Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugitani, I.; Miyauchi, A.; Sugino, K.; Okamoto, T.; Yoshida, A.; Suzuki, S. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J. Surg. 2012, 36, 1247–1254. [Google Scholar] [CrossRef]
- The JAES/JSTS Task Force on the Guidelines for Thyroid Tumors. Clinical Practice Guidelines on the Management of Thyroid Tumors 2018. J. JAES JSTS 2018, 35, 1–87. [Google Scholar]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef] [PubMed]
- Haymart, M.R.; Banerjee, M.; Yin, H.; Worden, F.; Griggs, J.J. Marginal treatment benefit in anaplastic thyroid cancer. Cancer 2013, 119, 3133–3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobin, L.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Yoshida, A.; Sugino, K.; Sugitani, I.; Miyauchi, A. Anaplastic thyroid carcinomas incidentally found on postoperative pathological examination. World J. Surg. 2014, 38, 2311–2316. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.G.; Oh, H.S.; Park, S.; Kwon, H.; Song, D.E.; Kim, T.Y.; Shong, Y.K.; Kim, W.B.; Sung, T.Y.; et al. Comparison of the seventh and eighth editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-Node-Metastasis Staging System for differentiated thyroid cancer. Thyroid 2017, 27, 1149–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugitani, I.; Kasai, N.; Fujimoto, Y.; Yanagisawa, A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J. Surg. 2001, 25, 617–622. [Google Scholar] [CrossRef]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; McMillan, A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005, 103, 1330–1335. [Google Scholar] [CrossRef]
- Akaishi, J.; Sugino, K.; Kitagawa, W.; Nagahama, M.; Kameyama, K.; Shimizu, K.; Ito, K.; Ito, K. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid 2011, 21, 1183–1189. [Google Scholar] [CrossRef]
- Besic, N.; Hocevar, M.; Zgajnar, J.; Pogacnik, A.; Grazio-Frkovic, S.; Grazio-Frkovic, S.; Auersperg, M. Prognostic factors in anaplastic carcinoma of the thyroid; a multivariate survival analysis of 188 patients. Langenbecks Arch. Surg. 2005, 390, 203–208. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, K.W.; Jung, T.S.; Kim, J.M.; Kim, S.W.; Chung, K.W.; Kim, E.Y.; Gong, G.; Oh, Y.L.; Cho, S.Y.; et al. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck 2007, 29, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hanamura, T.; Murayama, K.; Okada, T.; Watanabe, T.; Harada, M.; Ito, T.; Koyama, H.; Kanai, T.; Maeno, K.; et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: Improved survival in subgroups of patients with localized primary tumors. Head Neck 2012, 34, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Hasegawa, Y.; Sugasawa, M.; Tori, M.; Higashiyama, T.; Miyazaki, M.; Hosoi, H.; Orita, Y.; Kitano, H. Super-radical surgery for anaplastic thyroid carcinoma: A large cohort study using the Anaplastic Thyroid Carcinoma Research Consortium of Japan database. Head Neck 2014, 36, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Orita, Y.; Sugitani, I.; Amemiya, T.; Fujimoto, Y. Prospective application of our novel prognostic index in the treatment of anaplastic thyroid carcinoma. Surgery 2011, 150, 1212–1219. [Google Scholar] [CrossRef]
- Chen, J.; Tward, J.D.; Shrieve, D.C.; Hitchcock, Y.J. Surgery and radiotherapy improve survival in patients with anaplastic thyroid carcinoma. Analysis of the surveillance, epidemiology, and end results 1983–2002. Am. J. Clin. Oncol. 2008, 31, 460–464. [Google Scholar] [CrossRef]
- Wendler, J.; Kroiss, M.; Gast, K.; Kreissl, M.C.; Allelein, S.; Lichtenauer, U.; Blaser, R.; Spitzweg, C.; Fassnacht, M.; Schott, M.; et al. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: Results of a multicenter study in Germany. Eur. J. Endocrinol. 2016, 175, 521–529. [Google Scholar] [CrossRef]
- Onoda, N.; Sugino, K.; Higashiyama, T.; Kammori, M.; Toda, K.; Ito, K.; Yoshida, A.; Suganuma, N.; Nakashima, N.; Suzuki, S.; et al. The safety and efficacy of weekly paclitaxel administration for anaplastic thyroid cancer patients: A nationwide prospective study. Thyroid 2016, 26, 1293–1299. [Google Scholar] [CrossRef]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Balkissoon, J.; Lu, S.P.; Bal, C.; Marur, S.; Gramza, A.; Yosef, R.B.; Gitlitz, B.; et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 2014, 24, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019, 15, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Onoda, N.; Ito, K.; Sugitani, I.; Takahashi, S.; Yamaguchi, I.; Kabu, K.; Tsukada, K. Sorafenib in Japanese patients with locally advanced or metastatic medullary thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid 2017, 27, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Lu, C.; Ahmed, S.; Gule-Monroe, M.K.; Williams, M.D.; Sturgis, E.M.; et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma. Thyroid 2019, 29, 1036–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted therapy for advanced thyroid cancer: Kinase inhibitors and beyond. Endocr. Rev. 2019, 40, 1573–1604. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)–A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]




