How to Classify Pituitary Neuroendocrine Tumors (PitNET)s in 2020
Abstract
:1. Introduction
2. From Adenoma to Pituitary Neuroendocrine Tumor (PitNET): Evolution Not Revolution
3. Clinical and Pathological Classifications of PitNETs
3.1. Clinical Classifications
3.2. Neuroimaging Classification
Tumor Size and Invasion
3.3. Morphological and Functional Classification
3.3.1. Previous Classifications
3.3.2. WHO 2004 Classification
3.3.3. Morphological and Functional Classification in 2020
3.3.4. Lineage-Restricted Transcription Factors
3.3.5. Morphological Types and Subtypes
3.3.6. Plurihormonal Tumors
3.3.7. Immunonegative Type Rather Than “Null Cell Adenoma”
3.3.8. Difficult Differential Diagnosis
3.3.9. Prognostic Classifications
“Atypical Adenoma” and “High Risk Adenomas” of the WHO 2004 and 2017 Classifications
The French Five-Tiered Prognostic ClassificationCharacteristics of This Classification
Comments on the Criteria of the Grading
Prognostic Value of the Five-Tiered Classification
4. Aggressive Tumor and Pituitary Carcinoma
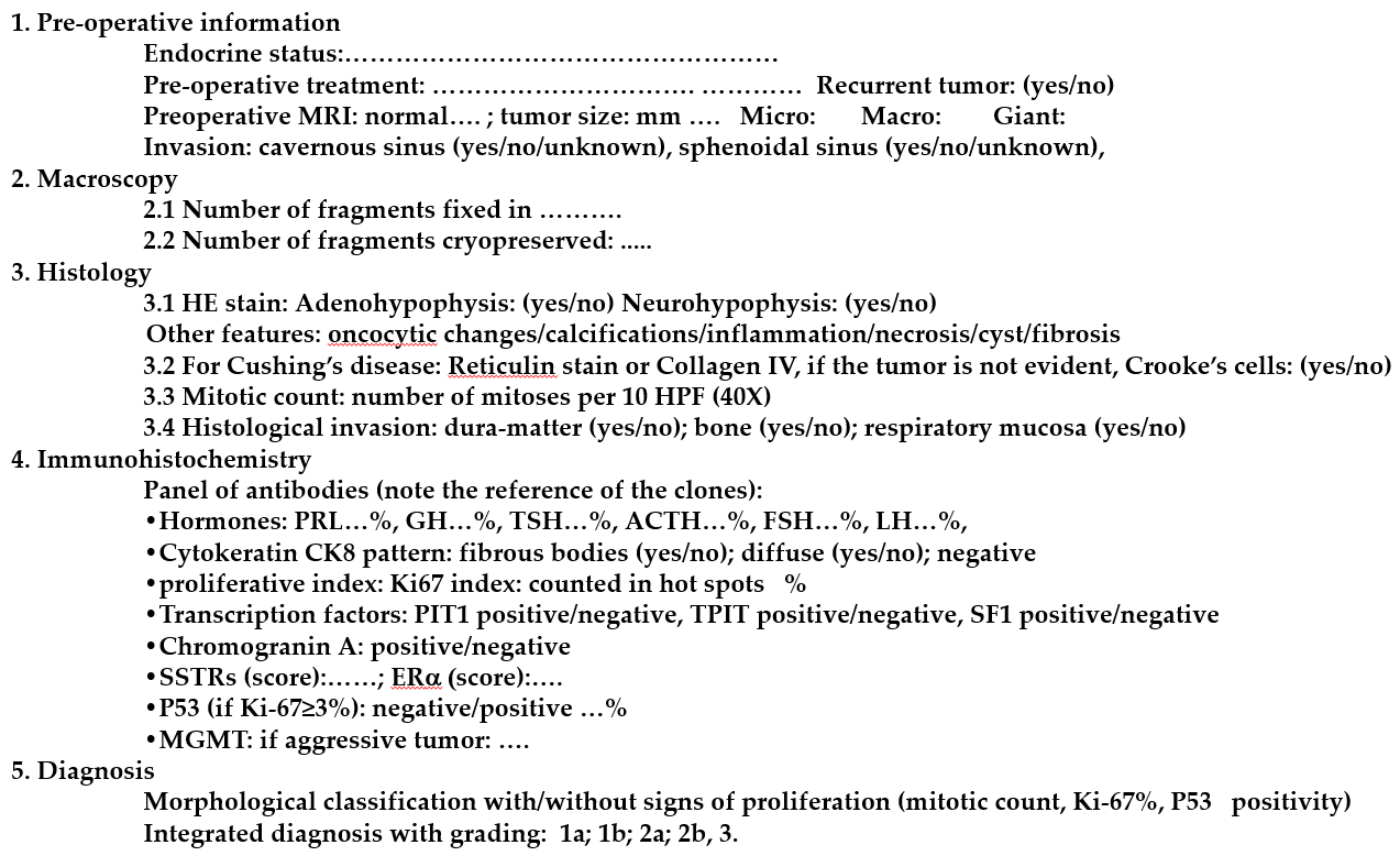
5. Standardized Histological Report of Pituitary Tumors
6. Conclusions
Funding
Conflicts of Interest
References
- Osamura, R.Y.; Grossman, A.; Korbonits, M.; Kovacs, K.; Lopes, M.B.S.; Matsuno, A.; Trouillas, J. Pituitary adenoma. In WHO Classification of Tumours of Endocrine Organs; Chapter 1: Tumors of the Pituitary Gland; WHO: Lyon, France, 2017; pp. 14–18. [Google Scholar]
- McCormack, A.; Dekkers, O.M.; Petersenn, S.; Popovic, V.; Trouillas, J.; Raverot, G.; Burman, P. ESE survey collaborators Treatment of aggressive pituitary tumours and carcinomas: Results of a European Society of Endocrinology (ESE) survey 2016. Eur. J. Endocrinol. 2018, 178, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouillas, J.; Burman, P.; McCormack, A.; Petersenn, S.; Popovic, V.; Dekkers, O.; Raverot, G. Aggressive pituitary tumours and carcinomas: Two sides of the same coin? Eur. J. Endocrinol. 2018, 178, C7–C9. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.Y.; Fleseriu, M.; Wass, J.; van der Lely, A.; Barkan, A.; Giustina, A.; Casanueva, F.F.; Heaney, A.P.; Biermasz, N.; Strasburger, C.; et al. A tale of pituitary adenomas: To NET or not to NET: Pituitary Society position statement. Pituitary 2019, 22, 569–573. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Nickel, B.; Semsarian, C.; Moynihan, R.; Barratt, A.; Jordan, S.; McLeod, D.; Brito, J.P.; McCaffery, K. Public perceptions of changing the terminology for low-risk thyroid cancer: A qualitative focus group study. BMJ Open 2019, 9, e025820. [Google Scholar] [CrossRef]
- Manojlovic-Gacic, E.; Engström, B.E.; Casar-Borota, O. Histopathological classification of non-functioning pituitary neuroendocrine tumors. Pituitary 2018, 21, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Drummond, J.; Roncaroli, F.; Grossman, A.B.; Korbonits, M. Clinical and Pathological Aspects of Silent Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 2473–2489. [Google Scholar] [CrossRef] [Green Version]
- Castinetti, F.; Dufour, H.; Gaillard, S.; Jouanneau, E.; Vasiljevic, A.; Villa, C.; Trouillas, J. Non-functioning pituitary adenoma: When and how to operate? What pathologic criteria for typing? Ann. Endocrinol. 2015, 76, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Raverot, G.; Wierinckx, A.; Jouanneau, E.; Auger, C.; Borson-Chazot, F.; Lachuer, J.; Pugeat, M.; Trouillas, J. Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing’s disease. Eur. J. Endocrinol. 2010, 163, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Chinezu, L.; Vasiljevic, A.; Trouillas, J.; Lapoirie, M.; Jouanneau, E.; Raverot, G. Silent somatotroph tumour revisited from a study of 80 patients with and without acromegaly and a review of the literature. Eur. J. Endocrinol. 2017, 176, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Capraru, O.M.; Gaillard, C.; Vasiljevic, A.; Lasolle, H.; Borson-Chazot, F.; Raverot, V.; Jouanneau, E.; Trouillas, J.; Raverot, G. Diagnosis, pathology, and management of TSH-secreting pituitary tumors. A single-center retrospective study of 20 patients from 1981 to 2014. Ann. Endocrinol. 2019, 80, 216–224. [Google Scholar]
