Antiparkinson Drug Benztropine Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis by Acting on SLC6A3/DAT and Reducing STAT3
Abstract
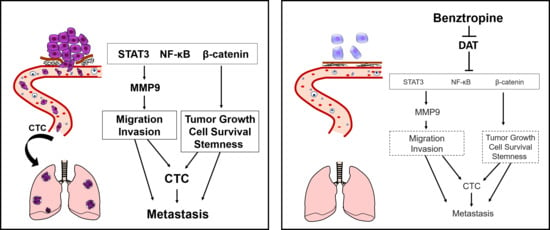
:1. Introduction
2. Results
2.1. Multiplex Drug screening to Target 3D Tumorigenicity, MMP9 Promoter Activity, and Cancer Cell Viability
2.2. Benztropine Suppresses Tumoroid Viability and MMP9 Promoter Activity
2.3. Benztropine Treatment Suppresses Migration and Invasion of Metastatic Cancer Cells
2.4. Benztropine Treatment Reduces STAT, NF-κB, β-catenin, and CD326 in Tumoroids
2.5. Anti-Tumor Effect of Benztropine through Inhibition of Dopamine Transporter SLC6A3
2.6. Benztropine Treatment Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis
2.7. Clinical Significance of DAT and STAT
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cell Culture
4.2. Three-Dimensional Tumoroid-Based Multiplex Reporter Assay
4.3. Chemicals and Drugs
4.4. Cell Viability Assay
4.5. Wound Healing Assay
4.6. Lactate Dehydrogenase Release Assay
4.7. Migration/Invasion Assays
4.8. Promoter Analysis
4.9. Protein Sample Fractionation
4.10. Western Blot Analysis
4.11. RT-qPCR
4.12. Genetic Alterations, Gene Expression, Kaplan-Meier Estimate
4.13. Coexpression Correlation Analysis
4.14. Tumor Allograft and CTC
4.15. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghossein, A.R.; Bhattacharya, S.; Rosai, J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin. Cancer Res. 1999, 5, 1950–1960. [Google Scholar] [PubMed]
- Sogawa, C.; Eguchi, T.; Okusha, Y.; Ono, K.; Ohyama, K.; Iizuka, M.; Kawasaki, R.; Hamada, Y.; Takigawa, M.; Sogawa, N.; et al. A Reporter System Evaluates Tumorigenesis, Metastasis, β-catenin/MMP Regulation, and Druggability. Tissue Eng. Part A 2019, 25, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.J.; Witty, J.P.; Rodgers, W.H.; Matrisian, L.M. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol. Carcinog. 1994, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Okusha, Y.; Eguchi, T.; Sogawa, C.; Okui, T.; Nakano, K.; Okamoto, K.; Kozaki, K.-I. The intranuclear PEX domain of MMP involves proliferation, migration, and metastasis of aggressive adenocarcinoma cells. J. Cell. Biochem. 2018, 119, 7363–7376. [Google Scholar] [CrossRef] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, T.; Calderwood, S.; Takigawa, M.; Kubota, S.; Kozaki, K.-I. Intracellular MMP3 PromotesHSPGene Expression in Collaboration With Chromobox Proteins. J. Cell. Biochem. 2016, 118, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel Transcription Factor-Like Function of Human Matrix Metalloproteinase 3 Regulating the CTGF/CCN2 Gene. Mol. Cell. Boil. 2008, 28, 2391–2413. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Tweardy, D.J.; Zhang, M.; Zhang, X.; Landua, J.; Petrovic, I.; Bu, W.; Roarty, K.; Hilsenbeck, S.G.; Rosen, J.M.; et al. STAT3 Signaling Is Activated Preferentially in Tumor-Initiating Cells in Claudin-Low Models of Human Breast Cancer. STEM CELLS 2014, 32, 2571–2582. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Clauser, K.R.; Tam, W.L.; Fröse, J.; Ye, X.; Eaton, E.N.; Reinhardt, F.; Donnenberg, V.S.; Bhargava, R.; Carr, S.A.; et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014, 16, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.-D.; Murshid, A.; Eguchi, T.; Gong, J.; Calderwood, S.K. HSF1 regulation of β-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene 2014, 34, 2178–2188. [Google Scholar] [CrossRef] [Green Version]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2017, 175, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, C.R.; Sullivan, D.J. New uses for old drugs. Nature 2007, 448, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.A.