Predictors of Postoperative Seizure Outcome in Low Grade Glioma: From Volumetric Analysis to Molecular Stratification
Abstract
:1. Introduction
2. Results
2.1. Study Population and Postoperative Seizure Outcome
2.2. Postoperative Seizure Outcome Analysis
2.3. ROC Analysis
3. Discussion
- (1)
- 70.97% of epileptic DLGG patients were in Engel Class IA 18 months after surgery;
- (2)
- Improved postoperative seizure outcome can be expected for EOR ≥ 85%, residual tumor ≤ 15 cm3, and preoperative ΔT2T1 MRI index ≤ 18 cm3.
- (3)
- Tumor infiltration index, expressed by ΔT2T1 MRI index, represents a quantitative evaluation of the diffusive and infiltrative tumor component as predictor of postoperative seizure outcome.
- (4)
- IDH1/2 mutation may represent the prevalent epileptogenic mechanism in presence of higher ΔT2T1 MRI index and consequent lower EOR.
3.1. The Role of EOR
3.2. The Tumor Growth Pattern Influences the Postoperative Seizure Outcome
3.3. It is a Matter of Interaction between EOR and Tumor Growth Pattern
3.4. Limitation and Future Directions
4. Materials and Methods
4.1. Study Population
4.2. Surgical Procedure
4.3. Volumetric Analysis
4.4. Histological and Molecular Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aronica, E.; Leenstra, S.; van Veelen, C.W.; van Rijen, P.C.; Hulsebos, T.J.; Tersmette, A.C.; Yankaya, B.; Troost, D. Glioneuronal tumors and medically intractable epilepsy: A clinical study with long-term follow-up of seizure outcome after surgery. Epilepsy. Res. 2001, 43, 179–191. [Google Scholar] [CrossRef]
- Rudà, R.; Trevisan, E.; Soffietti, R. Epilepsy and brain tumors. Curr. Opin. Oncol. 2010, 22, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.O.; Stupp, R. Epilepsy in brain tumor patients. Curr. Opin. Neurol. 2010, 23, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Rudà, R.; Bello, L.; Duffau, H.; Soffietti, R. Seizures in low-grade gliomas: Natural history, pathogenesis, and outcome after treatments. Neuro. Oncol. 2012, 14 (Suppl. 4), iv55–iv64. [Google Scholar] [CrossRef]
- Vecht, C.J.; Wagner, G.L.; Wilms, E.B. Treating seizures in patients with brain tumors: Drug interactions between antiepileptic and chemotherapeutic agents. Semin. Oncol. 2003, 30 (Suppl. 19), 49–52. [Google Scholar] [CrossRef]
- Huberfeld, G.; Vecht, C.J. Seizures and gliomas-towards a single therapeutic approach. Nat. Rev. Neurol. 2016, 12, 204–216. [Google Scholar] [CrossRef]
- Pallud, J.; Audureau, E.; Blonski, M.; Sanai, N.; Bauchet, L.; Fontaine, D.; Mandonnet, E.; Dezamis, E.; Psimaras, D.; Guyotat, J.; et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain 2014, 137 Pt 2, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Liubinas, S.V.; D’Abaco, G.M.; Moffat, B.M.; Gonzale, M.; Feleppa, F.; Nowell, C.J.; Gorelik, A.; Drummond, K.J.; O’Brien, T.J.; Kaye, A.H.; et al. IDH1 mutation is associated with seizures and protoplasmic subtype in patients with low-grade gliomas. Epilepsia 2014, 55, 1438–1443. [Google Scholar] [CrossRef]