- Mete, O.; Gomez-Hernandez, K.; Kucharczyk, W.; Ridout, R.; Zadeh, G.; Gentili, F.; Ezzat, S.; Asa, S.L. Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod. Pathol. 2016, 29, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, F.; Webb, S.M. Atypical pituitary adenomas: 10 years of experience in a reference centre in Portugal. Neurologia 2016, 31, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jaffrain-Rea, M.L.; Di Stefano, D.; Minniti, G.; Esposito, V.; Bultrini, A.; Ferretti, E.; Santoro, A.; Faticanti Scucchi, L.; Gulino, A.; Cantore, G. A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: Secreting versus non-secreting adenomas. Endocr. Relat. Cancer 2002, 9, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meij, B.P.; Lopes, M.-B.S.; Ellegala, D.B.; Alden, T.D.; Laws, E.R. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J. Neurosurg. 2002, 96, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Woodmansee, W.W.; Ramkissoon, S.; Amadio, J.; Nose, V.; Laws, E.R. Atypical pituitary adenomas: Incidence, clinical characteristics, and implications. J. Neurosurg. 2011, 114, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.-F.; Assaker, R.; Auger, C.; Brue, T.; et al. A new prognostic clinicopathological classification of pituitary adenomas: A multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013, 126, 123–135. [Google Scholar]
- Micko, A.S.G.; Wöhrer, A.; Wolfsberger, S.; Knosp, E. Invasion of the cavernous sinus space in pituitary adenomas: Endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 2015, 122, 803–811. [Google Scholar]
- Amar, A.P.; Hinton, D.R.; Krieger, M.D.; Weiss, M.H. Invasive pituitary adenomas: Significance of proliferation parameters. Pituitary 1999, 2, 117–122. [Google Scholar] [CrossRef]
- Raverot, G.; Dantony, E.; Beauvy, J.; Vasiljevic, A.; Mikolasek, S.; Borson-Chazot, F.; Jouanneau, E.; Roy, P.; Trouillas, J. Risk of Recurrence in Pituitary Neuroendocrine Tumors: A Prospective Study Using a Five-Tiered Classification. J. Clin. Endocrinol. Metab. 2017, 102, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Cortet-Rudelli, C.; Bonneville, J.-F.; Borson-Chazot, F.; Clavier, L.; Coche Dequéant, B.; Desailloud, R.; Maiter, D.; Rohmer, V.; Sadoul, J.L.; Sonnet, E.; et al. Post-surgical management of non-functioning pituitary adenoma. Ann. Endocrinol. 2015, 76, 228–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asa, S.L.; Kovacs, K. Histological classification of pituitary disease. Clin. Endocrinol. Metab. 1983, 12, 567–596. [Google Scholar] [CrossRef]
- Trouillas, J.; Cure, M.; Lheritier, M.; Guichard, Y.; Girod, C. Attempted at a cytofunctional classification of 19 pituitary adenomas using histological, immunocytochemical, biometric and ultrastructural data. Rev. D’oto-Neuro-Ophtalmologie 1974, 46, 223–236. [Google Scholar]
- Trouillas, J.; Girod, C. Pathology of pituitary adenomas. In Pituitary Adenomas; Landolt, A.M., Vance, M.L., Reilly, P.L., Eds.; New-York Publishers: New York, NY, USA, 1996; pp. 27–46. [Google Scholar]
- Snyder, P.J. Gonadotroph cell adenomas of the pituitary. Endocr. Rev. 1985, 6, 552–563. [Google Scholar] [CrossRef]
- Trouillas, J.; Girod, C.; Sassolas, G.; Claustrat, B.; Lhéritier, M.; Dubois, M.P.; Goutelle, A. Human pituitary gonadotropic adenoma; histological, immunocytochemical, and ultrastructural and hormonal studies in eight cases. J. Pathol. 1981, 135, 315–336. [Google Scholar] [CrossRef]
