Photo-Functionalized Magnetic Nanoparticles as a Nanocarrier of Photodynamic Anticancer Agent for Biomedical Theragnostics
Abstract
:1. Introduction
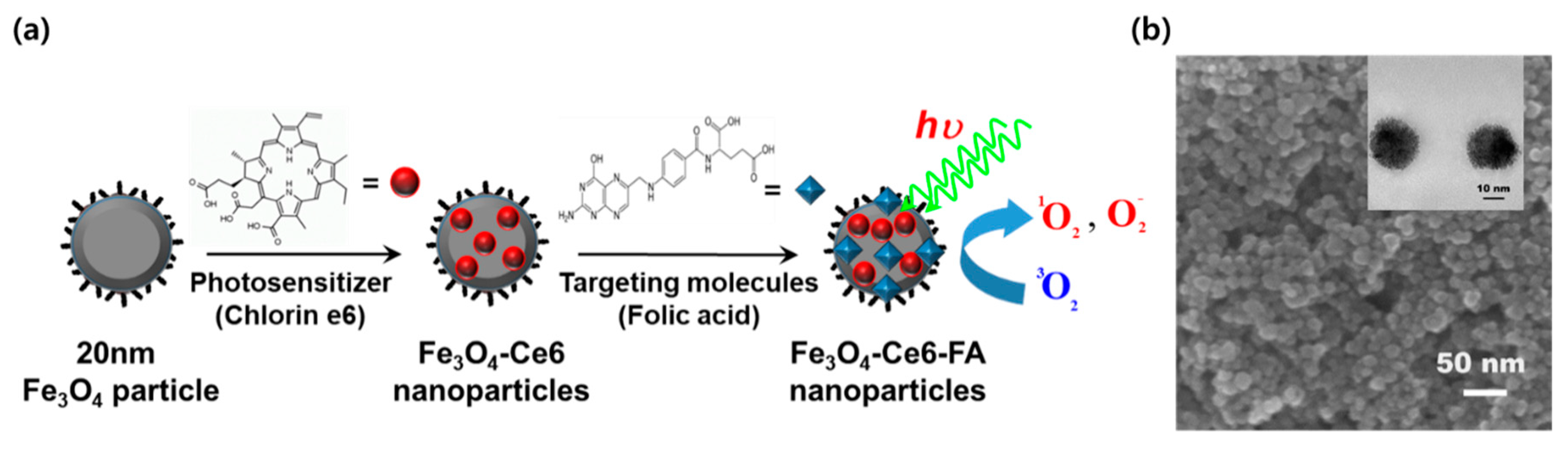
2. Results and Discussion
2.1. Cellular Uptake of FCF NPs by MCF-7 Cells
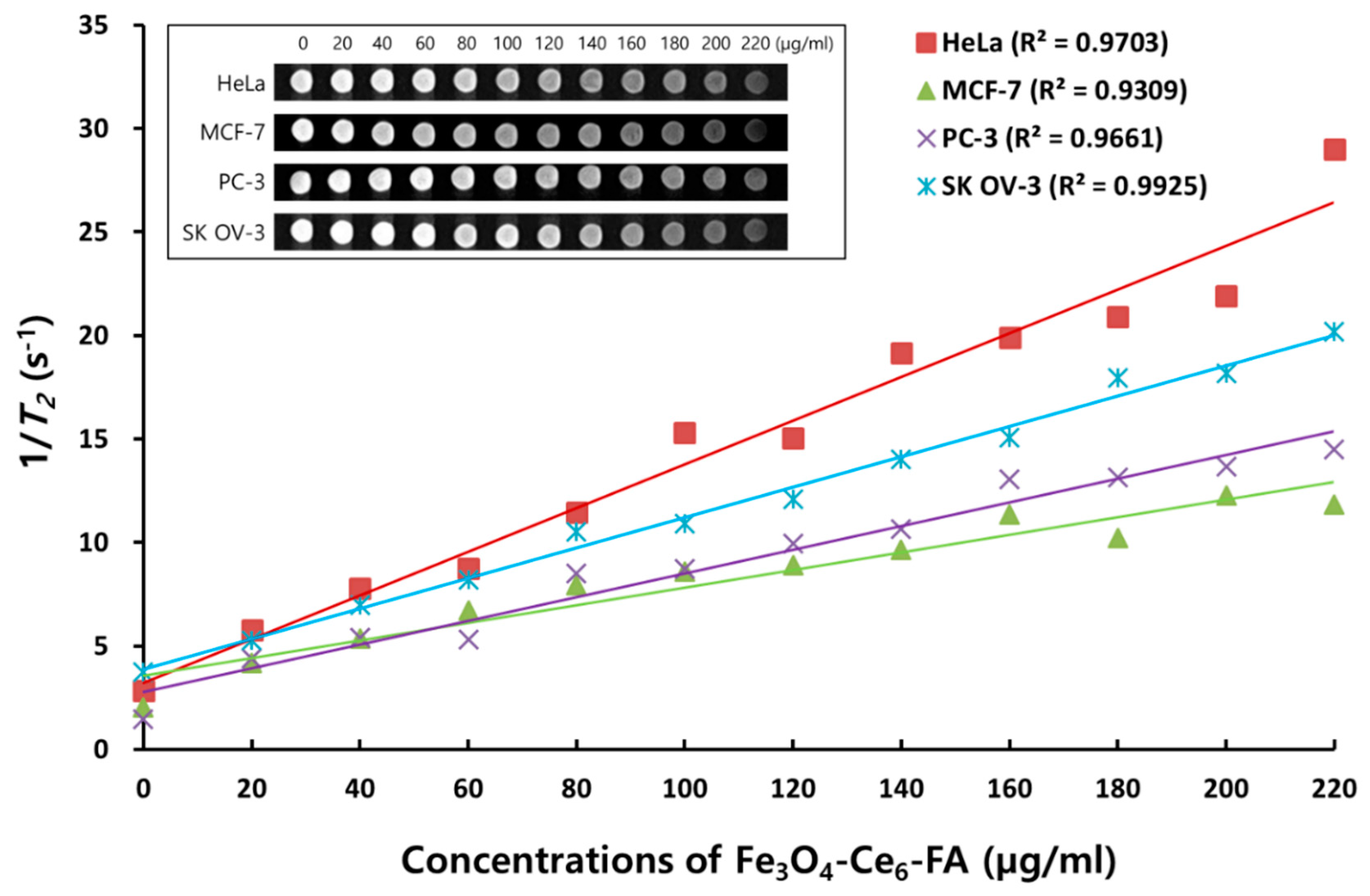
2.2. In Vitro MR Imaging of FCF NPs in Various Cancer Cells
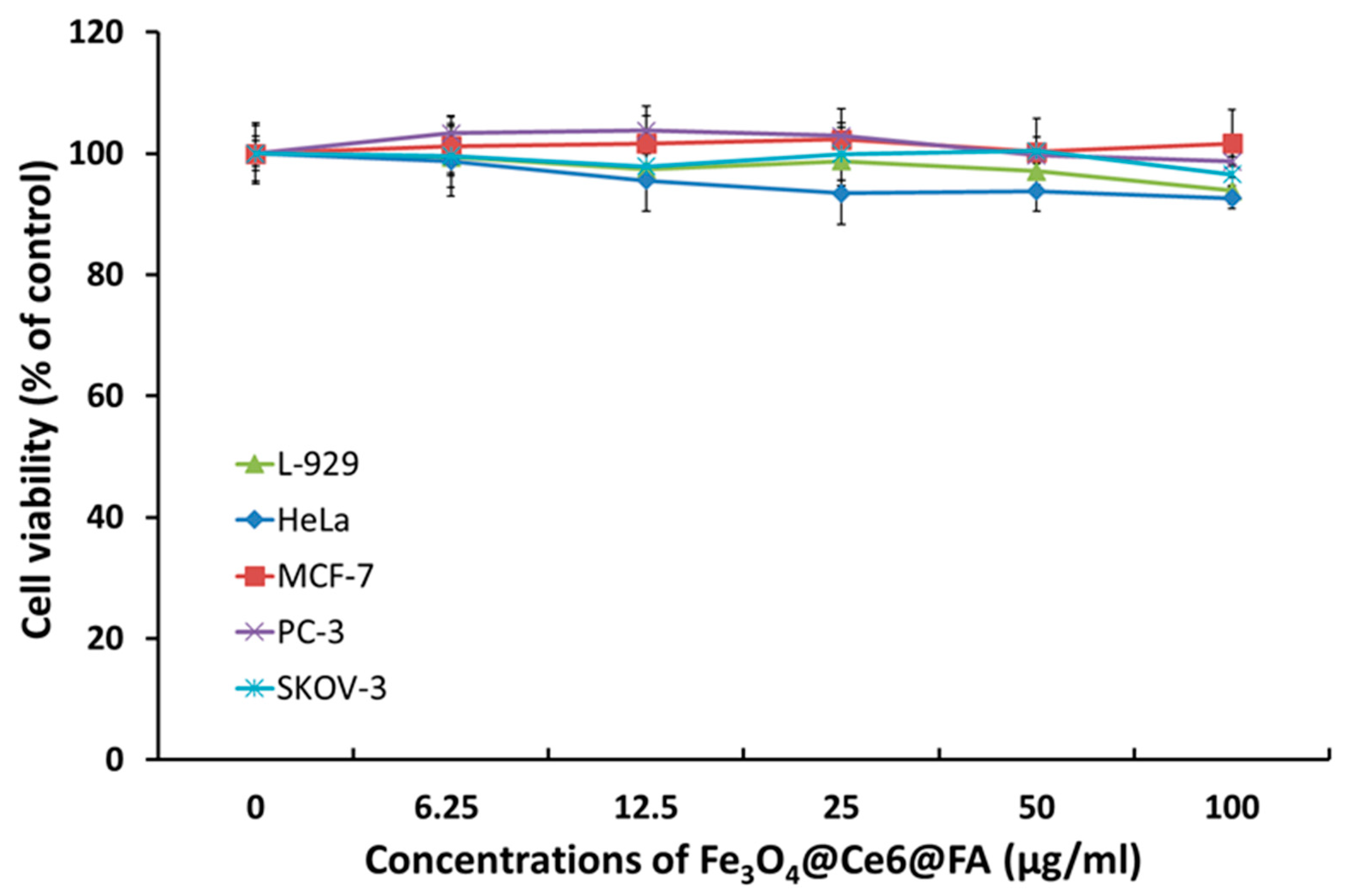
2.3. In Vitro Cytotoxicity of FCF NPs
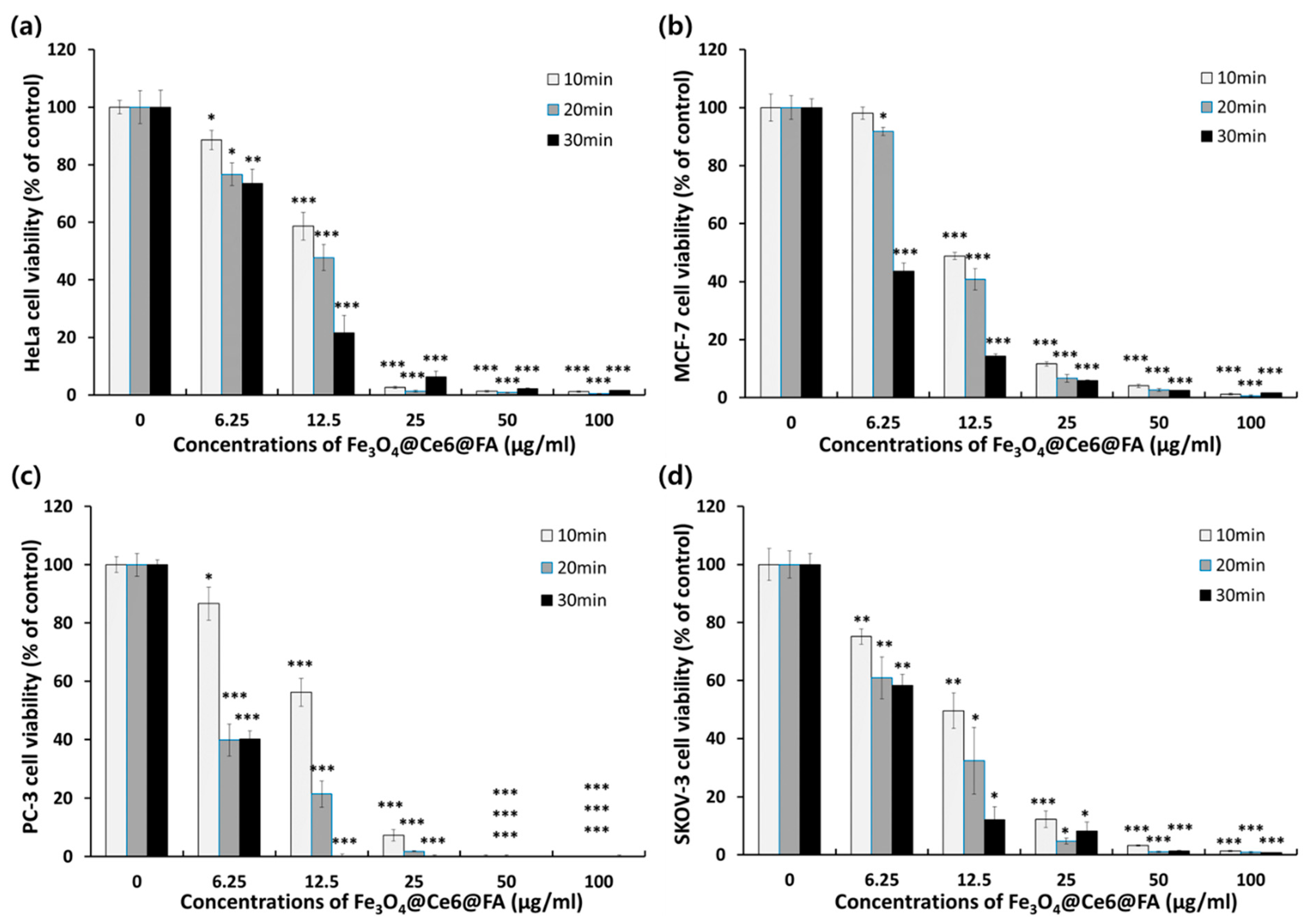
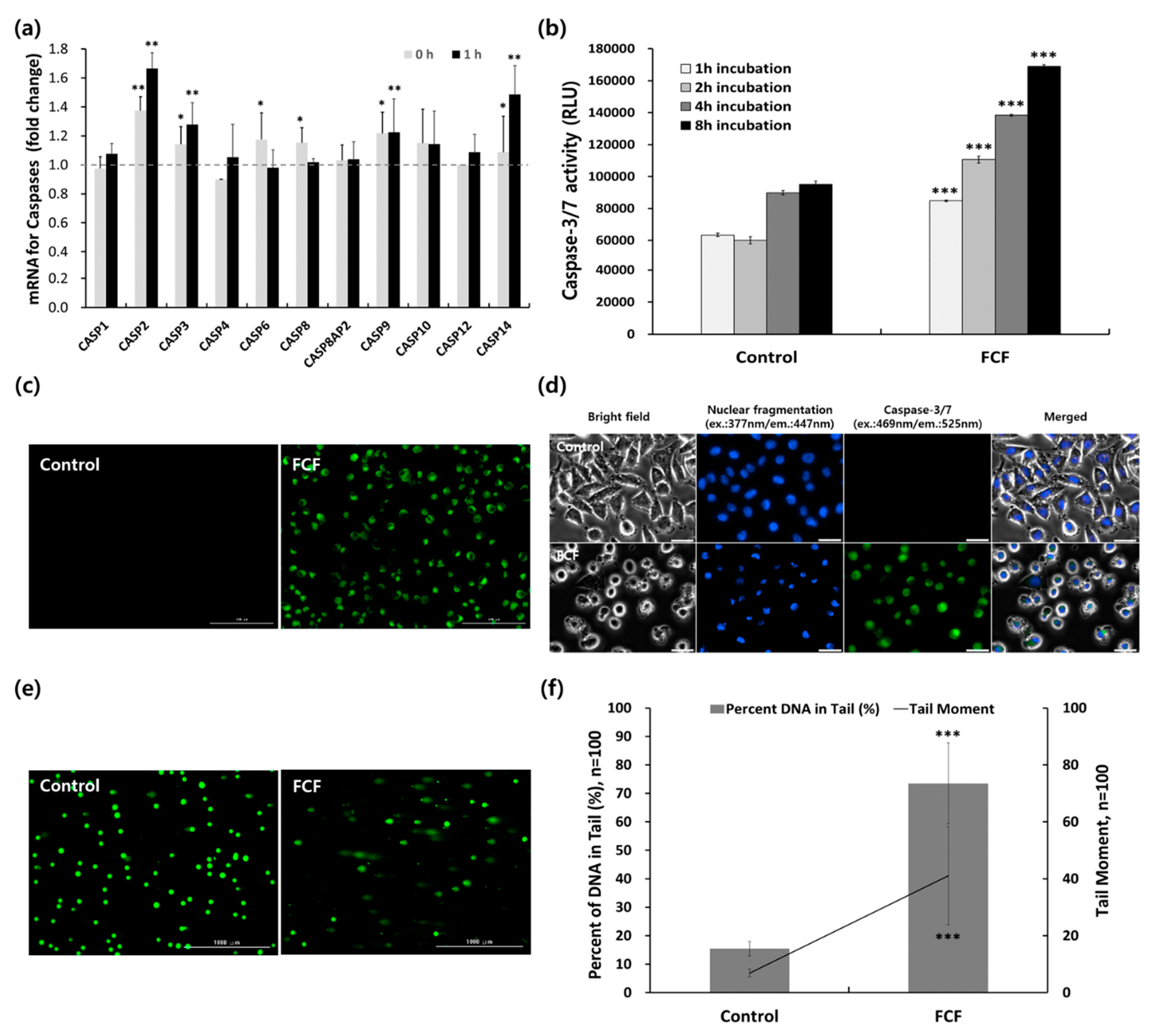
2.4. In Vitro Photodynamic Anticancer Activity of FCF NPs
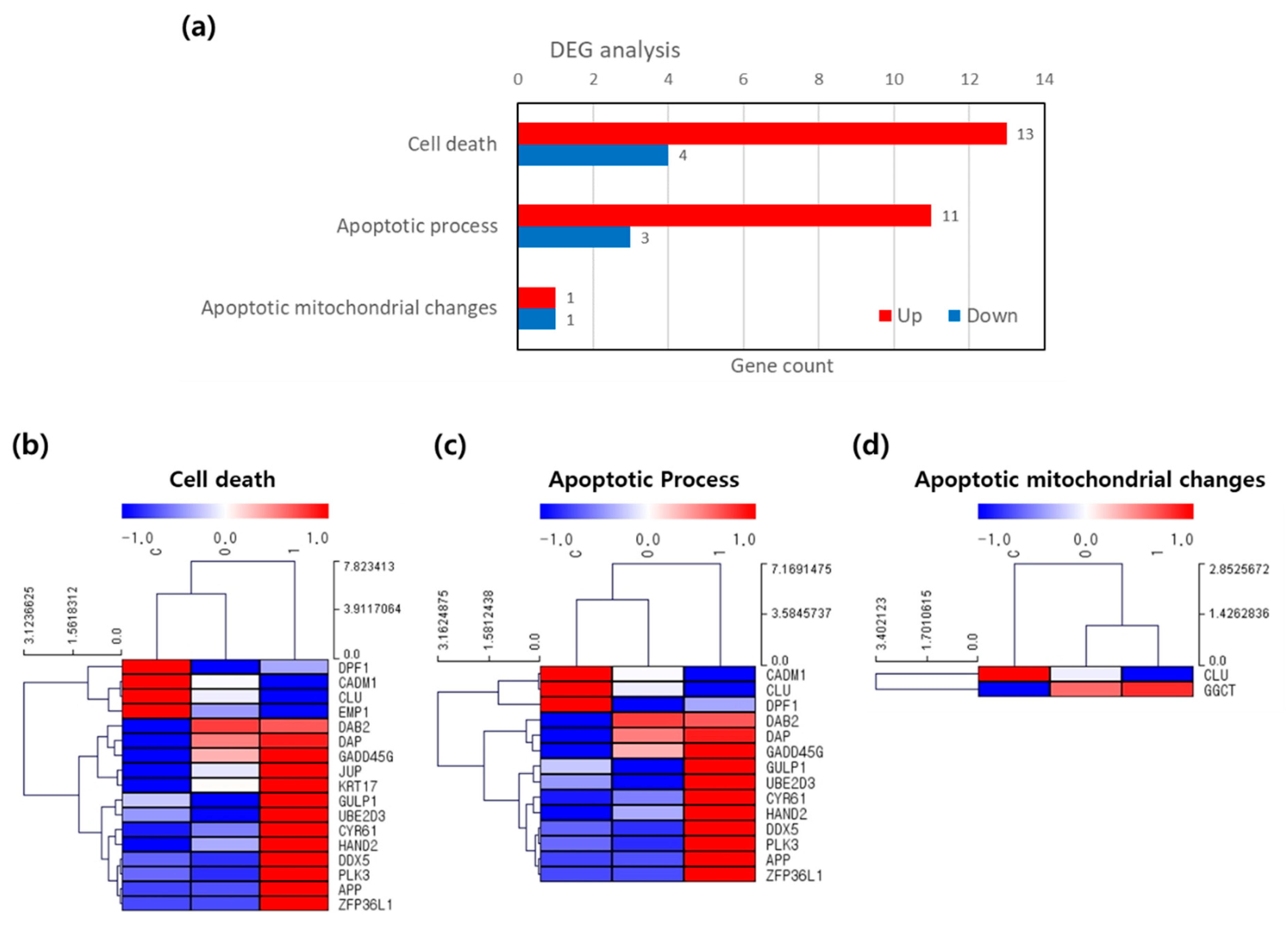
2.5. Analysis of FCF NP-Induced Apoptotic Cell Death in Cancer Cells
3. Materials and Methods
3.1. Preparation and Characterization of FCF NPs
3.2. Cells and Cell Cultures
3.3. Analysis of Cellular Uptake of FCF NPs in Cancer Cells
3.4. In Vitro MR Imaging of FCF NPs in Various Cancer Cells
3.5. In Vitro Cytotoxicity of FCF NPs
3.6. In Vitro Photodynamic Anticancer Activity of FCF NPs
3.7. Detection of Apoptotic Cell Death by PDT
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theragnostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lee, M.M.S.; Xu, W.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Theranostics based on AIEgens. Theranostics 2018, 8, 4925–4956. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, S.; Chen, S. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Singh, A.; Sharma, P.; Brown, S.; Moudgil, B. Nanoparticles as contrast agents for in-vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef]
- Na, H.B.; Hyeon, T. Nanostructured T1 MRI contrast agents. J. Mater. Chem. 2009, 19, 6267–6273. [Google Scholar] [CrossRef]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Bartlett, G.; Antoun, J.; Zgheib, N.K. Theranostics in primary care: Pharmacogenomics tests and beyond. Expert Rev. Mol. Diagn. 2012, 12, 841–855. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multifunctional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef]
