The Effect of Cholecystectomy on the Risk of Colorectal Cancer in Patients with Gallbladder Stones
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Ethics Statement
2.3. Data Sharing Statement
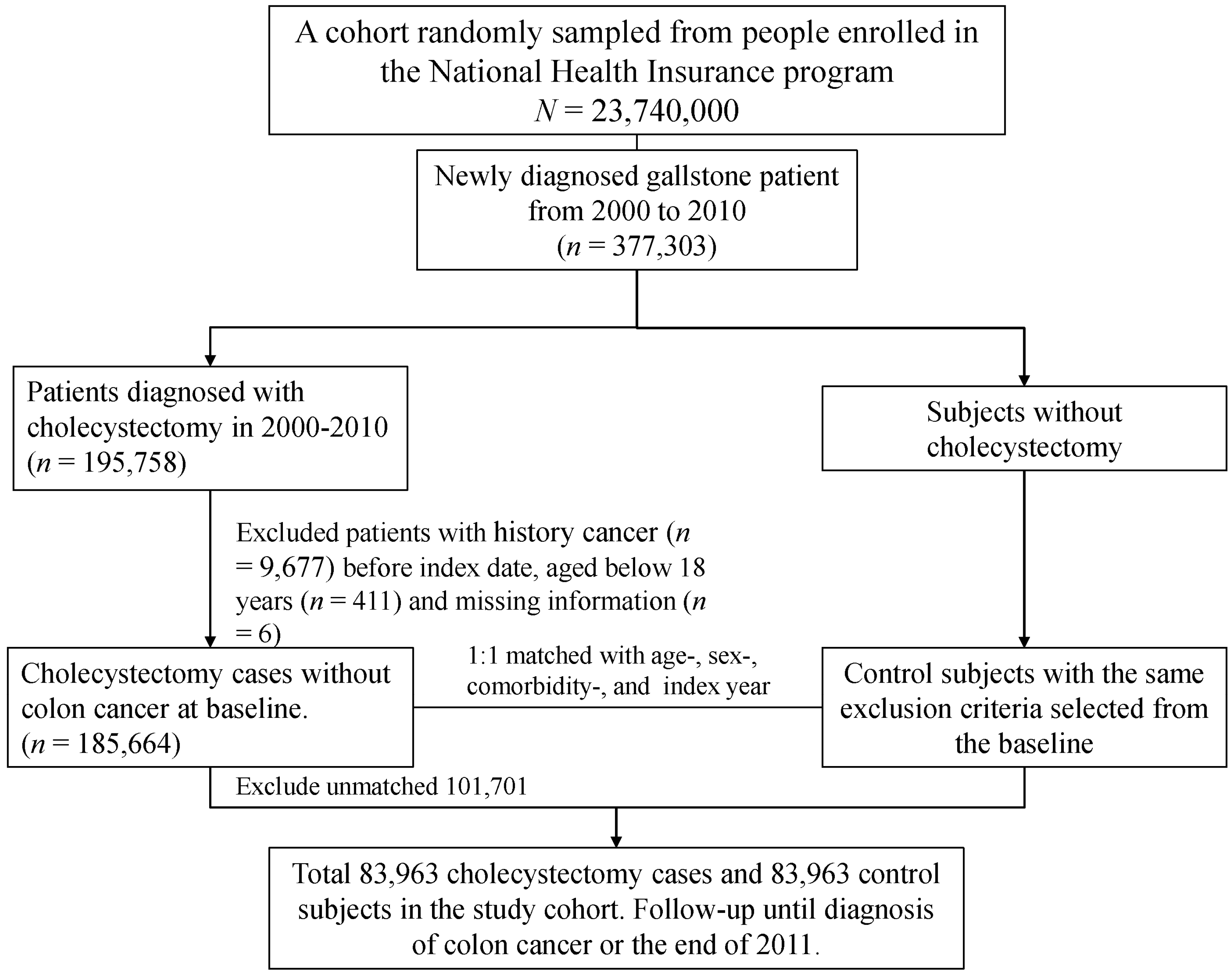
2.4. Sampled Patients
2.5. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| GBS | gallbladder stones |
| NHIRD | National Health Insurance Research Database |
| aHR | adjusted hazard ratio |
| CI | confidence interval |
| NHI | National Health Insurance |
| NHRI | National Health Research Institute |
| IRB | Institutional Review Board |
| CAD | coronary artery disease |
| COPD | chronic obstructive pulmonary disease |
| ICD-9-CM | International Classification of Diseases, Ninth Edition, Clinical Modification |
| SDs | standard deviations |
| HRs | hazard ratios |
| NNT | number needed to treat |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics, 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Health Promotion Administration Annual Report 2018. Available online: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/10231/File_11648.pdf (accessed on 13 February 2020).