- Trouillas, J.; Girod, C.; Sassolas, G.; Claustrat, B. The human gonadotropic adenoma: Pathologic diagnosis and hormonal correlations in 26 tumors. Semin. Diagn. Pathol. 1986, 3, 42–57. [Google Scholar]
- Kovacs, K.; Horvath, E.; Ryan, N.; Ezrin, C. Null cell adenoma of the human pituitary. Virchows Arch. A Pathol. Anat. Histol. 1980, 387, 165–174. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Brucker-Davis, F.; Persani, L.; Smallridge, R.C.; Weintraub, B.D. Thyrotropin-secreting pituitary tumors. Endocr. Rev. 1996, 17, 610–638. [Google Scholar]
- Bertholon-Grégoire, M.; Trouillas, J.; Guigard, M.P.; Loras, B.; Tourniaire, J. Mono- and plurihormonal thyrotropic pituitary adenomas: Pathological, hormonal and clinical studies in 12 patients. Eur. J. Endocrinol. 1999, 140, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, R.V.; Kovacs, K.; Young, W.F. Pitutary tumours: Introduction. In WHO Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs; DeLellis, R.A., Lloyd, R.V., Heitz, P.U., Eng, C., Eds.; Chapter 1: Tumours of Pituitary; WHO: Lyon, France, 2004; pp. 10–13. [Google Scholar]
- Asa, S.L.; Bamberger, A.M.; Cao, B.; Wong, M.; Parker, K.L.; Ezzat, S. The transcription activator steroidogenic factor-1 is preferentially expressed in the human pituitary gonadotroph. J. Clin. Endocrinol. Metab. 1996, 81, 2165–2170. [Google Scholar] [PubMed] [Green Version]
- Sanno, N.; Teramoto, A.; Sugiyama, M.; Matsuno, A.; Takumi, I.; Tahara, S.; Osamura, R.Y. Expression of Pit-1 mRNA and activin/inhibin subunits in clinically nonfunctioning pituitary adenomas. In situ hybridization and immunohistochemical analysis. Horm. Res. 1998, 50, 11–17. [Google Scholar] [PubMed]
- Umeoka, K.; Sanno, N.; Osamura, R.Y.; Teramoto, A. Expression of GATA-2 in human pituitary adenomas. Mod. Pathol. 2002, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Inoshita, N.; Mete, O.; Asa, S.L.; Hayashi, K.; Takeshita, A.; Fukuhara, N.; Yamaguchi-Okada, M.; Takeuchi, Y.; Yamada, S. The Complementary Role of Transcription Factors in the Accurate Diagnosis of Clinically Nonfunctioning Pituitary Adenomas. Endocr. Pathol. 2015, 26, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Kefeli, M.; Çalışkan, S.; Asa, S.L. GATA3 immunoreactivity expands the transcription factor profile of pituitary neuroendocrine tumors. Mod. Pathol. 2019, 32, 484–489. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Bollerslev, J.; Mulder, J.; Lindskog, C.; Pontén, F.; Casar-Borota, O. A specific antibody to detect transcription factor T-Pit: A reliable marker of corticotroph cell differentiation and a tool to improve the classification of pituitary neuroendocrine tumours. Acta Neuropathol. 2017, 134, 675–677. [Google Scholar] [CrossRef]
- McDonald, W.C.; Banerji, N.; McDonald, K.N.; Ho, B.; Macias, V.; Kajdacsy-Balla, A. Steroidogenic Factor 1, Pit-1, and Adrenocorticotropic Hormone: A Rational Starting Place for the Immunohistochemical Characterization of Pituitary Adenoma. Arch. Pathol. Lab. Med. 2017, 141, 104–112. [Google Scholar] [CrossRef]
- Villa, C.; Vasiljevic, A.; Jaffrain-Rea, M.L.; Ansorge, O.; Asioli, S.; Barresi, V.; Chinezu, L.; Gardiman, M.P.; Lania, A.; Lapshina, A.M.; et al. A standardised diagnostic approach to pituitary neuroendocrine tumours (PitNETs): A European Pituitary Pathology Group (EPPG) proposal. Virchows Arch. 2019, 475, 687–692. [Google Scholar] [CrossRef]
- Delgrange, E.; Vasiljevic, A.; Wierinckx, A.; François, P.; Jouanneau, E.; Raverot, G.; Trouillas, J. Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. Eur. J. Endocrinol. 2015, 172, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Obari, A.; Sano, T.; Ohyama, K.; Kudo, E.; Qian, Z.R.; Yoneda, A.; Rayhan, N.; Mustafizur Rahman, M.; Yamada, S. Clinicopathological features of growth hormone-producing pituitary adenomas: Difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr. Pathol. 2008, 19, 82–91. [Google Scholar] [CrossRef]