- Puri, A.; Blumenthal, R. Polymeric lipid assemblies as novel theranostic tools. Acc. Chem. Res. 2011, 44, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Huh, M.S.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.W.; Kim, I.S.; et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Paul, P.; Kumar, N. International conference on advances in manufacturing and materials engineering. Proc. Mater. Sci. 2014, 5, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.D.; Nagabhushana, H.; Thyagarajan, K.; Nagabhushana, B.M.; Jnaneshwara, D.M.; Sharma, S.C.; Shivakumara, C.; Gopal, N.O.; Ke, S.C.; Chakradhar, R.P.S. Magnetic and dielectric interactions in nano zinc ferrite powder: Prepared by self-sustainable propellant chemistry technique. J. Magn. Magn. Mater. 2014, 358, 132–141. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, J.; Randhawa, B.S. Essence of superparamagnetism in cadmium ferrite induced by various organic fuels via novel solution combustion method. Ceram Int. 2014, 40, 12235–12243. [Google Scholar] [CrossRef]
- Turcua, R.; Craciunescua, I.; Garamusb, V.M.; Jankoc, C.; Lyerc, S.; Tietzec, R.; Alexiouc, C.; Vekas, L. Magnetic microgels for drug targeting applications: Physical- chemical properties and cytotoxicity evaluation. J. Magn. Magn. Mater. 2015, 380, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Calero, M.; Gutierrez, L.; Salas, G.; Luengo, Y.; Lazaro, A.; Acedo, P.; Morales, M.P.; Miranda, R.; Villanueva, A. Efficient and safe internalization of magnetic iron oxide nanoparticles: Two fundamental requirements for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 733–743. [Google Scholar] [CrossRef]