- Iqbal, M.N.; Iqbal, M.A.; Javaid, R.; Abbas, M.W. Gall stones: A fundamental clinical review. Int. J. Res. Med. Sci. 2019, 7, 2869–2874. [Google Scholar] [CrossRef]
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.F.; Méndez-Sánchez, N.; Portincasa, P.; Van Erpecum, K.J.; Van Laarhoven, C.J.; Wang, D.Q.H. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [CrossRef]
- Hemminki, K.; Hemminki, O.; Forsti, A.; Sundquist, K.; Sundquist, J.; Li, X. Familial risks for gallstones in the population of Sweden. BMJ Open Gastroenterol. 2017, 4, e000188. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, M.H.; Yang, J.C.; Nien, C.K.; Etheredge, G.D.; Yang, C.C.; Yeh, Y.H.; Wu, H.S.; Der-Aur Chou Yueh, S.K. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J. Gastroenterol. Hepatol. 2006, 21, 1737–1743. [Google Scholar] [CrossRef]
- Chen, Y.K.; Yeh, J.H.; Lin, C.L.; Peng, C.L.; Sung, F.C.; Hwang, M.; Kao, C.H. Cancer risk in patients with cholelithiasis and after cholecystectomy: a nationwide cohort study. J. Gastroenetrol. 2014, 49, 923–931. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M.; Sorensen, L.T.; Jorgensen, T. Association between screen-detected gallstones disease and cancer in a cohort study. Gastroenterology 2017, 152, 1965–1974. [Google Scholar] [CrossRef]
- Coats, M.; Shimi, S.M. Cholecystectomy and the risk of alimentary tract cancers: A systematic review. World J. Gastroenterol. 2015, 21, 3679–3693. [Google Scholar] [CrossRef]
- Giovannucci, E.; Colditz, G.A.; Stampfer, K.J. A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology 1993, 105, 130–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Li, L.; Ai, M.; Gong, Z.; He, Y.; Dong, Y.; Xu, S.; Wang, J.; Jin, B.; et al. Cholecystectomy can increase the risk of colorectal cancer: A meta-analysis of 10 cohort studies. PLoS ONE 2017, 12, e0181852. [Google Scholar] [CrossRef] [PubMed]
- Kaibara, N.; Wakatsuki, T.; Mizusawa, K.; Sugesawa, A.; Kimura, O.; Koga, S. Negative correlation between cholecystectomy and the subsequent development of large bowel carcinoma in a low-risk Japanese population. Dis. Colon Rectum 1986, 29, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Database NHIR. Taiwan. Available online: http://nhird.nhri.org.tw/en/index.html (accessed on 1 March 1995).