- Casar-Borota, O.; Heck, A.; Schulz, S.; Nesland, J.M.; Ramm-Pettersen, J.; Lekva, T.; Alafuzoff, I.; Bollerslev, J. Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and long-term effects of octreotide. J. Clin. Endocrinol. Metab. 2013, 98, E1730–E1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinezu, L.; Vasiljevic, A.; Jouanneau, E.; François, P.; Borda, A.; Trouillas, J.; Raverot, G. Expression of somatostatin receptors, SSTR2A and SSTR5, in 108 endocrine pituitary tumors using immunohistochemical detection with new specific monoclonal antibodies. Hum. Pathol. 2014, 45, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Feelders, R.A.; van der Pas, R.; Kros, J.M.; Waaijers, M.; Sprij-Mooij, D.; Neggers, S.J.C.M.M.; van der Lelij, A.-J.; Minuto, F.; Lamberts, S.W.J.; et al. Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J. Clin. Endocrinol. Metab. 2013, 98, E66–E71. [Google Scholar] [CrossRef] [Green Version]
- Osamura, R.Y.; Grossman, A.; Nishioka, H.; Trouillas, J. Thyrotroph adenoma. Tumors of pituitary gland. In WHO Classifiaction of Tumours of Endocrine Organs; IARC: Lyon, France, 2017; pp. 28–29. [Google Scholar]
- Horvath, E.; Kovacs, K.; Smyth, H.S.; Cusimano, M.; Singer, W. Silent adenoma subtype 3 of the pituitary--immunohistochemical and ultrastructural classification: A review of 29 cases. Ultrastruct. Pathol. 2005, 29, 511–524. [Google Scholar] [CrossRef]
- Kageyama, K.; Ikeda, H.; Nigawara, T.; Sakihara, S.; Suda, T. Expression of adrenocorticotropic hormone, prolactin and transcriptional factors in clinically nonfunctioning pituitary adenoma. Endocr. J. 2007, 54, 961–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca, E.; Mattogno, P.P.; Porcelli, T.; Poliani, L.; Belotti, F.; Schreiber, A.; Maffezzoni, F.; Fontanella, M.M.; Doglietto, F. Plurihormonal ACTH-GH Pituitary Adenoma: Case Report and Systematic Literature Review. World Neurosurg. 2018, 114, e158–e164. [Google Scholar] [CrossRef]
- Balogun, J.A.; Monsalves, E.; Juraschka, K.; Parvez, K.; Kucharczyk, W.; Mete, O.; Gentili, F.; Zadeh, G. Null cell adenomas of the pituitary gland: An institutional review of their clinical imaging and behavioral characteristics. Endocr. Pathol. 2015, 26, 63–70. [Google Scholar] [CrossRef]
- Almeida, J.P.; Stephens, C.C.; Eschbacher, J.M.; Felicella, M.M.; Yuen, K.C.J.; White, W.L.; Mooney, M.A.; Bernat, A.L.; Mete, O.; Zadeh, G.; et al. Clinical, pathologic, and imaging characteristics of pituitary null cell adenomas as defined according to the 2017 World Health Organization criteria: A case series from two pituitary centers. Pituitary 2019, 22, 514–519. [Google Scholar] [CrossRef]
- Horvath, E.; Kovacs, K.; Singer, W.; Smyth, H.S.; Killinger, D.W.; Erzin, C.; Weiss, M.H. Acidophil stem cell adenoma of the human pituitary: Clinicopathologic analysis of 15 cases. Cancer 1981, 47, 761–771. [Google Scholar] [CrossRef]
- Mete, O.; Korbonits, M.; Trouillas, J.; Yamada, S. Somatotroph adenoma. In WHO Classification of Tumours of Endocrine Organs; Chapter 1: Tumors of the Pituitary Gland; WHO: Lyon, France, 2017; pp. 19–23. [Google Scholar]
- Nose, V.; Grossman, A.; Mete, O. Lactotroph Adenoma; Lloyd, R.V., Osamura, R.Y., Klöppel, G., Rosai, J., Eds.; Chapter 1: Tumours of Pituitary Gland; IARC: Lyon, France, 2017; pp. 24–27. [Google Scholar]
- Dénes, J.; Swords, F.; Rattenberry, E.; Stals, K.; Owens, M.; Cranston, T.; Xekouki, P.; Moran, L.; Kumar, A.; Wassif, C.; et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: Results from a large patient cohort. J. Clin. Endocrinol. Metab. 2015, 100, E531–E541. [Google Scholar] [CrossRef]