- Maleki, H.; Simchi, A.; Imani, M.; Costa, B.F.O. Size-controlled synthesis of superparamagnetic iron oxide nanoparticles and their surface coating by gold for biomedical applications. J. Magn. Magn. Mater. 2012, 324, 3997–4005. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutierrez, L.; Morales, M.P.; Bohm, I.B. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.P.; Brady, C.; Cowley, J.F.; McGlinchey, S.M.; McGoldrick, N.; Kinnear, D.J.; Andrews, G.P.; Jones, D.S. Triggered drug delivery from biomaterials. Expert Opin. Drug Deliv. 2010, 7, 605–616. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, G.S.; Wang, H.L.; Welsher, K.; Tabakman, S.M.; Sherlock, S.P.; Robinson, J.T.; Liang, Y.Y.; Dai, H.J. Graphite-coated magnetic nanoparticle microarray for few- cells enrichment and detection. ACS Nano 2012, 6, 1094–1101. [Google Scholar] [CrossRef]
- Shi, D.L.; Cho, H.S.; Chen, Y.; Xu, H.; Gu, H.C.; Lian, J.; Wang, W.; Liu, G.K.; Huth, C.; Wang, L.M. Fluorescent polystyrene- Fe3O4 composite nanospheres for in vivo imaging and hyperthermia. Adv. Mater. 2009, 21, 2170–2173. [Google Scholar] [CrossRef] [Green Version]
- Glockl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys. Condens. Matter 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Aguilar-Arteaga, K.; Rodriguez, J.A.; Barrado, E. Magnetic solids in analytical chemistry: A review. Anal. Chim. Acta 2010, 674, 157–165. [Google Scholar] [CrossRef]
- Beveridge, J.S.; Stephens, J.R.; Williams, M.E. The use of magnetic nanoparticles in analytical chemistry. Annu. Rev. Anal. Chem. 2011, 4, 251–273. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, Y.; Fan, Y.B.; Feng, Q.L.; Cui, F.Z.; Watari, F. Biocomposites reinforced by fibers or tubes, as scaffolds for tissue engineering or regenerative medicine. J. Biomed. Mater. Res. Part A 2014, 102, 1580–1594. [Google Scholar] [CrossRef]