- Chen, C.H.; Lin, C.L.; Kao, C.H. Association between gallbladder stone disease and prostate cancer: A nationwide population-based study. Oncotarget 2016, 7, 64380–64389. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, C.L.; Cheng, Y.S.; Jeng, L.B. Association between subtotal gastrectomy with Billroth II anastomosis and coronary heart disease. Obes. Surg. 2017, 27, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Chiong, C.; Cox, M.R.; Eslick, G.D. Gallstones are associated with colonic adenoma: A meta-analysis. World J. Surg. 2012, 36, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 14, 1–22. [Google Scholar]
- Chen, C.H.; Lin, C.L.; Jeng, L.B. Association between chronic pancreatitis and urolithiasis: A population-based cohort study. PLoS ONE 2018, 13, e0194019. [Google Scholar] [CrossRef]
- Wadhwa, V.; Jobanputra, Y.; Garg, S.K.; Patwardhan, S.; Mehta, D.; Sanaka, M.R. Nationwide trends of hospital admissions for acute cholecystitis in the United States. Gastroenterol. Rep. 2017, 5, 36–42. [Google Scholar] [CrossRef]
- Yokoe, M.; Takada, T.; Hwang, T.L.; Endo, I.; Akazawa, K.; Miura, F.; Mayumi, T.; Mori, R.; Chen, M.F.; Jan, Y.Y.; et al. Descriptive review of acute cholecystitis: Japan-Taiwan collaborative epidemiological study. J. Hepatobiliary Pancreat Sci. 2017, 24, 319–328. [Google Scholar] [CrossRef]
- Shaffer, E.A. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Shabanzadeh, D.M.; Holmboe, S.A.; Sorensen, L.T.; Linneberg, A.; Andersson, A.M.; Jorgensen, T. Are incident gallstones associated to sex-dependent changes with age? A cohort study. Andrology 2017, 5, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, F.; Wang, T.; Liu, Y.; Shen, Y.; Gong, J.; Dai, W.; Zhou, J.; Gu, J.; Tu, Y.; et al. Health-related lifestyle behaviors among male and female rural-to urban migrant workers in Shaghai, China. PLoS ONE 2015, 10, e0117946. [Google Scholar]
- Zhu, Q.; Sun, X.; Ji, X.; Zhu, L.; Xu, J.; Wang, C.; Zhang, C.; Xue, F.; Liu, Y. The association between gallstones and metabolic syndrome in urban Han Chinese: a longitudinal cohort study. Sci. Rep. 2016, 6, 29937. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.F.; Chuang, H.Y.; Lai, S.W. Metformin use correlates with reduced risk of gallstones in diabetic patients: A 12-year follow-up study. Front. Pharmacol. 2017, 8, 765. [Google Scholar] [CrossRef]