- Kovacs, K.; Horvath, E. Pituitary “chromophobe” adenoma composed of oncocytes. A light and electron microscopic study. Arch. Pathol. 1973, 95, 235–239. [Google Scholar] [PubMed]
- Trouillas, J.; Lheritier, M.; Cure, M.; Girod, C.; Guinet, P.; Tommasi, M.; Goutelle, A. Allegre GE L’oncocytome hypophysaire est-il une entité ? Discussion anatomoclinique à propos de 3 observations personnelles. Lyon Méd. 1975, 234, 25–35. [Google Scholar]
- Kaltsas, G.A.; Kolomodi, D.; Randeva, H.; Grossman, A. Nonneuroendocrine Neoplasms of the Pituitary Region. J. Clin. Endocrinol. Metab. 2019, 104, 3108–3123. [Google Scholar] [CrossRef]
- Hyrcza, M.D.; Ezzat, S.; Mete, O.; Asa, S.L. Pituitary Adenomas Presenting as Sinonasal or Nasopharyngeal Masses: A Case Series Illustrating Potential Diagnostic Pitfalls. Am. J. Surg. Pathol. 2017, 41, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Tihan, T.; Scheithauer, B.W.; Zhang, P.J.; Gonatas, N.K. Thyroid transcription factor 1 expression in sellar tumors: A histogenetic marker? J. Neuropathol. Exp. Neurol. 2009, 68, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Mete, O.; Lopes, M.B.; Asa, S.L. Spindle cell oncocytomas and granular cell tumors of the pituitary are variants of pituicytoma. Am. J. Surg. Pathol. 2013, 37, 1694–1699. [Google Scholar] [CrossRef]
- Casar-Borota, O.; Botling, J.; Granberg, D.; Stigare, J.; Wikström, J.; Boldt, H.B.; Kristensen, B.W.; Pontén, F.; Trouillas, J. Serotonin, ATRX, and DAXX Expression in Pituitary Adenomas: Markers in the Differential Diagnosis of Neuroendocrine Tumors of the Sellar Region. Am. J. Surg. Pathol. 2017, 41, 1238–1246. [Google Scholar] [CrossRef]
- Abushamat, L.A.; Kerr, J.M.; Lopes, M.B.S.; Kleinschmidt-DeMasters, B.K. Very Unusual Sellar/Suprasellar Region Masses: A Review. J. Neuropathol. Exp. Neurol. 2019. [Google Scholar] [CrossRef]
- Santos-Pinheiro, F.; Penas-Prado, M.; Kamiya-Matsuoka, C.; Waguespack, S.G.; Mahajan, A.; Brown, P.; Shah, K.; Fuller, G.N.; Mccutcheon, I. Treatment and long-term outcomes in pituitary carcinoma: A cohort study. Eur. J. Endocrinol. 2019, 181, 397–407. [Google Scholar] [CrossRef]
- Miermeister, C.P.; Petersenn, S.; Buchfelder, M.; Fahlbusch, R.; Lüdecke, D.K.; Hölsken, A.; Bergmann, M.; Knappe, H.U.; Hans, V.H.; Flitsch, J.; et al. Histological criteria for atypical pituitary adenomas - data from the German pituitary adenoma registry suggests modifications. Acta Neuropathol. Commun. 2015, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Chiloiro, S.; Doglietto, F.; Trapasso, B.; Iacovazzo, D.; Giampietro, A.; Di Nardo, F.; De Waure, C.; Lauriola, L.; Mangiola, A.; Anile, C.; et al. Typical and atypical pituitary adenomas: A single-center analysis of outcome and prognosis. Neuroendocrinology 2015, 101, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Saeger, W.; Lüdecke, D.K.; Buchfelder, M.; Fahlbusch, R.; Quabbe, H.-J.; Petersenn, S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur. J. Endocrinol. 2007, 156, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.E.; Divanlioglu, D.; Nacar, O.A.; Dursun, E.; Sahinoglu, M.; Unal, T.; Belen, A.D. Incidence, hormonal distribution and postoperative follow up of atypical pituitary adenomas. Turk. Neurosurg. 2013, 23, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Del Basso De Caro, M.; Solari, D.; Pagliuca, F.; Villa, A.; Guadagno, E.; Cavallo, L.M.; Colao, A.; Pettinato, G.; Cappabianca, P. Atypical pituitary adenomas: Clinical characteristics and role of ki-67 and p53 in prognostic and therapeutic evaluation. A series of 50 patients. Neurosurg. Rev. 2017, 40, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Fountas, A.; Lavrentaki, A.; Subramanian, A.; Toulis, K.A.; Nirantharakumar, K.; Karavitaki, N. Recurrence in silent corticotroph adenomas after primary treatment: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2018, 21. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Kovacs, K.; Killinger, D.W.; Smyth, H.S.; Platts, M.E.; Singer, W. Silent corticotropic adenomas of the human pituitary gland: A histologic, immunocytologic, and ultrastructural study. Am. J. Pathol. 1980, 98, 617–638. [Google Scholar] [PubMed]