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, T.; Sogawa, C.; Okusha, Y.; Uchibe, K.; Iinuma, R.; Ono, K.; Nakano, K.; Murakami, J.; Itoh, M.; Arai, K.; et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE 2018, 13, e0191109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namba, Y.; Sogawa, C.; Okusha, Y.; Kawai, H.; Itagaki, M.; Ono, K.; Murakami, J.; Aoyama, E.; Ohyama, K.; Asaumi, J.-I.; et al. Depletion of Lipid Efflux Pump ABCG1 Triggers the Intracellular Accumulation of Extracellular Vesicles and Reduces Aggregation and Tumorigenesis of Metastatic Cancer Cells. Front. Oncol. 2018, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Arai, K.; Eguchi, T.; Rahman, M.M.; Sakamoto, R.; Masuda, N.; Nakatsura, T.; Calderwood, S.K.; Kozaki, K.-I.; Itoh, M. A Novel High-Throughput 3D Screening System for EMT Inhibitors: A Pilot Screening Discovered the EMT Inhibitory Activity of CDK2 Inhibitor SU9516. PLoS ONE 2016, 11, e0162394. [Google Scholar] [CrossRef]
- Sharif, N.; Klimko, P.G. Prostaglandin FP receptor antagonists: Discovery, pharmacological characterization and therapeutic utility. Br. J. Pharmacol. 2018, 176, 1059–1078. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.G.M.; Anderson, E.; Lynch, G.S.; Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 1986, 319, 774–776. [Google Scholar] [CrossRef]
- Kaneko, I.; Fukumori, Y.; Fukuda, T.; Takeuchi, Y. Pharmacodynamics of Chlorzoxazone in Rats. J. Pharm. Sci. 1988, 77, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Woodward, N.; Coward, J. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madras, B.K.; Fahey, M.A.; Goulet, M.; Lin, Z.; Bendor, J.; Goodrich, C.; Meltzer, P.C.; Elmaleh, D.R.; Livni, E.; Bonab, A.A.; et al. Dopamine Transporter (DAT) Inhibitors Alleviate Specific Parkinsonian Deficits in Monkeys: Association with DAT Occupancy in Vivo. J. Pharmacol. Exp. Ther. 2006, 319, 570–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runyon, S.P.; Carroll, F.I. Dopamine transporter ligands: Recent developments and therapeutic potential. Curr. Top. Med. Chem. 2006, 6, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Zhen, J.; Kharkar, P.; Mishra, M.; Chen, N.; Dutta, A.K.; Reith, E.M. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: Molecular features that differentially determine antagonist-binding properties. J. Neurochem. 2008, 107, 928–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Joseph, D.B.; Bowen, W.D.; Flippen-Anderson, J.L.; Dersch, C.M.; Rothman, R.B.; Jacobson, A.E.; Rice, K.C. Synthesis and biological evaluation of tropane-like 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine (GBR 12909) analogues. J. Med. Chem. 2001, 44, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Darnowski, J.; Goulette, F.A.; Guan, Y.-J.; Chatterjee, D.; Yang, Z.-F.; Cousens, L.P.; Chin, Y.E. Stat3 Cleavage by Caspases. J. Boil. Chem. 2006, 281, 17707–17717. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, J.; DiGiovanni, J. Non-canonical Stat3 signaling in cancer. Mol. Carcinog. 2015, 55, 1889–1898. [Google Scholar] [CrossRef]
- Oishi, N.; Yamashita, T.; Kaneko, S. Molecular biology of liver cancer stem cells. Liver Cancer 2014, 3, 71–84. [Google Scholar] [CrossRef]
- Watanabe, M.; Tanigawara, Y.; Ko, R.; Wakuda, K.; Ono, A.; Imai, H.; Taira, T.; Naito, T.; Murakami, H.; Abe, M.; et al. Isolation and molecular analysis of circulating tumor cells from lung cancer patients using a microfluidic chip type cell sorter. Cancer Sci. 2018, 109, 2539–2548. [Google Scholar] [CrossRef] [Green Version]