- Li, X.M.; Huang, Y.; Zheng, L.S.; Liu, H.F.; Niu, X.F.; Huang, J.; Zhao, F.; Fan, Y.B. Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro. J. Biomed. Mater. Res. Part A 2014, 102, 1092–1101. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, X.M.; Fan, Y.B.; Zhang, G.P.; Li, D.M.; Dong, W.; Sha, Z.Y.; Yu, X.G.; Feng, Q.L.; Cui, F.Z.; et al. Repairing goat tibia segmental bone defect using scaffold cultured with mesenchymal stem cells. J. Biomed. Mater. Res. Part B 2010, 94, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ferain, E.; Legras, R. Templates for engineered nano-objects for use in microwave, electronic devices and biomedical sensing application. Nucl. Instrum. Methods Phys. Res. Sect. B 2009, 267, 1028–1031. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.X.; Hou, Y.F.; Ye, Z.H.; Wang, H.Y.; Koh, K.; Shen, Z.M.; Shu, Y.Q. Label-free surface plasmon resonance cytosensor for breast cancer cell detection based on nano- conjugation of monodisperse magnetic nanoparticle and folic acid. Sens. Actuators B 2014, 201, 433–438. [Google Scholar] [CrossRef]
- Philippova, O.; Barabanova, A.; Molchanov, V.; Khokhlov, A. Magnetic polymer beads: Recent trends and developments in synthetic design and applications. Eur. Polym. J. 2011, 47, 542–559. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.G.; Choi, U.S. Diverse applications of fibers surface-functionalized with nano- and microparticles. Compos. Sci. Technol. 2013, 79, 77–86. [Google Scholar] [CrossRef]
