mannelli, M.; et al. Effects of Germline CYP2W1*6 and CYP2B6*6 Single Nucleotide Polymorphisms on Mitotane Treatment in Adrenocortical Carcinoma: A Multicenter ENSAT Study. Cancers 2020, 12, 359. https://doi.org/10.3390/cancers12020359
Altieri B, Sbiera S, Herterich S, De Francia S, Della Casa S, Calabrese A, Pontecorvi A, Quinkler M, Kienitz T, Mannelli M, et al. Effects of Germline CYP2W1*6 and CYP2B6*6 Single Nucleotide Polymorphisms on Mitotane Treatment in Adrenocortical Carcinoma: A Multicenter ENSAT Study. Cancers. 2020; 12(2):359. https://doi.org/10.3390/cancers12020359
Chicago/Turabian StyleAltieri, Barbara, Silviu Sbiera, Sabine Herterich, Silvia De Francia, Silvia Della Casa, Anna Calabrese, Alfredo Pontecorvi, Marcus Quinkler, Tina Kienitz, Massimo Mannelli, and et al. 2020. "Effects of Germline CYP2W1*6 and CYP2B6*6 Single Nucleotide Polymorphisms on Mitotane Treatment in Adrenocortical Carcinoma: A Multicenter ENSAT Study" Cancers 12, no. 2: 359. https://doi.org/10.3390/cancers12020359
APA StyleAltieri, B., Sbiera, S., Herterich, S., De Francia, S., Della Casa, S., Calabrese, A., Pontecorvi, A., Quinkler, M., Kienitz, T., Mannelli, M., Canu, L., Angelousi, A., Chortis, V., Kroiss, M., Terzolo, M., Fassnacht, M., & Ronchi, C. L. (2020). Effects of Germline CYP2W1*6 and CYP2B6*6 Single Nucleotide Polymorphisms on Mitotane Treatment in Adrenocortical Carcinoma: A Multicenter ENSAT Study. Cancers, 12(2), 359. https://doi.org/10.3390/cancers12020359