- Parashar, D.; Geethadevi, A.; Aure, M.R.; Mishra, J.; George, J.; Chen, C.; Mishra, M.K.; Tahiri, A.; Zhao, W.; Nair, B.; et al. miRNA551b-3p Activates an Oncostatin Signaling Module for the Progression of Triple-Negative Breast Cancer. Cell Rep. 2019, 29, 4389–4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Hollmén, M.; Li, L.; Chen, Y.; Proulx, S.; Reker, D.; Schneider, G.; Detmar, M. New use of an old drug: Inhibition of breast cancer stem cells by benztropine mesylate. Oncotarget 2016, 8, 1007–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omara-Opyene, A.L.; Qiu, J.; Shah, G.V.; Iczkowski, A.K. Prostate cancer invasion is influenced more by expression of a CD44 isoform including variant 9 than by Muc18. Lab. Investig. 2004, 84, 894–907. [Google Scholar] [CrossRef]
- Munz, M.; Baeuerle, P.A.; Gires, O. The Emerging Role of EpCAM in Cancer and Stem Cell Signaling. Cancer Res. 2009, 69, 5627–5629. [Google Scholar] [CrossRef] [Green Version]
- Cerles, O.; Gonçalves, T.C.; Chouzenoux, S.; Benoit, E.; Schmitt, A.; Saidu, N.; Kavian, N.; Chéreau, C.; Gobeaux, C.; Weill, B.; et al. Preventive action of benztropine on platinum-induced peripheral neuropathies and tumor growth. Acta Neuropathol. Commun. 2019, 7, 9. [Google Scholar] [CrossRef]
- Schrödter, S.; Braun, M.; Syring, I.; Klümper, N.; Deng, M.; Schmidt, D.; Perner, S.; Müller, S.; Ellinger, J. Identification of the dopamine transporter SLC6A3 as a biomarker for patients with renal cell carcinoma. Mol. Cancer 2016, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Andersen, P.H. Biochemical and Pharmacological Characterization of [3H]GBR 12935 Binding In Vitro to Rat Striatal Membranes: Labeling of the Dopamine Uptake Complex. J. Neurochem. 1987, 48, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Groshan, K.; Blakely, R.D.; Richelson, E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997, 340, 249–258. [Google Scholar] [CrossRef]
- Kornhuber, J.; Muehlbacher, M.; Trapp, S.; Pechmann, S.; Friedl, A.; Reichel, M.; Mühle, C.; Terfloth, L.; Groemer, T.; Spitzer, G.M.; et al. Identification of Novel Functional Inhibitors of Acid Sphingomyelinase. PLoS ONE 2011, 6, e23852. [Google Scholar] [CrossRef] [Green Version]
- Pollak, M. Investigating Metformin for Cancer Prevention and Treatment: The End of the Beginning. Cancer Discov. 2012, 2, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Brooks, D.J. Molecular imaging of dopamine transporters. Ageing Res. Rev. 2016, 30, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, C.; Mitsuhata, C.; Kumagai-Morioka, K.; Sogawa, N.; Ohyama, K.; Morita, K.; Kozai, K.; Dohi, T.; Kitayama, S. Expression and Function of Variants of Human Catecholamine Transporters Lacking the Fifth Transmembrane Region Encoded by Exon 6. PLoS ONE 2010, 5, e11945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, K.; Resat, H. Constitutive activation of STAT3 in breast cancer cells: A review. Int. J. Cancer 2015, 138, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-S.; Tseng, H.-Y.; Chen, Y.-A.; Shen, P.-C.; Al Haq, A.T.; Chen, L.-M.; Tung, Y.-C.; Hsu, H.-L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Yu, T.; Dong, N.; Wang, B.; Sun, F.; Jiang, D.-H. Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 289. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.R.; Watson, S.M.R.; Tevendale, M.C.L.; Watson, C.J.; Clarke, A. Caspase-dependent proteolytic cleavage of STAT3α in ES cells, in mammary glands undergoing forced involution and in breast cancer cell lines. BMC Cancer 2007, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.-H.; Kim, Y.-J.; Kim, Y.-G.; Joh, T.H.; Beal, M.F.; Kim, Y.-S. Role of Matrix Metalloproteinase 3-mediated α-Synuclein Cleavage in Dopaminergic Cell Death. J. Boil. Chem. 2011, 286, 14168–14177. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-N.; Zhang, H.-D.; Yuan, S.-J.; Tian, Z.-Y.; Wang, L.; Sun, Z.-X. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/β-catenin pathway. Int. J. Cancer 2007, 121, 1360–1365. [Google Scholar] [CrossRef]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. EBioMedicine 2014, 2, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Sakata, K.; Kozaki, K.; Iida, K.; Tanaka, R.; Yamagata, S.; Utsumi, K.R.; Saga, S.; Shimizu, S.; Matsuyama, M. Establishment and Characterization of High- and Low-lung-metastatic Cell Lines Derived from Murine Colon Adenocarcinoma 26 Tumor Line. Jpn. J. Cancer Res. 1996, 87, 78–85. [Google Scholar] [CrossRef]
- Eguchi, T.; Kubota, S.; Takigawa, M.; Takigawa, M. Promoter Analyses of CCN Genes. Adv. Struct. Saf. Stud. 2016, 1489, 177–185. [Google Scholar] [CrossRef]
- Boeva, V.; Surdez, D.; Guillon, N.; Tirode, F.; Fejes, A.P.; Delattre, O.; Barillot, E. De novo motif identification improves the accuracy of predicting transcription factor binding sites in ChIP-Seq data analysis. Nucleic Acids Res. 2010, 38, e126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihle, J.N. STATs: Signal Transducers and Activators of Transcription. Cell 1996, 84, 331–334. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Skanderup, A.J.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Skanderup, A.J.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]








| Data Set | TCGA PanCancer Atlas | A Curated Set of Non-Redundant Studies |
|---|---|---|
| Number of patients | 10,953 patients | 44,313 patients |
| Number of samples | 10,967 samples | 46,641 samples |
| Overall survival, P-value | 0.0268 | 0.136 |
| Disease/Progression-free survival, P-value | 0.382 | 0.792 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogawa, C.; Eguchi, T.; Tran, M.T.; Ishige, M.; Trin, K.; Okusha, Y.; Taha, E.A.; Lu, Y.; Kawai, H.; Sogawa, N.; et al. Antiparkinson Drug Benztropine Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis by Acting on SLC6A3/DAT and Reducing STAT3. Cancers 2020, 12, 523. https://doi.org/10.3390/cancers12020523
Sogawa C, Eguchi T, Tran MT, Ishige M, Trin K, Okusha Y, Taha EA, Lu Y, Kawai H, Sogawa N, et al. Antiparkinson Drug Benztropine Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis by Acting on SLC6A3/DAT and Reducing STAT3. Cancers. 2020; 12(2):523. https://doi.org/10.3390/cancers12020523
Chicago/Turabian StyleSogawa, Chiharu, Takanori Eguchi, Manh Tien Tran, Masayuki Ishige, Kilian Trin, Yuka Okusha, Eman Ahmed Taha, Yanyin Lu, Hotaka Kawai, Norio Sogawa, and et al. 2020. "Antiparkinson Drug Benztropine Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis by Acting on SLC6A3/DAT and Reducing STAT3" Cancers 12, no. 2: 523. https://doi.org/10.3390/cancers12020523
APA StyleSogawa, C., Eguchi, T., Tran, M. T., Ishige, M., Trin, K., Okusha, Y., Taha, E. A., Lu, Y., Kawai, H., Sogawa, N., Takigawa, M., Calderwood, S. K., Okamoto, K., & Kozaki, K.-i. (2020). Antiparkinson Drug Benztropine Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis by Acting on SLC6A3/DAT and Reducing STAT3. Cancers, 12(2), 523. https://doi.org/10.3390/cancers12020523