- Chatterjee, K.; Sarkar, S.; Rao, K.J.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.M.; Jo, J.; Cabral, H.; Bakalova, R.; Aoki, I. MR imaging techniques for nano-pathophysiology and theranostics. Adv. Drug Deliv. Rev. 2014, 74, 75–94. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yon, J.; Wu, C.; Dai, Y.; Shi, M.; Dong, L.; Xu, K. T1-T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents. Int. J. Nanomed. 2018, 13, 4607–4625. [Google Scholar] [CrossRef] [Green Version]
- Park, B.J.; Choi, K.H.; Nam, K.I.; Ali, A.; Min, J.E.; Son, H.; Uhm, H.S.; Kim, H.J.; Jung, J.S.; Choi, E.U. Photodynamic anticancer activities of multifunctional cobalt ferrite nanoparticles in various cancer cells. J. Biomed. Nanotechnol. 2015, 11, 226–235. [Google Scholar] [CrossRef]
- Liao, J.; Qi, T.; Chu, B.; Peng, J.; Luo, F.; Qian, Z. Multifunctional nanostructured materials for multimodal cancer imaging and therapy. J. Nanosci. Nanotechnol. 2014, 14, 175–189. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Nam, K.C.; Cho, G.; Jung, J.S.; Park, B.J. Enhanced photodynamic anticancer activities of multifunctional magnetic nanoparticles (Fe3O4) conjugated with chlorin e6 and folic acid in prostate and breast cancer cells. Nanomaterials 2018, 8, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.H.; Nam, K.C.; Malkinski, L.; Choi, E.H.; Jung, J.S.; Park, B.J. Size-dependent photodynamic anticancer activity of biocompatible multifunctional magnetic submicron particles in prostate cancer cells. Molecules 2016, 21, 1187. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Nam, K.C.; Kim, U.H.; Cho, G.; Jung, J.S.; Park, B.J. Optimized photodynamic therapy with multifunctional cobalt magnetic nanoparticles. Nanomaterials 2017, 7, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Choi, K.H.; Park, K.M.; Lee, J.M.; Park, B.J.; Park, K.D. In situ forming and H2O2-releasing hydrogels for treatment of drug-resistant bacterial infections. ACS Appl. Mater. Interfaces 2017, 9, 16890–16899. [Google Scholar] [CrossRef]