- Langlois, F.; Lim, D.S.T.; Yedinak, C.G.; Cetas, I.; McCartney, S.; Cetas, J.; Dogan, A.; Fleseriu, M. Predictors of silent corticotroph adenoma recurrence; a large retrospective single center study and systematic literature review. Pituitary 2018, 21, 32–40. [Google Scholar] [CrossRef]
- Bradley, K.J.; Wass, J.A.H.; Turner, H.E. Non-functioning pituitary adenomas with positive immunoreactivity for ACTH behave more aggressively than ACTH immunonegative tumours but do not recur more frequently. Clin. Endocrinol. 2003, 58, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Karavitaki, N.; Ansorge, O.; Wass, J.A.H. Silent corticotroph adenomas. Arq. Bras. Endocrinol. Metabol. 2007, 51, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Kovács, G.L.; Góth, M.; Rotondo, F.; Scheithauer, B.W.; Carlsen, E.; Saadia, A.; Hubina, E.; Kovács, L.; Szabolcs, I.; Nagy, P.; et al. ACTH-secreting Crooke cell carcinoma of the pituitary. Eur. J. Clin. Investig. 2013, 43, 20–26. [Google Scholar] [CrossRef]
- Asioli, S.; Righi, A.; Iommi, M.; Baldovini, C.; Ambrosi, F.; Guaraldi, F.; Zoli, M.; Mazzatenta, D.; Faustini-Fustini, M.; Rucci, P.; et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: Retrospective analysis on 566 patients from a tertiary care centre. Eur. J. Endocrinol. 2019, 180, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kiseljak-Vassiliades, K.; Carlson, N.E.; Borges, M.T.; Kleinschmidt-DeMasters, B.K.; Lillehei, K.O.; Kerr, J.M.; Wierman, M.E. Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine 2015, 49, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Langlois, F.; Woltjer, R.; Cetas, J.S.; Fleseriu, M. Silent somatotroph pituitary adenomas: An update. Pituitary 2018, 21, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Delgrange, E.; Wierinckx, A.; Vasiljevic, A.; Jouanneau, E.; Burman, P.; Raverot, G. Clinical, Pathological, and Molecular Factors of Aggressiveness in Lactotroph Tumours. Neuroendocrinology 2019, 109, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Jouanneau, E.; Trouillas, J. Management of endocrine disease: Clinicopathological classification and molecular markers of pituitary tumours for personalized therapeutic strategies. Eur. J. Endocrinol. 2014, 170, R121–R132. [Google Scholar] [CrossRef] [Green Version]
- Trouillas, J. In search of a prognostic classification of endocrine pituitary tumors. Endocr. Pathol. 2014, 25, 124–132. [Google Scholar] [CrossRef]
- Raverot, G.; Vasiljevic, A.; Jouanneau, E.; Trouillas, J. A prognostic clinicopathologic classification of pituitary endocrine tumors. Endocrinol. Metab. Clin. N. Am. 2015, 44, 11–18. [Google Scholar] [CrossRef]
- Vasiljevic, A.; Jouanneau, E.; Trouillas, J.; Raverot, G. Clinicopathological prognostic and theranostic markers in pituitary tumors. Minerva Endocrinol. 2016, 41, 377–389. [Google Scholar] [PubMed]
- Lelotte, J.; Mourin, A.; Fomekong, E.; Michotte, A.; Raftopoulos, C.; Maiter, D. Both invasiveness and proliferation criteria predict recurrence of non-functioning pituitary macroadenomas after surgery: A retrospective analysis of a monocentric cohort of 120 patients. Eur. J. Endocrinol. 2018, 178, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef]
