Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. CD47 and PD-L1 Expression in BC Cell Lines
2.2. Patients
2.3. CD47 and PD-L1 Expression on CTCs of Patients with Early, Recurrent, and de Novo Metastatic BC
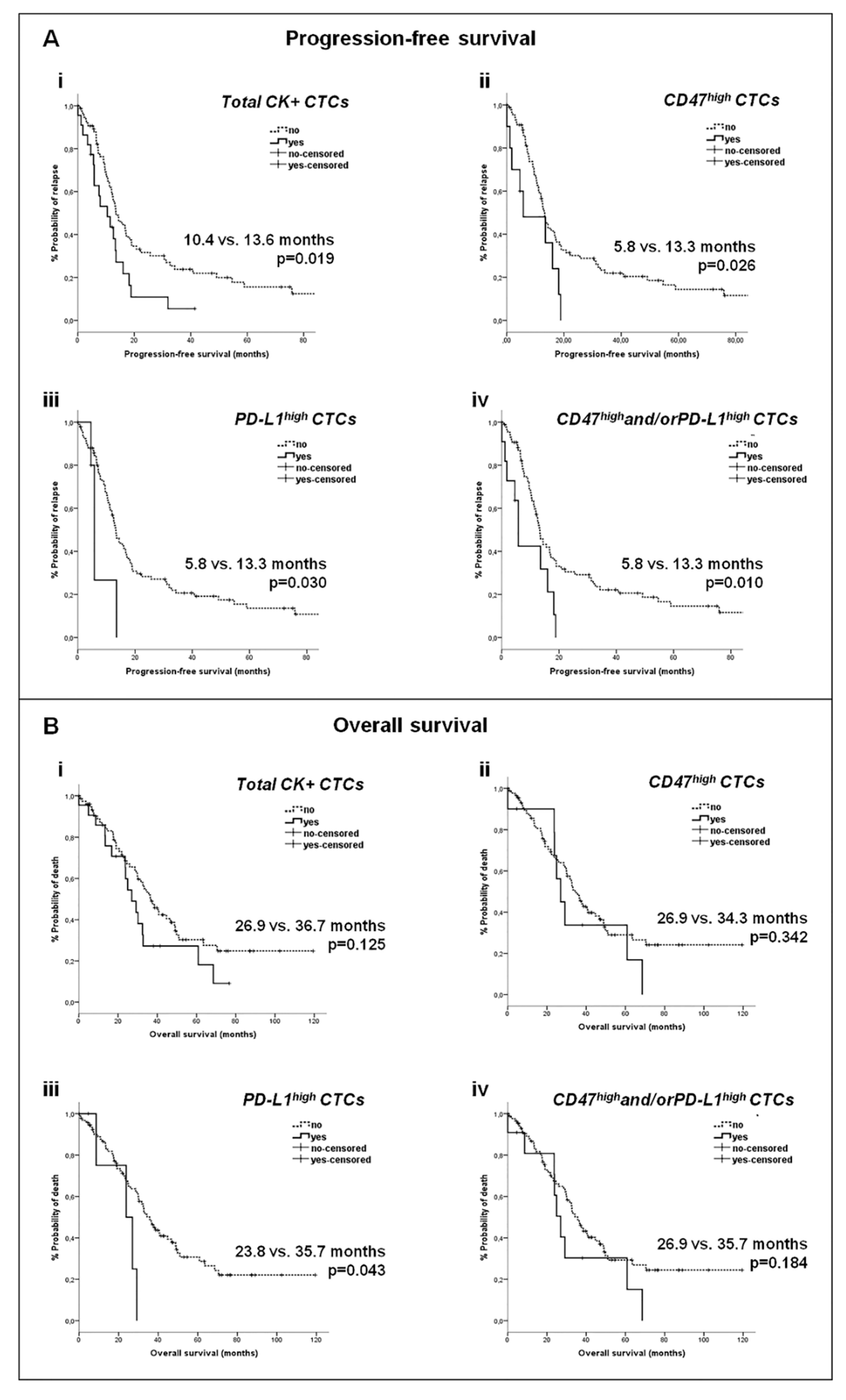
2.4. Clinical Relevance of CD47 and PD-L1 Expression on CTCs in Metastatic BC
2.4.1. Correlation of CTC Subsets with Clinicopathological Parameters and Response to First-Line Treatment
2.4.2. Correlation of CTC Subsets with Survival Measures
2.5. Clinical Relevance of CD47 and PD-L1 Expression on CTCs in Early BC
2.6. Comparative Analysis of CD47 and PD-L1 Expression on Tumor and Immune Cells in Matched Peripheral Blood and Tissue Samples
2.6.1. CD47 and PD-L1 Expression on Tumor and Immune Cells within Peripheral Blood (CTC-Positive Patients, n = 36)
2.6.2. PD-L1 Expression on Tumor and Immune Cells within Tumor Tissue
2.6.3. CD47 and PD-L1 Expression on Tumor Cells in Matched Peripheral Blood and Tumor Tissue Samples
2.6.4. PD-L1 Expression on Immune Cells in Matched Peripheral Blood and Tumor Tissue Samples
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Cell Culture
4.3. Enrichment of CTCs in Blood Samples
4.4. Immunofluorescence (IF)
4.5. Evaluation of CD47 and PD-L1 Expression in BC Cell Lines
4.6. Evaluation of CD47 and PD-L1 Expression in CTCs and PBMCs
4.7. Immunohistochemistry (IHC)
4.8. Evaluation of CD47 and PD-L1 Expression on Tissue Samples
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Austreid, E.; Lonning, P.E.; Eikesdal, H.P. The emergence of targeted drugs in breast cancer to prevent resistance to endocrine treatment and chemotherapy. Expert Opin. Pharmacother. 2014, 15, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Eyles, J.; Puaux, A.-L.; Wang, X.; Toh, B.; Prakash, C.; Hong, M.; Tan, T.G.; Zheng, L.; Ong, L.C.; Jin, Y.; et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Investig. 2010, 120, 2030–2039. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay AN, Van den Berg TK: The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 2014, 32, 25–50. [CrossRef]
- Oldenborg, P.-A. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Baccelli, I.; Stenzinger, A.; Vogel, V.; Pfitzner, B.M.; Klein, C.; Wallwiener, M.; Scharpff, M.; Saini, M.; Holland-Letz, T.; Sinn, H.-P.; et al. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal-type breast cancer patients. Oncotarget 2014, 5, 8147–8160. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Matikas, A.; Zerdes, I.; Lovrot, J.; Richard, F.; Sotiriou, C.; Bergh, J.; Valachis, A.; Foukakis, T. Prognostic Implications of PD-L1 Expression in Breast Cancer: Systematic Review and Meta-analysis of Immunohistochemistry and Pooled Analysis of Transcriptomic Data. Clin. Cancer Res. 2019, 25, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gutgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, L.; Ren, Z.; Yang, K.; Xu, H.; Luan, Y.; Fu, K.; Guo, J.; Peng, H.; Zhu, M.; et al. Dual Targeting of Innate and Adaptive Checkpoints on Tumor Cells Limits Immune Evasion. Cell Rep. 2018, 24, 2101–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinidou, A.; Alifieris, C.; Trafalis, D.T. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 2019, 194, 84–106. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw, W.G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Kang, Y.; Pantel, K. Tumor cell dissemination: Emerging biological insights from animal models and cancer patients. Cancer Cell 2013, 23, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Alix-Panabieres, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallergi, G.; Papadaki, M.A.; Politaki, E.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011, 13, R59. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Mak, M.P.; Tong, P.; Diao, L.; Cardnell, R.J.; Gibbons, D.L.; William, W.N.; Skoulidis, F.; Parra, E.R.; Rodriguez-Canales, J.; Wistuba, I.I.; et al. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin. Cancer Res. 2016, 22, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Steinert, G.; Schölch, S.; Niemietz, T.; Iwata, N.; García, S.A.; Behrens, B.; Voigt, A.Y.; Kloor, M.; Benner, A.; Bork, U.; et al. Immune Escape and Survival Mechanisms in Circulating Tumor Cells of Colorectal Cancer. Cancer Res. 2014, 74, 1694–1704. [Google Scholar] [CrossRef] [Green Version]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabieres, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Bian, Z.; Shi, L.; Niu, S.; Ha, B.; Tremblay, A.; Li, L.; Zhang, X.; Paluszynski, J.; Liu, M.; et al. Loss of Cell Surface CD47 Clustering Formation and Binding Avidity to SIRPalpha Facilitate Apoptotic Cell Clearance by Macrophages. J. Immunol. 2015, 195, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Polioudaki, H.; Chantziou, A.; Kalyvianaki, K.; Malamos, P.; Notas, G.; Mavroudis, D.; Kampa, M.; Castanas, E.; Theodoropoulos, P.A. Nuclear localization of PD-L1: Artifact or reality? Cell. Oncol. 2019, 42, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gaule, P.; Smithy, J.W.; Toki, M.; Rehman, J.; Patell-Socha, F.; Cougot, D.; Collin, P.; Morrill, P.; Neumeister, V.; Rimm, D.L. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol. 2017, 3, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Lee, Y.K.; Koo, J.S. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J. Transl. Med. 2016, 14, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmgren, J.A.; Mayer, M.; Atwood, M.K.; Kaplan, H.G. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res. Treat. 