- Hu, D.; Sheng, Z.; Fang, S.; Wang, Y.; Gao, D.; Zhang, P.; Gong, P.; Ma, Y.; Cai, L. Folate receptor-targeting gold nanoclusters as fluorescence enzyme mimetic nanoprobes for tumor molecular colocalization diagnosis. Theranostics 2014, 4, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Huang, P.; Gao, G.; Shapter, J.G.; Shen, Y.; Sun, R.; Yue, C.; Zhang, C.; Liu, Y.; Zhou, S.; et al. Superparamagnetic Fe3O4-PEG2k-FA@Ce6 nanoprobes for in vivo dual-mode imaging and targeted photodynamic therapy. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zekavati, A.; Nasir, A.; Alcaraz, A.; Aldrovandi, M.; Marsh, P.; Norton, J.D.; Murphy, J.J. Post-transcriptional tegulation of BCL2 mRNA by the RNA-binding protein ZFP36L1 in malignant B cells. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Suk, F.M.; Chang, C.C.; Lin, R.J.; Lin, S.Y.; Liu, S.C.; Jau, C.F.; Liang, Y.C. ZFP36L1 and ZFP36L2 inhibit cell proliferation in a cyclin D-dependent and p53-independent manner. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vogel, K.U.; Bell, L.S.; Galloway, A.; Ahlfors, H.; Turner, M. The RNA-binding proteins Zfp36l1 and Zfp36l2 enforce the thymic b-selection checkpoint by limiting DNA damage response signaling and cell cycle progression. J. Immunol. 2016, 197, 2673–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, J.I.; Lau, L.F. The matricellular protein CCN1/CYR61 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010, 12, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Chen, C.C.; Hay, N.; Lau, L.F. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J. Cell Biol. 2005, 171, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.A. Senescence, apoptosis and therapy--cutting the lifelines of cancer. Nat. Rev. Cancer 2003, 3, 286–295. [Google Scholar] [CrossRef]