- Zhang, C.B.; Bao, Z.S.; Wang, H.J.; Yan, W.; Liu, Y.W.; Li, M.Y.; Zhang, W.; Chen, L.; Jiang, T. Correlation of IDH1/2 mutation with clinicopathologic factors and prognosis in anaplastic gliomas: A report of 203 patients from China. J. Cancer. Res. Clin. Oncol. 2014, 140, 45–51. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Surgical oncology for gliomas: The state of the art. Nat. Rev. Clin. Oncol. 2018, 15, 112–125. [Google Scholar] [CrossRef]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: A series of 1097 cases: Clinical article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ius, T.; Isola, M.; Budai, R.; Pauletto, G.; Tomasino, B.; Fadiga, L.; Skrap, M. Low-grade glioma surgery in eloquent areas: Volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: Clinical article. J. Neurosurg. 2012, 117, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Chaichana, K.L.; Attenello, F.J.; Weingart, J.D.; Than, K.; Burger, P.C.; Olivi, A.; Brem, H.; Quinoñes-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008, 63, 700–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Pauletto, G.; Isola, M.; Gregoraci, G.; Budai, R.; Lettieri, C.; Eleopra, R.; Fadiga, L.; Skrap, M. Surgery for insular low-grade glioma: Predictors of postoperative seizure outcome. J. Neurosurg. 2014, 120, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Still, M.E.H.; Roux, A.; Huberfeld, G.; Bauchet, L.; Baron, M.H.; Fontaine, D.; Blonski, M.; Mandonnet, E.; Guillevin, R.; Guyotat, J.; et al. Extent of Resection and Residual Tumor Thresholds for Postoperative Total Seizure Freedom in Epileptic Adult Patients Harboring a Supratentorial Diffuse Low-Grade Glioma. Neurosurgery 2018, 85, 332–340. [Google Scholar] [CrossRef]
- Xu, D.S.; Awad, A.W.; Mehalechko, C.; Wilson, J.R.; Ashby, L.S.; Coons, S.W.; Sanai, N. An extent of resection threshold for seizure freedom in patients with low-grade gliomas. J. Neurosurg. 2018, 128, 1084–1090. [Google Scholar] [CrossRef]
- Englot, D.J.; Wang, D.D.; Rolston, J.D.; Shih, T.T.V.; Chang, E.F. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: A systematic review and meta-analysis. J. Neurosurg. 2012, 116, 1042–1048. [Google Scholar] [CrossRef]
- Chen, H.; Judkins, J.; Thomas, C.; Wu, M.; Khoury, L.; Benjamin, C.G.; Pacione, D.; Golfinos, J.G.; Kumthekar, P.; Ghamsari, F.; et al. Mutant IDH1 and seizures in patients with glioma. Neurology 2017, 88, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, L.; Ryan, E.; O’Brien, M.; Looby, S.; Heffernan, J.; O’Sullivan, J.; Clarke, M.; Buckley, P.; O’Brien, D.; Farrell, M.; et al. Genetic features of oligodendrogliomas and presence of seizures. The relationship of seizures and genetics in LGOs. Clin. Neuropathol. 2014, 33, 292–298. [Google Scholar] [CrossRef]