2018, 167, 579–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, D.; Brufsky, A.; Cobleigh, M.; Jahanzeb, M.; Kaufman, P.A.; Mason, G.; O’Shaughnessy, J.; Rugo, H.S.; Swain, S.M.; Yardley, D.A.; et al. De Novo Versus Recurrent HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from the SystHERs Registry. Oncologist 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Castro, A.C.; Spurr, L.; Hughes, M.E.; Li, Y.Y.; Cherniack, A.D.; Bychkovsky, B.L.; Barroso-Sousa, R.; Di Lascio, S.; Files, J.; Kumari, P.; et al. Genomic landscape of de novo stage IV breast cancer. J. Clin. Oncol. 2019, 37, 1022. [Google Scholar] [CrossRef]
- Yuan, J.; He, H.; Chen, C.; Wu, J.; Rao, J.; Yan, H. Combined high expression of CD47 and CD68 is a novel prognostic factor for breast cancer patients. Cancer Cell Int. 2019, 19, 238. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Shi, X.; Chen, C.; He, H.; Liu, L.; Wu, J.; Yan, H. High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis. Oncol. Lett. 2019, 18, 3249–3255. [Google Scholar] [CrossRef] [Green Version]
- Kowanetz, M.; Zou, W.; Gettinger, S.N.; Koeppen, H.; Kockx, M.; Schmid, P.; Kadel, E.E., III; Wistuba, I.; Chaft, J.; Rizvi, N.A.; et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc. Natl. Acad. Sci. USA 2018, 115, E10119–E10126. [Google Scholar] [CrossRef] [Green Version]
- Cimino-Mathews, A.; Thompson, E.; Taube, J.M.; Ye, X.; Lu, Y.; Meeker, A.; Xu, H.; Sharma, R.; Lecksell, K.; Cornish, T.C.; et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2016, 47, 52–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobral-Leite, M.; Van d, V.; Michaut, M.; van der Linden, R.; Hooijer, G.K.J.; Horlings, H.M.; Severson, T.M.; Mulligan, A.M.; Weerasooriya, N.; Sanders, J.; et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018, 7, e1509820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalper, K.A.; Velcheti, V.; Carvajal, D.; Wimberly, H.; Brown, J.; Pusztai, L.; Rimm, D.L. In Situ Tumor PD-L1 mRNA Expression Is Associated with Increased TILs and Better Outcome in Breast Carcinomas. Clin. Cancer Res. 2014, 20, 2773–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017, 168, 487–502. [Google Scholar] [CrossRef]
- Kallergi, G.; Agelaki, S.; Papadaki, M.A.; Nasias, D.; Matikas, A.; Mavroudis, D.; Georgoulias, V. Expression of truncated human epidermal growth factor receptor 2 on circulating tumor cells of breast cancer patients. Breast Cancer Res. 2015, 17, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, M.A.; Kallergi, G.; Zafeiriou, Z.; Manouras, L.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Co-expression of putative stemness and epithelial-to-mesenchymal transition markers on single circulating tumour cells from patients with early and metastatic breast cancer. BMC Cancer 2014, 14, 651. [Google Scholar] [CrossRef] [Green Version]