- Aune, D.; Vatten, L.J. Diabetes mellitus and the risk of gallbladder disease: a systematic review and meta-analysis of prospective studies. J. Diabetes Complicat. 2016, 30, 368–373. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Significant association between gallstone disease and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig. Dis. Sci. 2016, 61, 2389–2396. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M.; Sorensen, L.T.; Jorgensen, T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016, 150, 156–167. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M. Incidence of gallstone disease and complications. Curr. Opin. Gastroenterol. 2018, 34, 81–89. [Google Scholar] [CrossRef]
- Anderson, J.C.; Baron, J.A.; Ahnen, D.J.; Barry, E.L.; Bostick, R.M.; Burke, C.A.; Bresalier, R.S.; Church, T.R.; Cole, B.F.; Cruz-Correa, M.; et al. Factors associated with shorter colonoscopy surveillance intervals for patients with low-risk colorectal adenomas and effect on outcome. Gastroenterology 2017, 152, 1819–1821. [Google Scholar] [CrossRef]
- Lagergren, J.; Ye, W.; Ekbom, A. Intestinal cancer after cholecystectomy: Is bile involved in carcinogenesis? Gastroenterology 2001, 131, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Wang, D.Q.H.; Protincasa, P. Cholecystectomy and risk of metabolic syndrome. Eur. J. Int. Med. 2018, 53, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.A.; Veysey, M.J.; Bathgate, T.; King, A.; French, G.; Smeeton, N.C.; Murphy, G.M.; Dowling, R.H. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology 2000, 119, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, H.N.; Park, E.; Ryu, S.; Chang, Y.; Shin, H.; Kim, H.L.; Yi, S.Y. The impact of cholecystectomy on the gut microbiota: A case-control study. J. Clin. Med. 2019, 8, 79. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mirzaei, H.; Alikhani, M.Y.; Sholeh, M.; Arabestani, M.R.; Saeedijam, M.; Karampoor, S.; Ahmadyousefi, Y.; Moghadam, M.S.; Irajian, G.R.; et al. Bacterial biofilm in colorectal cancer: What is the real mechanism of action? Microb. Pathog. 2020, 8, 104052. [Google Scholar] [CrossRef]
- Carini, F.; Mazzola, M.; Rappa, F.; Jurjus, A.; Geagea, A.G.; Al Kattar, S.; Bou-Assi, T.; Jurjus, R.; Damiani, P.; Leone, A.; et al. Colorectal carcinogenesis: Role of oxidative stress and antioxidants. Anticancer Res. 2017, 37, 4759–4766. [Google Scholar]
- Lee, G.H.; Malietzis, G.; Askari, A.; Bernardo, D.; Al-Hassi, H.O.; Clark, S.K. Is right-sided colon cancer different to left-sided colorectal cancer? A systematic review. Eur. J. Surg. Oncol. 2015, 41, 300–308. [Google Scholar] [CrossRef]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef]