- Thapar, K.; Kovacs, K.; Scheithauer, B.W.; Stefaneanu, L.; Horvath, E.; Pernicone, P.J.; Murray, D.; Laws, E.R. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery 1996, 38, 99–106. [Google Scholar] [CrossRef]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 protein as a tumour proliferation marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; La Rosa, S. Correction to: Ki67 labeling index: Assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018, 472, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gejman, R.; Swearingen, B.; Hedley-Whyte, E.T. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum. Pathol. 2008, 39, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, B.W.; Gaffey, T.A.; Lloyd, R.V.; Sebo, T.J.; Kovacs, K.T.; Horvath, E.; Yapicier, O.; Young, W.F.; Meyer, F.B.; Kuroki, T.; et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 2006, 59, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Agati, P.; Sisto, A.; Frank, G.; Faustini-Fustini, M.; Agati, R.; Mazzatenta, D.; Farnedi, A.; Menetti, F.; Marucci, G.; et al. A classification tree approach for pituitary adenomas. Hum. Pathol. 2012, 43, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Di Ieva, A.; Rotondo, F.; Syro, L.V.; Cusimano, M.D.; Kovacs, K. Aggressive pituitary adenomas—Diagnosis and emerging treatments. Nat. Rev. Endocrinol. 2014, 10, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M. European Society of Endocrinology European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Delgrange, E.; Raverot, G.; Bex, M.; Burman, P.; Decoudier, B.; Devuyst, F.; Feldt-Rasmussen, U.; Andersen, M.; Maiter, D. Giant prolactinomas in women. Eur. J. Endocrinol. 2014, 170, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Raverot, G.; Castinetti, F.; Jouanneau, E.; Morange, I.; Figarella-Branger, D.; Dufour, H.; Trouillas, J.; Brue, T. Pituitary carcinomas and aggressive pituitary tumours: Merits and pitfalls of temozolomide treatment. Clin. Endocrinol. 2012, 76, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Roncaroli, F.; Kovacs, K.; Lloyd, R.V.; Matsuno, A.; Righi, A. Pituitary carcinoma. In WHO Classification of Tumours of Endocrine Organs; Lloyd, R.V., Osamura, R.Y., Klöpel, G., Rosai, J., Eds.; Chapter 1: Tumours of Pituitary Gland; IARC: Lyon, France, 2017; pp. 41–44. [Google Scholar]
- Dudziak, K.; Honegger, J.; Bornemann, A.; Horger, M.; Müssig, K. Pituitary carcinoma with malignant growth from first presentation and fulminant clinical course--case report and review of the literature. J. Clin. Endocrinol. Metab. 2011, 96, 2665–2669. [Google Scholar] [CrossRef] [Green Version]
- Alshaikh, O.M.; Asa, S.L.; Mete, O.; Ezzat, S. An Institutional Experience of Tumor Progression to Pituitary Carcinoma in a 15-Year Cohort of 1055 Consecutive Pituitary Neuroendocrine Tumors. Endocr. Pathol. 2019, 30, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, G.A.; Nomikos, P.; Kontogeorgos, G.; Buchfelder, M.; Grossman, A.B. Clinical review: Diagnosis and management of pituitary carcinomas. J. Clin. Endocrinol. Metab. 2005, 90, 3089–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncaroli, F.; Scheithauer, B.W.; Horvath, E.; Erickson, D.; Tam, C.K.; Lloyd, R.V.; Kovacs, K. Silent subtype 3 carcinoma of the pituitary: A case report. Neuropathol. Appl. Neurobiol. 2010, 36, 90–94. [Google Scholar] [PubMed]
- Zemmoura, I.; Wierinckx, A.; Vasiljevic, A.; Jan, M.; Trouillas, J.; François, P. Aggressive and malignant prolactin pituitary tumors: Pathological diagnosis and patient management. Pituitary 2013, 16, 515–522. [Google Scholar] [CrossRef]
- Jaffrain-Rea, M.L.; Rotondi, S.; Alesse, E. New Insights in the Pathogenesis of Pituitary Tomours. In Hot Topics in Endocrine and Endocrine-Related Diseases; Fedele, M., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1080-4. [Google Scholar] [CrossRef]