- Lau, L.F. CCN1/CYR61: The very model of a modern matricellular protein. Cell Mol. Life Sci. 2011, 68, 3149–3163. [Google Scholar] [CrossRef] [Green Version]
- Franzen, C.A.; Chen, C.C.; Todorovic, V.; Juric, V.; Monzon, R.I.; Lau, L.F. The matrix protein CCN1 is critical for prostate carcinoma cell proliferation and TRAIL-induced apoptosis. Mol. Cancer Res. 2009, 7, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Cretu, A.; Sha, X.; Tront, J.; Hoffman, B.; Liebermann, D.A. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009, 7, 268–276. [Google Scholar]
- Ying, J.; Srivastava, G.; Hsieh, W.S.; Gao, A.; Murray, P.; Shuen Kuei Liao, S.K.; Ambinder, R.; Tao, Q. The stress-responsive gene GADD45G is a functional tumor wuppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin. Cancer Res. 2005, 11, 6442–6449. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Park, S.M.; Hwang, E.M.; Baek, K.E.; Kim, I.K.; Nam, I.K.; Im, M.J.; Park, S.H.; Bae, S.; Park, J.Y.; et al. Gadd45b mediates Fas-induced apoptosis by enhancing the interaction between p38 and retinoblastoma tumor suppressor. J. Biol. Chem. 2010, 285, 25500–25505. [Google Scholar] [CrossRef] [Green Version]
- Nettersheim, D.; Jostes, S.; Fabry, M.; Honecker, F.; Schumacher, V.; Kirfel, J.; Kristiansen, G.; Schorle, H. A signaling cascade including ARID1A, GADD45B, and DUSP1 induces apoptosis and affects the cell cycle of germ cell cancers after romidepsin treatment. Oncotarget 2016, 7, 74931–74946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhou, B.; Fan, K.; Heung, E.; Xue, L.; Liu, X.; Kirschbaum, M.; Yen, Y. A sequential treatment of depsipeptide followed by 5-azacytidine enhances Gadd45beta expression in hepatocellular carcinoma cells. Anticancer Res. 2007, 27, 3783–3789. [Google Scholar] [PubMed]