- Neal, A.; Kwan, P.; O’Brien, T.J.; Buckland, M.E.; Gonzales, M.; Morokoff, A. IDH1 and IDH2 mutations in postoperative diffuse glioma-associated epilepsy. Epilepsy. Behav. 2018, 78, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, Q.; Wang, X.; Liu, Y.; Mao, Y.; Zhou, Q.; Luo, J. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J. Clin. Neurosci. 2016, 31, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, Z.; Wang, Y.; You, G.; Jiang, T. IDH1/2 mutation is associated with seizure as an initial symptom in low-grade glioma: A report of 311 Chinese adult glioma patients. Epilepsy. Res. 2015, 109, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, F.; Duffau, H. Intractable epilepsy in paralimbic Word Health Organization Grade II gliomas: Should the hippocampus be resected when not invaded by the tumor? J. Neurosurg. 2012, 116, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Roessler, K.; Heynold, E.; Buchfelder, M.; Stefa, H.; Hamer, H.M. Current value of intraoperative electrocorticography (iopECoG). Epilepsy. Behav. 2019, 91, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.F.; Fric-Shamji, E.C.; Benoit, B.G. Brain tumors and epilepsy: Pathophysiology of peritumoral changes. Neurosurg. Rev. 2009, 32, 275–284. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Sha, Z.; Jiang, T. The pathogenesis of tumor-related epilepsy and its implications for clinical treatment. Seizure 2012, 21, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Skrap, M.; Mondani, M.; Tomasino, B.; Weis, L.; Budai, R.; Pauletto, G.; Eleopra, R.; Fadiga, L.; Ius, T. Surgery of insular nonenhancing gliomas: Volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery 2012, 70, 1081–1094. [Google Scholar] [CrossRef] [Green Version]
- Mandonnet, E.; Capelle, L.; Duffau, H. Extension of paralimbic low grade gliomas: Toward an anatomical classification based on white matter invasion patterns. J. Neurooncol. 2006, 78, 179–185. [Google Scholar] [CrossRef]
- Pallud, J.; Le Van Quyen, M.; Bielle, F.; Pellegrino, C.; Varlet, P.; Cresto, N.; Baulac, M.; Duyckaerts, C.; Kourdougli, N.; Chazal, G.; et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci. Transl. Med. 2014, 6, 244–289. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Ellison, D.W.; Brat, D.J.; Aldape, K.; Capper, D.; Hawkins, C.; Paulus, W.; Perry, A.; Reifenberger, G.; Figarella-Branger, D.; et al. cIMPACT-NOW: A practical summary of diagnostic points from Round 1 updates. Brain Pathol. 2019, 29, 469–472. [Google Scholar] [PubMed] [Green Version]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanella, F.; Fabbro, F.; Ius, T.; Shallice, T.; Skrap, M. Acute effects of surgery on emotion and personality of brain tumor patients: Surgery impact, histological aspects, and recovery. Neuro. Oncol. 2015, 17, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maschio, M.; Aguglia, U.; Avanzini, G.; Banfi, P.; Buttinelli, C.; Capovilla, G.; Casazza, M.M.L.; Colicchio, G.; Coppola, A.; Costa, C.; et al. Brain Tumor-related Epilepsy study group of Italian League Against Epilepsy (LICE). Management of epilepsy in brain tumors. Neurol. Sci. 2019, 40, 2217–2234. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Engel, J., Jr.; Burchfiel, J.; Ebersole, J.; Gates, J.; Gotman, J.; Homan, R.; Ives, J.; King, D.; Lieb, J.; Sato, S.; et al. Long-term monitoring for epilepsy. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 437–458. [Google Scholar] [CrossRef]