- Spiliotaki, M.; Mavroudis, D.; Kokotsaki, M.; Vetsika, E.K.; Stoupis, I.; Matikas, A.; Kallergi, G.; Georgoulias, V.; Agelaki, S. Expression of insulin-like growth factor-1 receptor in circulating tumor cells of patients with breast cancer is associated with patient outcomes. Mol. Oncol. 2018, 12, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.L.; Adams, D.K.; He, J.; Kalhor, N.; Zhang, M.; Xu, T.; Gao, H.; Reuben, J.M.; Qiao, Y.; Komaki, R.; et al. Sequential Tracking of PD-L1 Expression and RAD50 Induction in Circulating Tumor and Stromal Cells of Lung Cancer Patients Undergoing Radiotherapy. Clin. Cancer Res. 2017, 23, 5948–5958. [Google Scholar] [CrossRef] [Green Version]
- Fehm, T.; Müller, V.; Aktas, B.; Janni, W.; Schneeweiss, A.; Stickeler, E.; Lattrich, C.; Löhberg, C.R.; Solomayer, E.; Rack, B.; et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: A prospective, multicenter trial. Breast Cancer Res. Treat. 2010, 124, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Wallwiener, M.; Hartkopf, A.D.; Riethdorf, S.; Nees, J.; Sprick, M.R.; Schönfisch, B.; Taran, F.-A.; Heil, J.; Sohn, C.; Pantel, K.; et al. The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: A retrospective study in 107 patients. BMC Cancer 2015, 15, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutkin, D.W.; Shurin, M.R. Clinical evaluation of systemic and local immune responses in cancer: Time for integration. Cancer Immunol. Immunother. 2014, 63, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.; Volkmer, J.P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.M.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Early BC Patients (n = 100) | n (%) | Metastatic BC Patients (n = 98) | n (%) |
|---|---|---|---|
| Age, years | Age, yrs | ||
| Median (range) | 55 (32–81) | Median (range) | 59 (29–84) |
| PS | PS | ||
| 0–1 | 97 (97) | 0–1 | 92 (93.9) |
| 2 | 3 (3) | 2 | 3 (3.1) |
| Unknown | 3 (3.1) | ||
| Histology | Histology | ||
| Ductal | 83 (83) | Ductal | 80 (81.6) |
| Lobular | 11 (11) | Lobular | 10 (10.2) |
| Mixed | 2 (2) | Mixed | 4 (4.1) |
| Unknown | 4 (4) | Unknown | 4 (4.1) |
| Grade | Stage at diagnosis | ||
| I–II | 44 (44) | I–III | 71 (70.4) |
| III | 43 (43) | IV | 27 (27.6) |
| Unknown | 13 (13) | ||
| Stage | Subtype | ||
| I | 22 (22) | HR+/HER2− | 63 (64.3) |
| ΙΙ | 60 (60) | HER2+ | 22 (22.5) |
| IΙΙ | 14 (14) | Triple-negative | 12 (12.2) |
| Unknown | 4 (4) | Unknown | 1 (1) |
| Subtype | Prior adjuvant treatment | ||
| HR+/HER2− | 71 (71) | Yes | 66 (67.3) |
| HER2+ | 17 (17) | No | 28 (28.6) |
| Triple-negative | 10 (10) | Unknown | 4 (4.1) |
| Unknown | 2 (2) | ||
| Adjuvant treatment a Chemotherapy Hormone therapy | 97 (97) 77 (77) | Organs affected Breast Bones CNS Lung Liver LNs Other | 26 (26.5) 36 (36.7) 10 (10.2) 41 (41.8) 34 (34.7) 39 (39.8) 9 (9.2) |