- Shao, T.; Yang, Y.X. Cholecystectomy and the risk of colorectal cancer. Am. J. Gastroenterol. 2005, 100, 1813–1820. [Google Scholar] [CrossRef]
- Schmidt, M.; Smastuen, M.C.; Sondenaa, K. Increased cancer incidence in some gallstone diseases, and equivocal effect of cholecystectomy: a long-term analysis of cancer and mortality. Scand. J. Gastroenterol. 2012, 47, 1467–1474. [Google Scholar] [CrossRef]
- Ward, H.A.; Murphy, N.; Weiderpass, E.; Leitzmann, M.F.; Aglago, E.; Gunter, M.J.; Freisling, H.; Jenab, M.; Boutron-Ruault, M.C.; Severi, G.; et al. Gallstones and incident colorectal cancer in a large pan-European cohort study. Int. J. Cancer 2019, 145, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Lai, M.S.; Gau, S.S.F.; Wang, S.C.; Tsai, H.J. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS ONE 2014, 9, e112257. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Lee, C.H.; Chen, P.S.; Li, Y.H.; Lin, S.J.; Yang, Y.H.K. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.D. Natural history of asymptomatic and symptomatic gallstones. Am. J. Surg. 1993, 165, 399–404. [Google Scholar] [CrossRef]
| Variables | Cholecystectomy | p-Value | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| (N = 83,963) | (N = 83,963) | ||||
| n | % | n | % | ||
| Age, Year | 0.003 | ||||
| ≤49 | 32,560 | 38.8 | 31,962 | 38.1 | |
| 50–64 | 21,334 | 25.4 | 21,316 | 25.4 | |
| ≥ 65 | 30,069 | 35.8 | 30,685 | 36.6 | |
| Mean (SD) # | 56.5 | 17.1 | 56.8 | 17.2 | 0.002 |
| Gender | 0.42 | ||||
| Female | 38,434 | 46.0 | 38,599 | 46.0 | |
| Male | 45,529 | 54.2 | 45,364 | 54.0 | |
| Occupation | 0.12 | ||||
| White collar | 19,844 | 23.6 | 19,825 | 23.6 | |
| Blue collar | 44,186 | 52.6 | 43,854 | 52.2 | |
| Others ‡ | 19,933 | 23.7 | 20,284 | 24.2 | |
| Urbanization Level † | 0.63 | ||||
| 1 (highest) | 18,136 | 21.6 | 18,002 | 21.4 | |
| 2 | 24,031 | 28.6 | 24,103 | 28.7 | |
| 3 | 13,628 | 16.2 | 13,506 | 16.1 | |
| 4(lowest) | 28,168 | 33.6 | 28,352 | 33.8 | |
| Comorbidity | |||||
| Hypertension | 18,197 | 21.7 | 18,663 | 22.2 | 0.01 |
| Hyperlipidemia | 3713 | 4.42 | 3645 | 4.34 | 0.42 |
| Cirrhosis | 8639 | 10.3 | 8497 | 10.1 | 0.25 |
| Stroke | 5636 | 6.71 | 5730 | 6.82 | 0.36 |
| CAD | 7405 | 8.82 | 7577 | 9.02 | 0.14 |
| Diabetes mellitus | 11,400 | 13.6 | 11,452 | 13.6 | 0.71 |
| COPD | 4061 | 4.84 | 4904 | 5.84 | 0.001 |
| Chronic kidney diseases | 3217 | 3.83 | 3216 | 3.83 | 0.99 |