- Berger, M.S.; Ojemann, G.A. Intraoperative brain mapping techniques in neuro-oncology. Stereotact. Funct. Neurosurg. 1992, 58, 153–161. [Google Scholar] [CrossRef]
- Skrap, M.; Marin, D.; Ius, T.; Fabbro, F.; Tomasino, B. Brain mapping: A novel intraoperative neuropsychological approach. J. Neurosurg. 2016, 125, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Ius, T.; Angelini, E.; Thiebaut de Schotten, M.; Mandonnet, E.; Duffau, H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain”. Neuroimage 2011, 56, 992–1000. [Google Scholar] [CrossRef]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An open-source soft- ware for navigating in multidimensional DICOM images. J. Digit. Imaging 2004, 17, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preusser, M.; Berghoff, A.S.; Manzl, C.; Filipits, M.; Weinhäusel, A.; Pulverer, W.; Dieckmann, K.; Widhalm, G.; Wöhrer, A.; Knosp, E.; et al. Clinical Neuropathology practice news 1-2014: Pyrosequencing meets clinical and analytical performance criteria for routine testing of MGMT promoter methylation status in glioblastoma. Clin. Neuropathol. 2014, 33, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Value (N and %, Mean ± s.d. or Median and Range) |
|---|---|
| No. of patients | 155 |
| Sex | |
| Female | 59 (38.06%) |
| Male | 96 (61.94%) |
| Age (years) | 37 18-73) |
| Tumor side | |
| Left | 88 (56.77%) |
| Right | 67 (43.23%) |
| Tumor site | |
| Frontal | 50 (32.26%) |
| Parietal | 13 (8.39%) |
| Temporal | 24 (15.48%) |
| Insular | 65 (41.94%) |
| Occipital | 3 (1.93%) |
| Preoperative tumor volume computed on T2-weighted MRI images, cm3 | 48 (6–144) |
| Preoperative ΔT2T1 MRI index, cm3 | 12 (0-55) |
| Preoperative ΔVT2T1 MRI index | |
| <18 cm3 | 88 (56.67%) |
| ≥18 cm3 | 67 (43.23%) |
| EOR% | 88 (38–100) |
| EOR% | |
| 100 | 30 (19.36%) |
| 99–90 | 43 (27.74%) |
| 70–89 | 45 (29.03%) |
| ≤69 | 37 (23.87%) |
| Postoperative residual tumor volume computed on T2-weighted MRI images, cm3 | 10 (0–44) |
| Molecular class | |
| Oligodendroglioma IDH1/2 mutated 1p-19q codeleted | 44 (28.39%) |
| Astrocytoma IDH 1/2 mutated 1p-19q non codeleted | 93 (60.00%) |
| Astrocytoma IDH 1/2 wild type | 18 (11.61%) |
| MGMT Methylation status yes vs. no | 136 vs. 19 (87.74% vs. 12.26%) |
| Intraoperative protocol | |
| Awake surgery | 113 (72.90%) |
| General anesthesia | 42 (27.10%) |
| Time between seizure onset and surgery | 6 months (range 4–20 months) |
| Parameter | N (%) |
|---|---|
| Onset Seizure Features | |
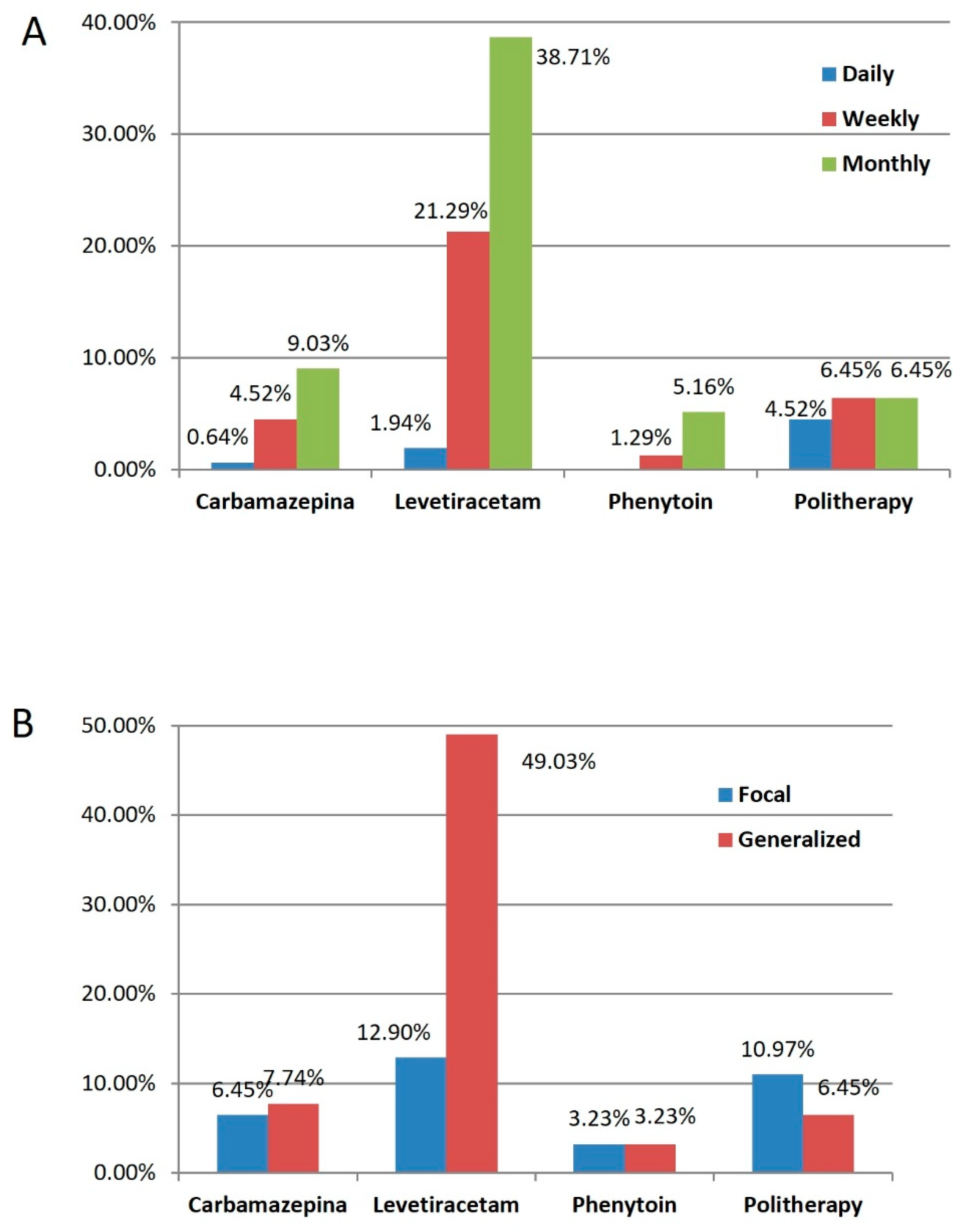
| Focal | 52 (33.55%) |
| Motor | 18 |
| Non motor sensory | 17 |
| Non motor emotional | 2 |
| Non motor cognitive | 11 |
| Non motor autonomic | 4 |
| Generalized | 103 (66.45%) |
| Motor | 76 |
| Focal to bilateral | 14 |
| Absence | 9 |
| Non motor cognitive | 2 |
| Non motor emotional | 1 |
| Non motor sensory | 1 |
| Seizure Frequency | |
| Monthly | 92 (59.35%) |