| Disease sites 1–2 >2 Unknown | 61 (62.2) 33 (33.7) 4 (4.1) | ||
| First line treatment a Chemotherapy Hormone therapy Unknown | 88 (89.8) 9 (9.2) 1 (1) | ||
| Response to treatment PR SD PD NE | 41 (41.8) 31 (31.6) 18 (18.4) 8 (8.2) |
| CTC Populations | CTC Detection According to BC Subtype (% of Patients) | p Value | ||
|---|---|---|---|---|
| Triple-Negative | HR+/HER2− | HER2+ | ||
| Total CTCs | 50 | 20.6 | 13.6 | 0.053 |
| CD47+ | 50 | 17.5 | 13.6 | 0.045 * |
| CD47high | 25 | 11.1 | 0 | 0.049 * |
| PD-L1+ | 25 | 4.8 | 0 | 0.025 * |
| PD-L1high | 16.7 | 4.8 | 0 | 0.097 |
| CD47+and/orPD-L1+ | 50 | 17.5 | 13.6 | 0.045 * |
| CD47highand/orPD-L1high | 33.3 | 11.1 | 0 | 0.015 * |
| CTC Populations | CTC Detection According to Response to Treatment (% of Patients) | p Value | |
|---|---|---|---|
| PD | PR/SD | ||
| Total CTCs | 44.4 | 16.7 | 0.011 * |
| CD47+ | 38.9 | 15.3 | 0.025 * |
| CD47high | 22.2 | 5.6 | 0.026 * |
| PD-L1+ | 11.1 | 2.8 | 0.177 |
| PD-L1high | 11.1 | 1.4 | 0.101 |
| CD47+and/orPD-L1+ | 38.9 | 15.3 | 0.025 * |
| CD47highand/orPD-L1high | 27.8 | 5.6 | 0.005 * |
| Cox Regression Analysis | Progression-Free Survival (PFS) | Overall Survival (OS) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Covariates | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age (>59) | 0.811 (0.512–1.283) | 0.370 | 0.677 (0.409–1.120) | 0.129 | ||||
| Performance status (0–1) | 0.431 (0.133–1.402) | 0.162 | 0.363 (0.112–1.178) | 0.091 | ||||
| Recurrent disease | 1.575 (0.944–2.626) | 0.082 | 1.837 (1.013–3.331) | 0.045 * | 4.072 (1.970–8.418) | 0.000 * | ||
| Molecular subtype of tumor | ||||||||
| HR-positive | 0.910 (0.522–1.587) | 0.740 | 0.918 (0.504–1.675) | 0.781 | ||||
| HER2-positive | 1.607 (0.923–2.798) | 0.094 | 1.794 (0.951–3.385) | 0.071 | ||||
| Triple-negative | 1.559 (0.796–3.054) | 0.195 | 1.401 (0.688–2.850) | 0.353 | ||||
| No of organs affected (>2) | 1.453 (0.914–2.310) | 0.115 | 2.084 (1.255–3.461) | 0.005 * | 3.456 (1.897–6.294) | 0.000 * | ||
| Metastatic sites | ||||||||
| Liver | 1.613 (1.017–2.560) | 0.042 * | 1.816 (1.126–2.930) | 0.014 * | 2.002 (1.195–3.353) | 0.008 * | 1.999 (1.142–3.499) | 0.015 * |
| Lung | 0.995 (0.631–1.568) | 0.982 | 0.825 (0.497–1.368) | 0.455 | ||||
| Bones | 1.287 (0.809–2.049) | 0.287 | 1.513 (0.911–2.514) | 0.110 | ||||
| Lymph nodes | 0.825 (0.524–1.297) | 0.404 | 0.688 (0.418–1.135) | 0.143 | ||||
| CNS | 0.872 (0.434–1.751) | 0.700 | 0.901 (0.427–1.901) | 0.785 | ||||
| Skin | 0.841 (0.364–1.943) | 0.685 | 0.716 (0.308–1.666) | 0.439 | ||||
| Total CTCs | 1.866 (1.100–3.168) | 0.021 * | 1.558 (0.880–2.758) | 0.128 | ||||
| CD47high CTCs | 2.192 (1.079–4.452) | 0.030 * | 1.433 (0.680–3.018) | 0.344 | ||||