| Alcohol-related illness | 1464 | 1.74 | 1357 | 1.62 | 0.04 |
| Colorectal adenomas | 280 | 0.33 | 290 | 0.35 | 0.67 |
| Inflammatory bowel disease | 112 | 0.13 | 188 | 0.22 | 0.001 |
| Irritable bowel syndrome | 89 | 0.11 | 100 | 0.12 | 0.42 |
| Obesity | 26 | 0.03 | 28 | 0.03 | 0.79 |
| Variables | Cholecystectomy | Crude HR (95% CI) | Adjusted HR § (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Event | PY | Rate # | Event | PY | Rate # | |||
| All | 623 | 40,812 | 15.3 | 82 | 41,702 | 1.97 | 7.68 (6.10, 9.67) *** | 7.90 (6.27, 9.94) *** |
| Gender | ||||||||
| Female | 275 | 18,778 | 14.6 | 30 | 19,203 | 1.56 | 9.29 (6.37, 13.5) *** | 9.75 (6.69, 14.2) *** |
| Male | 348 | 22,034 | 15.8 | 52 | 22,499 | 2.31 | 6.75 (5.04, 9.03) *** | 6.86 (5.13, 9.18) *** |
| Age, Year | ||||||||
| ≤49 | 34 | 16,112 | 2.11 | 4 | 15,953 | 0.25 | 8.39 (2.98, 23.7) *** | 8.23 (2.92, 23.2) *** |
| 50–64 | 160 | 10,476 | 15.3 | 15 | 10,614 | 1.41 | 10.7 (6.32, 18.2) *** | 10.7 (6.32, 18.2) *** |
| ≥65 | 429 | 14,224 | 30.2 | 63 | 15,135 | 4.16 | 7.08 (5.44, 9.23) *** | 7.10 (5.45, 9.25) *** |
| Occupation | ||||||||
| White collar | 75 | 9795 | 7.66 | 7 | 9887 | 0.71 | 10.8 (4.96, 23.4) *** | 10.4 (4.80, 22.6) *** |
| Blue collar | 392 | 21,429 | 18.3 | 47 | 21,775 | 2.16 | 8.36 (6.18, 11.3) *** | 8.63 (6.38, 11.7) *** |
| Others ‡ | 156 | 9588 | 16.3 | 28 | 10,039 | 2.79 | 5.76 (3.85, 8.61) *** | 5.94 (3.97, 8.88) *** |
| Urbanization Level † | ||||||||
| 1 (highest) | 115 | 8905 | 12.9 | 16 | 8957 | 1.79 | 7.19 (4.26, 12.1) *** | 7.27 (4.31, 12.3) *** |
| 2 | 156 | 11,707 | 13.3 | 20 | 11,982 | 1.67 | 7.91 (4.97, 12.6) *** | 8.05 (5.06, 12.8) *** |
| 3 | 96 | 6625 | 14.5 | 13 | 6705 | 1.94 | 7.39 (4.14, 13.2) *** | 7.71 (4.32, 13.8) *** |
| 4(lowest) | 256 | 13,574 | 18.9 | 33 | 14,058 | 2.35 | 7.91 (5.51, 11.4) *** | 8.16 (5.68, 11.7) *** |
| Comorbidity & | ||||||||
| No | 283 | 25,098 | 11.3 | 41 | 25,777 | 1.61 | 6.95 (5.01, 9.64) *** | 7.12 (5.13, 9.89) *** |
| Yes | 340 | 15,714 | 21.6 | 41 | 15,925 | 2.52 | 8.44 (6.10, 11.7) *** | 8.62 (6.24, 11.9) *** |
| Follow-Up Period | ||||||||
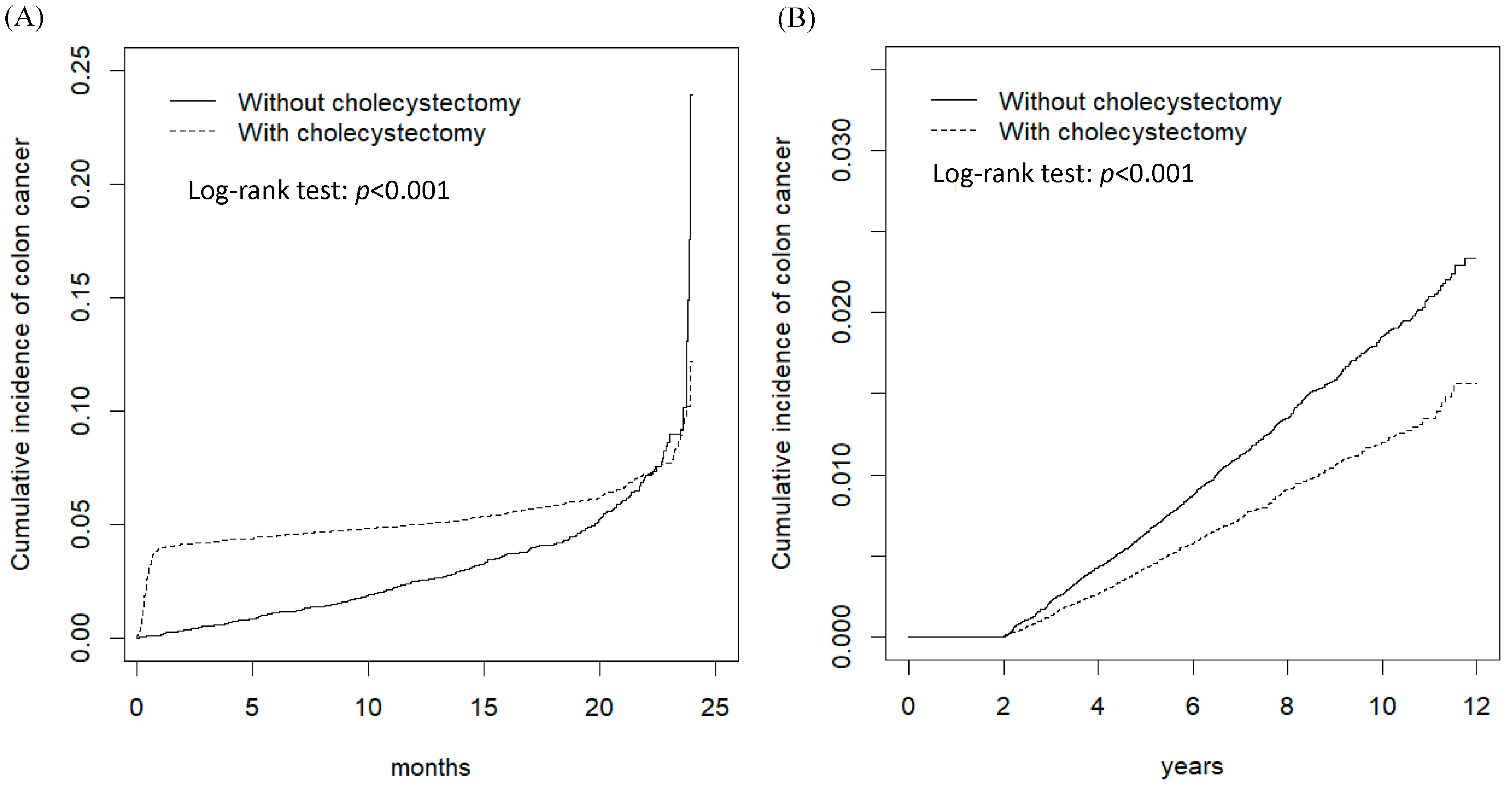
| <3 months | 589 | 20,574 | 28.6 | 39 | 20,923 | 1.86 | 15.2 (11.0, 21.1) *** | 15.5 (11.2, 21.5) *** |
| 3–6 months | 34 | 40,375 | 0.84 | 43 | 41,484 | 1.04 | 0.81 (0.52, 1.27) | 0.85 (0.54, 1.33) |
| Variables | Cholecystectomy | Crude HR (95% CI) | Adjusted HR § (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Event | PY | Rate # | Event | PY | Rate # | |||