| Stage | 7th Edition | 8th Edition |
|---|---|---|
| IV A | T4a, any N and M0 T4a: The cancer is any size but confined to the thyroid | T1–3a, N0 and M0 T1: Tumor ≤ 2 cm in greatest dimension limited to the thyroid T2: Tumor > 2 cm but ≤ 4 cm in greatest dimension limited to the thyroid T3a: Tumor > 4 cm limited to the thyroid |
| IV B | T4b, any N and M0 T4b: The cancer is extended outside of the thyroid gland to any extent | T1–3a, N1 and M0 Or T3b–T4b, any N and M0 T3b: Gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size T4a: Gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve from a tumor of any size T4b: Gross extrathyroidal extension invading prevertebral fascia or encasing a carotid artery or mediastinal vessels from a tumor of any size |
| IV C | Any T, any N and M1 | Any T, any N and M1 |
| Factor | No. | % | Median Overall Survival Months 95%CI (Range) | Log-Rank Test p-Value | |
|---|---|---|---|---|---|
| Age: | Median 71.0 (28–96) yrs | ||||
| <70 yrs | 349 | 46.1% | 5.0 (4.3–6.0) | 0.01 | |
| ≥70 yrs | 408 | 53.9% | 4.6 (3.8–5.3) | ||
| Sex: | |||||
| Male | 305 | 40.3% | 4.9 (3.8–5.8) | 0.74 | |
| Female | 452 | 59.7% | 4.6 (4.1–5.4) | ||
| T (7th edn.): | |||||
| 4a | 168 | 22.2% | 7.5 (5.7–8.9) | < 0.01 | |
| 4b | 589 | 77.8% | 4.3 (3.7–4.7) | ||
| T (8th edn.): | |||||
| 1 | 8 | 1.1% | 12.5 (0.9–16.8) | n.s. 1 vs. others | |
| 2 | 43 | 5.7% | 10.9 (8.2–17.7) | 0.03 vs. T3a <0.01 vs. T3b and T4 | |
| 3a | 117 | 15.5% | 5.7 (4.3–7.3) | <0.01 vs. T3b 0.03 vs. T4 | |
| 3b | 438 | 57.9% | 3.9 (3.4–4.5) | 0.66 vs. T4 | |
| 4a and 4b | 151 | 19.9% | 5.0(3.8–6.4) | ||
| N: | |||||
| 0 | 224 | 29.6% | 5.9 (4.8–7.0) | <0.01 | |
| 1 | 533 | 70.4% | 4.3 (3.7–4.9) | ||
| M: | |||||
| 0 | 467 | 61.7% | 6.6 (5.9–7.3) | <0.01 | |
| 1 | 290 | 38.3% | 2.8 (2.3–3.3) | ||
| Stage (7th edn.): | |||||
| IV A | 108 | 14.3% | 10.6 (7.7–13.3) | <0.01 between each | |
| IV B | 359 | 47.4% | 6.0 (5.2–6.6) | ||
| IV C | 290 | 38.3% | 2.8 (2.3–3.3) | ||
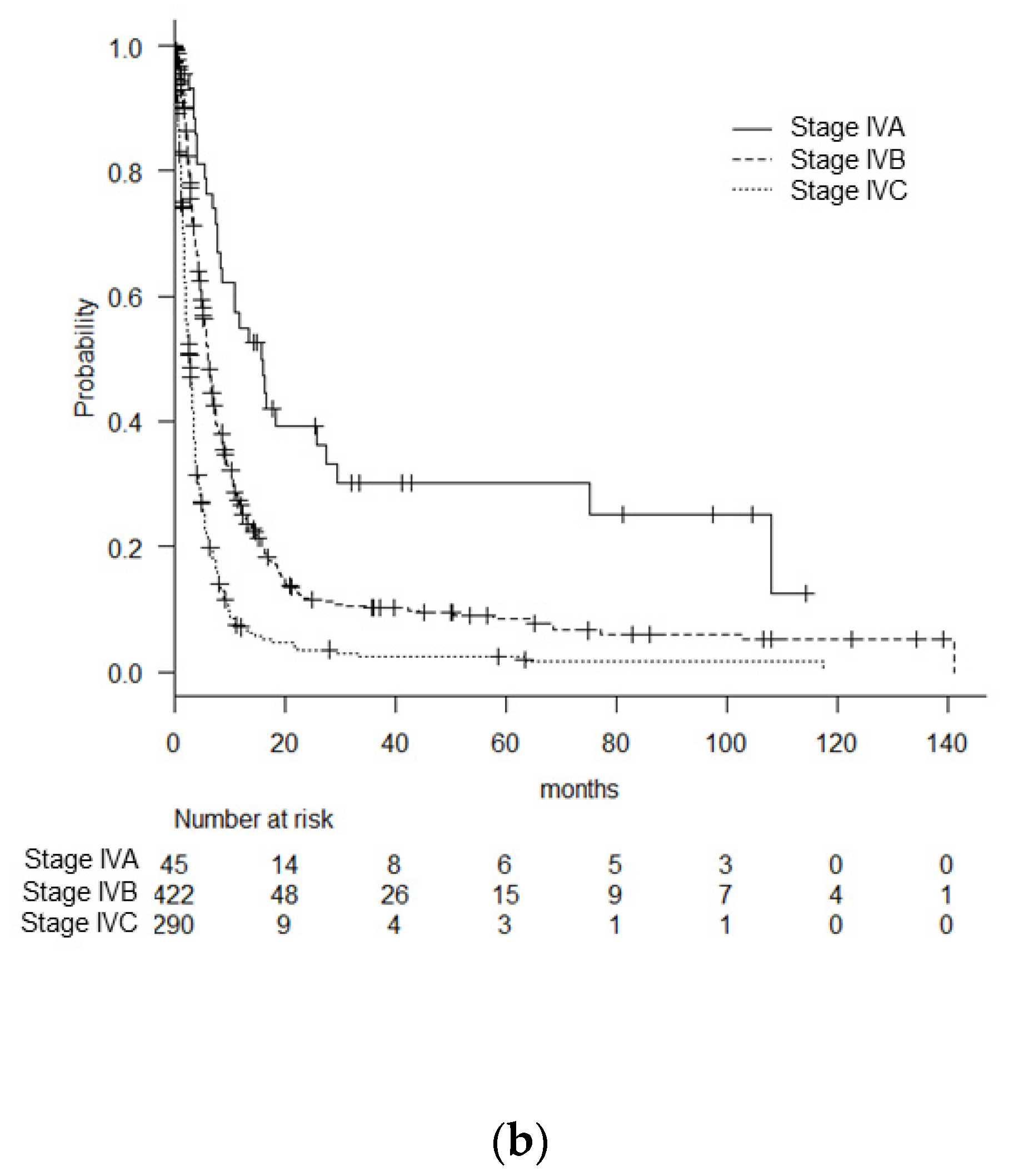
| Stage (8th edn.): | |||||
| IV A | 45 | 5.9% | 15.8 (8.5–27.6) | <0.01 between each | |
| IV B | 422 | 55.7% | 6.1 (5.7–6.8) | ||
| IV C | 290 | 38.3% | 2.8 (2.3–3.3) | ||
| T | 3 Mos. | 6 Mos. | 12 Mos. |
|---|---|---|---|
| 1 | 71.4% ± 17.1% | 71.4% ± 17.1% | 42.9% ± 18.7% |
| 2 | 80.2 % ± 6.3% | 72.7% ± 7.0% | 47.1% ± 8.0% |
| 3a | 74.7% ± 4.1% | 45.7% ± 4.7% | 25.9% ± 4.2% |
| 3b | 61.4% ± 2.4% | 35.7% ± 2.4% | 17.1% ± 1.9% |
| 4 | 65.1% ± 4.0% | 41.6% ± 4.3% | 16.9% ± 3.3% |
| Stage | 3 Mos. | 6 Mos. | 12 Mos. |
|---|---|---|---|