- Tenta, R.; Katopodis, H.; Chatziioannou, A.; Pilalis, E.; Calvo, E.; Luu-The, V.; Labrie, F.; Kolisis, F.; Koutsilieris, M. Microarray analysis of survival pathways in human PC-3 prostate cancer cells. Cancer Genom. Proteom. 2007, 4, 309–318. [Google Scholar]
- Hu, Y.; Sun, H.; Drake, J.; Kittrell, F.; Abba, M.C.; Deng, L.; Gaddis, S.; Sahin, A.; Baggerly, K.; Medina, D.; et al. From mice to humans: Identification of commonly deregulated genes in mammary cancer via comparative SAGE studies. Cancer Res. 2004, 64, 7748–7755. [Google Scholar] [CrossRef] [Green Version]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–29. [Google Scholar] [CrossRef]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 2019, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.C.; Han, Y.S.; Lee, J.-M.; Kim, S.C.; Cho, G.; Park, B.J. Photo-Functionalized Magnetic Nanoparticles as a Nanocarrier of Photodynamic Anticancer Agent for Biomedical Theragnostics. Cancers 2020, 12, 571. https://doi.org/10.3390/cancers12030571
Nam KC, Han YS, Lee J-M, Kim SC, Cho G, Park BJ. Photo-Functionalized Magnetic Nanoparticles as a Nanocarrier of Photodynamic Anticancer Agent for Biomedical Theragnostics. Cancers. 2020; 12(3):571. https://doi.org/10.3390/cancers12030571
Chicago/Turabian StyleNam, Ki Chang, Yong Soo Han, Jong-Min Lee, Si Chan Kim, Guangsup Cho, and Bong Joo Park. 2020. "Photo-Functionalized Magnetic Nanoparticles as a Nanocarrier of Photodynamic Anticancer Agent for Biomedical Theragnostics" Cancers 12, no. 3: 571. https://doi.org/10.3390/cancers12030571
APA StyleNam, K. C., Han, Y. S., Lee, J.-M., Kim, S. C., Cho, G., & Park, B. J. (2020). Photo-Functionalized Magnetic Nanoparticles as a Nanocarrier of Photodynamic Anticancer Agent for Biomedical Theragnostics. Cancers, 12(3), 571. https://doi.org/10.3390/cancers12030571