| All | 638 | 423,992 | 1.50 | 1170 | 530,121 | 2.21 | 0.69 (0.63, 0.76) *** | 0.66 (0.60, 0.73) *** |
| Gender | ||||||||
| Female | 253 | 200,207 | 1.26 | 487 | 262,402 | 1.86 | 0.69 (0.59, 0.80) *** | 0.66 (0.57, 0.77) *** |
| Male | 385 | 223,786 | 1.72 | 683 | 267,719 | 2.55 | 0.68 (0.60, 0.77) *** | 0.66 (0.58, 0.75) *** |
| Age, Year | ||||||||
| ≤49 | 53 | 176,539 | 0.30 | 231 | 235,703 | 0.98 | 0.33 (0.25, 0.45) *** | 0.33 (0.24, 0.45) *** |
| 50–64 | 177 | 113,021 | 1.57 | 355 | 139,776 | 2.54 | 0.63 (0.52, 0.75) *** | 0.62 (0.52, 0.75) *** |
| ≥65 | 408 | 134,432 | 3.03 | 584 | 154,642 | 3.78 | 0.81 (0.71, 0.91) *** | 0.80 (0.71, 0.91) *** |
| Occupation | ||||||||
| White collar | 227 | 161,496 | 1.41 | 227 | 161,496 | 1.41 | 0.53 (0.41, 0.69) *** | 0.53 (0.41, 0.69) *** |
| Blue collar | 651 | 267,670 | 2.43 | 651 | 267,670 | 2.43 | 0.71 (0.63, 0.81) *** | 0.70 (0.62, 0.79) *** |
| Others ‡ | 292 | 100,955 | 2.89 | 292 | 100,955 | 2.89 | 0.65 (0.54, 0.79) *** | 0.66 (0.54, 0.79) *** |
| Urbanization Level † | ||||||||
| 1 (highest) | 108 | 94,205 | 1.15 | 251 | 126,211 | 1.99 | 0.59 (0.47, 0.74) *** | 0.57 (0.45, 0.71) *** |
| 2 | 165 | 123,195 | 1.34 | 343 | 157,654 | 2.18 | 0.62 (0.52, 0.75) *** | 0.59 (0.49, 0.71) *** |
| 3 | 109 | 68,301 | 1.60 | 205 | 85,985 | 2.38 | 0.69 (0.55, 0.87) ** | 0.66 (0.53, 0.84) *** |
| 4(lowest) | 256 | 138,291 | 1.85 | 371 | 160,270 | 2.31 | 0.80 (0.68, 0.94) ** | 0.79 (0.67, 0.92) ** |
| Comorbidity & | ||||||||
| No | 313 | 278,547 | 1.12 | 715 | 362,620 | 1.97 | 0.58 (0.51, 0.66) *** | 0.55 (0.48, 0.63) *** |
| Yes | 325 | 145,445 | 2.23 | 455 | 167,501 | 2.72 | 0.83 (0.72, 0.95) ** | 0.82 (0.71, 0.95) ** |
| Follow-Up Period | ||||||||
| 6–12 months | 56 | 39,942 | 1.40 | 84 | 41,114 | 2.04 | 0.69 (0.49, 0.96) * | 0.70 (0.50, 0.99) * |
| 1–3 years | 203 | 138,722 | 1.46 | 303 | 151,779 | 2.00 | 0.73 (0.61, 0.88) *** | 0.74 (0.62, 0.88) *** |
| 3–6 years | 211 | 142,551 | 1.48 | 393 | 180,127 | 2.18 | 0.68 (0.58, 0.80) *** | 0.64 (0.54, 0.76) *** |
| >6 years | 168 | 102,778 | 1.63 | 390 | 157,099 | 2.48 | 0.66 (0.55, 0.79) *** | 0.60 (0.50, 0.73) *** |
| Outcome | Cholecystectomy | Crude HR (95% CI) | Adjusted HR § (95% CI) | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Event | Rate # | Event | Rate # | |||
| Follow-Up Period ≤ 6 Months | ||||||
| All | ||||||
| Proximal colon cancer | 90 | 2.21 | 9 | 0.22 | 10.1 (5.09, 20.0) *** | 10.4 (5.23, 25.6) *** |
| Distal colon cancer | 52 | 1.27 | 7 | 0.17 | 7.51 (3.41, 16.5) *** | 7.70 (3.50, 17.0) *** |