| IV A, 7th edn. | 86.5% ± 3.3% | 67.7% ± 4.6% | 43.6% ± 5.0% |
| IV B, 7th edn. | 74.5% ± 2.4% | 48.8% ± 2.7% | 24.6% ± 2.4% |
| IV A, 8th edn. | 90.7% ± 4.4% | 74.0% ± 6.8% | 52.5% ± 7.7% |
| IV B, 8th edn. | 75.9% ± 2.1% | 50.8% ± 2.5% | 26.6% ± 2.3% |
| IV C, 7th and 8th edn. | 46.0% ± 3.0% | 21.1% ± 2.5% | 6.6% ± 1.6% |
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age < 70 vs. ≥ 70 yrs. | 1.3 | 1.1–1.5 | 0.002 | 1.3 | 1.1–1.5 | 0.002 |
| Male vs. female | 1.1 | 0.9–1.3 | 0.323 | |||
| T4b vs. T4a | 1.5 | 1.1–2.1 | 0.008 | 1.6 | 1.3–2.1 | 0.004 |
| N1 vs. N0 | 1.2 | 1.0–1.5 | 0.016 | |||
| M1 vs. M0 | 2.5 | 1.7–3.5 | 0.000 | |||
| Stage IV B vs. IV A | 1.1 | 0.7–1.6 | 0.688 | 1.1 | 0.73–1.6 | 0.744 |
| Stage IV C vs. IV A | NA 1 | NA–NA | NA | 2.5 | 1.73–3.5 | < 0.001 |
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age <70 vs. ≥ 70 yrs. | 1.3 | 1.1–1.6 | 0.001 | 1.3 | 1.1–1.5 | 0.002 |
| Male vs. female | 1.1 | 0.9–1.3 | 0.306 | |||
| T2 vs. T1 | 0.6 | 0.3–1.4 | 0.273 | |||
| T3a vs. T1 | 1.0 | 0.5–2.2 | 0.934 | |||
| T3b vs. T1 | 1.4 | 0.7–3.0 | 0.369 | |||
| T4b vs. T1 | 1.2 | 0.5–2.5 | 0.703 | |||
| N1 vs. N0 | 1.2 | 1.0–1.5 | 0.030 | |||
| M1 vs. M0 | 3.0 | 1. 9–4.6 | 0.000 | |||
| Stage IV B vs. IV A | 1.3 | 0.8–2.0 | 0.279 | 2.1 | 1.4–3.0 | < 0.001 |
| Stage IV C vs. IV A | NA 1 | NA–NA | NA | 4.5 | 3.0–6.6 | < 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onoda, N.; Sugitani, I.; Ito, K.-i.; Suzuki, A.; Higashiyama, T.; Fukumori, T.; Suganuma, N.; Masudo, K.; Nakayama, H.; Uno, A.; et al. Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma. Cancers 2020, 12, 552. https://doi.org/10.3390/cancers12030552
Onoda N, Sugitani I, Ito K-i, Suzuki A, Higashiyama T, Fukumori T, Suganuma N, Masudo K, Nakayama H, Uno A, et al. Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma. Cancers. 2020; 12(3):552. https://doi.org/10.3390/cancers12030552
Chicago/Turabian StyleOnoda, Naoyoshi, Iwao Sugitani, Ken-ichi Ito, Akifumi Suzuki, Takuya Higashiyama, Tatsuya Fukumori, Nobuyasu Suganuma, Katsuhiko Masudo, Hirotaka Nakayama, Atsuhiko Uno, and et al. 2020. "Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma" Cancers 12, no. 3: 552. https://doi.org/10.3390/cancers12030552
APA StyleOnoda, N., Sugitani, I., Ito, K.-i., Suzuki, A., Higashiyama, T., Fukumori, T., Suganuma, N., Masudo, K., Nakayama, H., Uno, A., Yane, K., Yoshimoto, S., Ebina, A., Kawasaki, Y., Maeda, S., Iwadate, M., & Suzuki, S. (2020). Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma. Cancers, 12(3), 552. https://doi.org/10.3390/cancers12030552