| PD-L1high CTCs | 2.977 (1.058–8.375) | 0.039 * | 2.793 (0.986–7.914) | 0.053 | ||||
| CD47highand/orPD-L1high CTCs | 2.373 (1.203–4.682) | 0.013 * | 2.719 (1.302–5.677) | 0.008 * | 1.610 (0.792–3.272) | 0.189 | 2.398 (1.071–5.371) | 0.034 * |
| PD-L1 Distribution among Tumor and Immune Cells | Peripheral Blood Patients (%) | Primary Tumor Patients (%) | ||||
|---|---|---|---|---|---|---|
| CTCs | PBMCs | Positivity Concordance | Tumor Cells | TILs | Positivity Concordance | |
| PD-L1 expression | 27.8 | 22.2 | 11.1 | 34.6 | 65.4 | 34.6 |
| PD-L1high expression | 19.4 | 5.6 | 2.8 | 11.5 | 42.3 | 11.5 |
| CD47 in Tumor cells | CD47 Expression patients (%) | CD47high Expression patients (%) | ||||
| Primary Tumor | CTCs | Positivity Concordance | Primary Tumor | CTCs | Positivity Concordance | |
| All patients | 88.5 | 84.6 | 76.9 | 19.2 | 53.8 | 11.5 |
| Early | 80 | 70 | 60 | 20 | 60 | 20 |
| Metastatic | 93.8 | 93.8 | 87.5 | 18.8 | 50 | 6.2 |
| PD-L1 in Tumor cells | PD-L1 expression patients (%) | PD-L1high expression patients (%) | ||||
| Primary tumor | CTCs | Positivity concordance | Primary tumor | CTCs | Positivity concordance | |
| All patients | 34.6 | 30.8 | 7.7 | 11.5 | 23.1 | 3.8 |
| Early | 30 | 30 | 0 | 20 | 20 | 0 |
| Metastatic | 37.5 | 31.2 | 12.5 | 6.2 | 25 | 6.2 |
| PD-L1 in Immune cells | PD-L1 expression patients (%) | PD-L1high expression patients (%) | ||||
| TILs | PBMCs | Positivity concordance | TILs | PBMCs | Positivity concordance | |
| All patients | 64 | 20 | 16 | 44 | 8 | 4 |
| Early | 60 | 40 | 30 | 40 | 10 | 0 |
| Metastatic | 66.7 | 6.7 | 6.7 | 46.7 | 6.7 | 6.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C.; et al. Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer. Cancers 2020, 12, 376. https://doi.org/10.3390/cancers12020376
Papadaki MA, Koutsopoulos AV, Tsoulfas PG, Lagoudaki E, Aggouraki D, Monastirioti A, Koutoulaki C, Apostolopoulou CA, Merodoulaki AC, Papadaki C, et al. Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer. Cancers. 2020; 12(2):376. https://doi.org/10.3390/cancers12020376
Chicago/Turabian StylePapadaki, Maria A., Anastasios V. Koutsopoulos, Panormitis G. Tsoulfas, Eleni Lagoudaki, Despoina Aggouraki, Alexia Monastirioti, Chara Koutoulaki, Christina A. Apostolopoulou, Aikaterini C. Merodoulaki, Chara Papadaki, and et al. 2020. "Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer" Cancers 12, no. 2: 376. https://doi.org/10.3390/cancers12020376
APA StylePapadaki, M. A., Koutsopoulos, A. V., Tsoulfas, P. G., Lagoudaki, E., Aggouraki, D., Monastirioti, A., Koutoulaki, C., Apostolopoulou, C. A., Merodoulaki, A. C., Papadaki, C., Mavroudis, D., & Agelaki, S. (2020). Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer. Cancers, 12(2), 376. https://doi.org/10.3390/cancers12020376