| Weekly | 52 (33.55%) |
| Daily | 11 (7.10%) |
| Duration | |
| <1 year | 133 (85.81%) |
| >1 year | 22 (14.19%) |
| Preoperative AEDs | |
| Levetiracetam | 96 (61.94%) |
| Polytherapy | 27 (17.42%) |
| Carbamazepine | 22 (14.19%) |
| Phenytoin | 10 (6.45%) |
| Postoperative Engel Class | |
| IA | 110 (70.97%) |
| IB, IC, ID | 16 (10.32%) |
| II, III | 23 (14.84%) |
| IV | 6 (3.87%) |
| Postoperative AEDs | |
| Levetiracetam | 105 (67.74%) |
| Polytherapy | 31 (20.00%) |
| Oxcarbamazepina | 6 (3.86%) |
| Carbamazepine | 5 (3.23%) |
| Valproic Acid | 5 (3.23%) |
| Lacosamide | 3 (1.94%) |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| Age (yrs) | 1.042 | 1.010–1.074 | 0.009 | 1.056 | 1.010–1.103 | 0.014 |
| Sex | ||||||
| Male | 1 | |||||
| Female | 1.456 | 0.718–2.950 | 0.297 | |||
| Tumor side | ||||||
| Left | 1 | |||||
| Right | 1.044 | 0.290–3.758 | 0.947 | |||
| Tumor Site | ||||||
| Pre-central | 1 | |||||
| Retro-central | 0.755 | 0.207–2.752 | 0.671 | |||
| Temporal | 0.497 | 0.159–1.552 | 0.229 | |||
| Insular | 0.723 | 0.328–1.591 | 0.421 | |||
| Onset seizure features | ||||||
| Generalized | 1 | |||||
| Focal | 1.057 | 0.324–2.267 | 0.013 | |||
| Seizure frequency | ||||||
| Monthly | 1 | |||||
| Weekly | 1.457 | 0.690–3.076 | 0.323 | |||
| Daily | 2.500 | 0.697–8.966 | 0.160 | |||
| Duration | ||||||
| <1 yr | 1 | |||||
| >1 yr | 0.857 | 0.324–2.267 | 0.756 | |||
| Preoperative tumor volume computed on T2-weighted images, cm3 | 1.116 | 1.069–1.185 | <0.0001 | |||
| ΔT2T1 MRI index | 1.156 | 1.066–1.195 | <0.0001 | 1.077 | 1.102–1.134 | 0.016 |
| Molecular Class | ||||||
| Astrocytoma IDH1/2 mutated 1p-19q non codeleted | 1 | |||||
| Astrocytoma IDH1/2 wild type | 0.430 | 0.154–1.200 | 0.107 | |||
| Oligodendroglioma IDH1/2 mutated 1p-19q codeleted | 0.222 | 0.669–0.747 | 0.014 | |||
| MGMT Methylation yes vs. no | 2.382 | 0.658–8.619 | 0.186 | |||
| % EOR Continuous variable | 0–929 | 0.903–0.955 | <0.0001 | 0.957 | 0.920–0.995 | 0.030 |
| Postoperative residual tumor volume computed on T2 weighted MRI images, cm3 | 1.057 | 0.324–2.267 | 0.001 | |||
| Authors | N of Cases | Age at Surgery (years) | Location | Histology | Preoperative Tumor Volume cm3 | EOR | Preoperative Seizures | Postoperative Seizures (Engel Class I Outcome) | IDH1/2 Mutation | 1p/19q Codeletion | MGMT Methylation | P53+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neal A et Al. 2018 [21] | 70 HGG and 30 LGG | 50.2 ± 17.5 | Frontal 48; occipital 1; parietal 11; temporal 26 | 70 HGG 20 A 10 O/OA | NA | 15 PR 42 ST 37 GTR 6 unknown | 52 cases (52%) | 58 cases (58%) | 35 cases (35%) | NA | NA | NA |
| Still M.E.H. et al. 2018 [16] | 346 LGG | 35.0 | Frontal 192, temporal 70, insular 41, parietal 27, other 16 | 48 A 298 O | NA | 100% 50 cases; 90%–99% 92 cases; 50%–89% 134 cases; <50% 70 cases | 346 cases (100%) | 227 cases (65.60%) | 19 (21 cases tested) (90.47%) | 65 (206 cases tested) (31.55%) | NA | NA |