| Adenoma Types | Immunophenotypes | Transcription Factors | |
|---|---|---|---|
| Somatrotroph Adenoma | Densely granulated adenoma | GH ± PRL ± α-su LMW CK: diffuse | Pit-1 |
| Sparsely granulated adenoma | GH ± PRL LMW CK: fibrous bodies | Pit-1 | |
| Mammosomatotroph adenoma | GH + PRL (in same cell) ± α-su | Pit-1, ERɑ | |
| Mixed Somatotroph-Lactotroph adenoma | GH + PRL (in different cells) ± α-su | Pit-1, ERɑ | |
| Lactotroph Adenoma | Sparsely granulated adenoma | PRL | Pit-1, ERɑ |
| Densely granulated adenoma | PRL | Pit-1, ERɑ | |
| Acidophilic stem cell adenoma | PRL, GH (focal and inconstant) | ||
| Thyrotroph Adenoma | βTSH, α-su | Pit-1, GATA2 | |
| Corticotroph Adenoma | Densely granulated adenoma | ACTH, LMW CK: diffuse pattern | Tpit |
| Sparsely granulated adenoma | ACTH, LMW CK: diffuse pattern | Tpit | |
| Crooke’s cell adenoma | ACTH, LMW CK: ring-like pattern | Tpit | |
| Gonadotroph adenoma | Sparsely granulated | βFSH, βLH, α-su (various combinations) | SF-1, GATA2, ERɑ (variable) |
| Null cell adenoma | None | None | |
| Plurihormonal adenomas | Pit-1 positive plurihormonal adenoma (previously termed Silent subtype 3 adenoma) | GH, PRL, βTSH ± α-su | Pit-1 |
| Adenomas with unusual immunohistochemical combinations | Various combinations | ||
| Double adenomas | Distinct adenomas | Usually PRL and ACTH adenomas | Pit-1 and Tpit, respectively |
| Secretion | Immunophenotype | Type | |
|---|---|---|---|
| Functioning | Acromegaly + Hyperthyroidism | GH±TSH ±PRL | Somatotroph plurihormonal |
| Hyperthyroidism | TSH ±GH±PRL | Thyrotroph plurihormonal | |
| Variable | PRL-ACTH * | Double/triple tumors | |
| Non functioning | No secretion | GH-TSH-PRL (Scarcely IR cells) | Silent plurihormonal/Poorly differentiated PIT1 |
| Tumor Types | Frequency % | Cytological Aspects | Immunophenotypes | Transcription Factors and Receptors |
|---|---|---|---|---|
| Densely granulated | GH ± PRL ± α-subunit | PIT1, SSTR2-3-5 | ||
| LMW CK: diffuse | ||||
| Somatotroph Tumor | 27 | Sparsely granulated | GH ± PRL | PIT1 ± SSTR2-3-5 |
| LMW CK: fibrous bodies | ||||
| Somatolactotroph | GH + PRL (in different cells) ± α-subunit | PIT1, ERɑ, ± SSTR | ||
| Lactotroph Tumor | 21 | Sparsely granulated | PRL | PIT1, ERɑ |
| Thyrotroph Tumor | 2 | Sparsely granulated | βTSH, α-subunit | PIT1, GATA2, SSTR2-5 |
| Corticotroph Tumor | 13 | Densely granulated * | ACTH, βend; | TPIT, ± SSTR5 |
| LMW CK: diffuse | ||||
| Sparsely granulated | ACTH, βend; | TPIT | ||
| LMW CK: diffuse | ||||
| Gonadotroph Tumor | 35 | Sparsely granulated | βFSH, βLH, α-subunit | SF1, GATA2, ERɑ, ±SSTR |
| (various combinations) | ||||
| Immunonegative Tumor ** | 2 | Sparsely granulated | None | None |
| Plurihormonal | Various aspects | GH, PRL, βTSH ± | PIT1 | |
| Tumor *** | α-SU | |||
| Double/Triple Tumors | Exceptional | Distinct tumors | PRL and ACTH | PIT1/TPIT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trouillas, J.; Jaffrain-Rea, M.-L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. https://doi.org/10.3390/cancers12020514
Trouillas J, Jaffrain-Rea M-L, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to Classify Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers. 2020; 12(2):514. https://doi.org/10.3390/cancers12020514
Chicago/Turabian StyleTrouillas, Jacqueline, Marie-Lise Jaffrain-Rea, Alexandre Vasiljevic, Gérald Raverot, Federico Roncaroli, and Chiara Villa. 2020. "How to Classify Pituitary Neuroendocrine Tumors (PitNET)s in 2020" Cancers 12, no. 2: 514. https://doi.org/10.3390/cancers12020514
APA StyleTrouillas, J., Jaffrain-Rea, M.-L., Vasiljevic, A., Raverot, G., Roncaroli, F., & Villa, C. (2020). How to Classify Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers, 12(2), 514. https://doi.org/10.3390/cancers12020514