| Rectal cancer | 102 | 2.50 | 13 | 0.31 | 7.93 (4.45, 14.1) *** | 8.06 (4.53, 14.4) *** |
| Female | ||||||
| Proximal colon cancer | 49 | 2.61 | 4 | 0.21 | 12.4 (4.48, 34.4) *** | 13.0 (4.69, 36.0) *** |
| Distal colon cancer | 22 | 1.17 | 3 | 0.16 | 7.43 (2.23, 24.8) *** | 7.73 (2.31, 25.8) *** |
| Rectal cancer | 43 | 2.29 | 2 | 0.10 | 21.8 (5.28, 89.9) *** | 22.5 (5.46, 93.0) *** |
| Male | ||||||
| Proximal colon cancer | 44 | 2.00 | 9 | 0.40 | 4.94 (2.41, 10.1) *** | 4.99 (2.44, 10.2) *** |
| Distal colon cancer | 37 | 1.68 | 27 | 1.20 | 1.40 (0.85, 2.29) | 1.42 (0.86, 2.33) |
| Rectal cancer | 81 | 3.68 | 42 | 1.87 | 1.96 (1.35, 2.85) *** | 2.00 (1.38, 2.91) *** |
| Follow-Up Period > 6 Months | ||||||
| All | ||||||
| Proximal colon cancer | 70 | 0.17 | 121 | 0.23 | 0.77 (0.57, 1.03) | 0.72 (0.54, 0.97) * |
| Distal colon cancer | 93 | 0.22 | 173 | 0.33 | 0.70 (0.55, 0.90) ** | 0.65 (0.51, 0.84) *** |
| Rectal cancer | 149 | 0.35 | 289 | 0.55 | 0.66 (0.54, 0.81) *** | 0.63 (0.51, 0.77) *** |
| Female | ||||||
| Proximal colon cancer | 35 | 0.17 | 59 | 0.22 | 0.82 (0.54, 1.24) | 0.76 (0.50, 1.15) |
| Distal colon cancer | 32 | 0.16 | 62 | 0.24 | 0.70 (0.46, 1.08) | 0.66 (0.43, 1.02) |
| Rectal cancer | 56 | 0.28 | 116 | 0.44 | 0.67 (0.48, 0.92) * | 0.64 (0.46, 0.88) ** |
| Male | ||||||
| Proximal colon cancer | 32 | 0.14 | 58 | 0.22 | 0.71 (0.46, 1.10) | 0.68 (0.44, 1.04) |
| Distal colon cancer | 54 | 0.24 | 88 | 0.33 | 0.79 (0.56, 1.11) | 0.73 (0.52, 1.03) |
| Rectal cancer | 71 | 0.32 | 142 | 0.53 | 0.63 (0.47, 0.84) ** | 0.59 (0.44, 0.79) *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lin, C.-L.; Kao, C.-H. The Effect of Cholecystectomy on the Risk of Colorectal Cancer in Patients with Gallbladder Stones. Cancers 2020, 12, 550. https://doi.org/10.3390/cancers12030550
Chen C-H, Lin C-L, Kao C-H. The Effect of Cholecystectomy on the Risk of Colorectal Cancer in Patients with Gallbladder Stones. Cancers. 2020; 12(3):550. https://doi.org/10.3390/cancers12030550
Chicago/Turabian StyleChen, Chien-Hua, Cheng-Li Lin, and Chia-Hung Kao. 2020. "The Effect of Cholecystectomy on the Risk of Colorectal Cancer in Patients with Gallbladder Stones" Cancers 12, no. 3: 550. https://doi.org/10.3390/cancers12030550
APA StyleChen, C.-H., Lin, C.-L., & Kao, C.-H. (2020). The Effect of Cholecystectomy on the Risk of Colorectal Cancer in Patients with Gallbladder Stones. Cancers, 12(3), 550. https://doi.org/10.3390/cancers12030550