| Xu DS et al. 2018 [17] | 128 LGG | 40.8 | Frontal 74, parietal 34, temporal 45, occipital 8, insular 17, deep 6 | 18 A 86 O 24 OA | 57,5 | 90%–99% 64 cases; 80%–89% 11 cases | 128 cases (100%) | 105 cases (82.03%) | NA | 25 cases (19.53%) | NA | NA |
| Chen H et al. 2017 [19] | 712 GLIOMA | 54 (60.7–53.4) | Temporal 191 non temporal 521 | 77 WHO II, 128 WHO III, 507 WHO IV | NA | NA | 276 cases (38.76%) | NA | 177 cases (16.43%) | 644 cases (90.44%) | NA | NA |
| Zhong Z. et al. 2015 [23] | 311 LGG | 38 | NA | 140 A 140 OA 31 O | NA | NA | 183 cases (58.84%) | 211 cases (67.84%) | 257 cases (82.63%) | NA | NA | NA |
| Yang Y. et al. 2015 [22] | 6 LGG 106 HGG | 34 (39.8–42.2) | 88 frontal; 74 temporal; 45 parietal; 11 occipital; 17 insular | 64 WHO II 58 WHO III 48 WHO IV | 4.7 cm (5.6–6.4 cm) | NA | 74 cases (42.3%) | NA | 41 WHO II cases (64.0%); 33 WHO III cases (56.8%); 10 WHO IV cases (20.8%) | NA | NA | 24 WHO II cases (37.5%); 28 WHO III cases (48.2%); 25 WHO IV cases (52.0%) |
| Ius et al. 2014 [15] | 52 LGG | 38.73 | Insula; left 36, right 16 | 32 A 11 OA 9 O | 75.42 | 87% >90% 21 cases 70–89% 23 cases <70% 8 cases | NA | 35 cases (67.30%) | NA | NA | NA | NA |
| Mulligan L. et al. 2014 [20] | 62 LGG | NA | NA | 62 O | 4 groups: 45 mm 46 mm 56 mm 37.5 mm | NA | 48 cases (77.41%) | NA | 48 cases (77.41%) | 39 cases (62.90%) | NA | 24 cases (38.70%) |
| Liubinas SV et al. 2014 [8] | 30 LGG | 35.4 years | NA | 22 A 6 OA 1 mixed OA and protoplasmic astrocytoma 1 O | 4 groups: 45 mm, 46 mm, 56 mm, 37.5 mm | NA | 23 cases (76.66%) | NA | 17 cases (56.66% | NA | NA | NA |
| Pallud J et al. 2014 [7] | 1509 LGG | <30 yrs = 390 cases, 30–45 yrs = 726 cases | NA | 327 A 781 OA 280 mixed glioma 121 missing | NA | <100 cm3 808 cases (53.54%), >100 cm3 346 cases (22.92%), missing cases 355 (23,54) | NA | NA | NA | NA | NA | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ius, T.; Pauletto, G.; Tomasino, B.; Maieron, M.; Budai, R.; Isola, M.; Cesselli, D.; Lettieri, C.; Skrap, M. Predictors of Postoperative Seizure Outcome in Low Grade Glioma: From Volumetric Analysis to Molecular Stratification. Cancers 2020, 12, 397. https://doi.org/10.3390/cancers12020397
Ius T, Pauletto G, Tomasino B, Maieron M, Budai R, Isola M, Cesselli D, Lettieri C, Skrap M. Predictors of Postoperative Seizure Outcome in Low Grade Glioma: From Volumetric Analysis to Molecular Stratification. Cancers. 2020; 12(2):397. https://doi.org/10.3390/cancers12020397
Chicago/Turabian StyleIus, Tamara, Giada Pauletto, Barbara Tomasino, Marta Maieron, Riccardo Budai, Miriam Isola, Daniela Cesselli, Christian Lettieri, and Miran Skrap. 2020. "Predictors of Postoperative Seizure Outcome in Low Grade Glioma: From Volumetric Analysis to Molecular Stratification" Cancers 12, no. 2: 397. https://doi.org/10.3390/cancers12020397
APA StyleIus, T., Pauletto, G., Tomasino, B., Maieron, M., Budai, R., Isola, M., Cesselli, D., Lettieri, C., & Skrap, M. (2020). Predictors of Postoperative Seizure Outcome in Low Grade Glioma: From Volumetric Analysis to Molecular Stratification. Cancers, 12(2), 397. https://doi.org/10.3390/cancers12020397





