Age-Dependent Presentation and Clinical Course of 1465 Patients Aged 0 to Less than 18 Years with Ovarian or Testicular Germ Cell Tumors; Data of the MAKEI 96 Protocol Revisited in the Light of Prenatal Germ Cell Biology
Abstract
:1. Introduction
2. Results
2.1. Source and Number of Patients with TER or MGCT and Additional Findings
Histology and Stage Editors
2.2. Follow-Up of Events
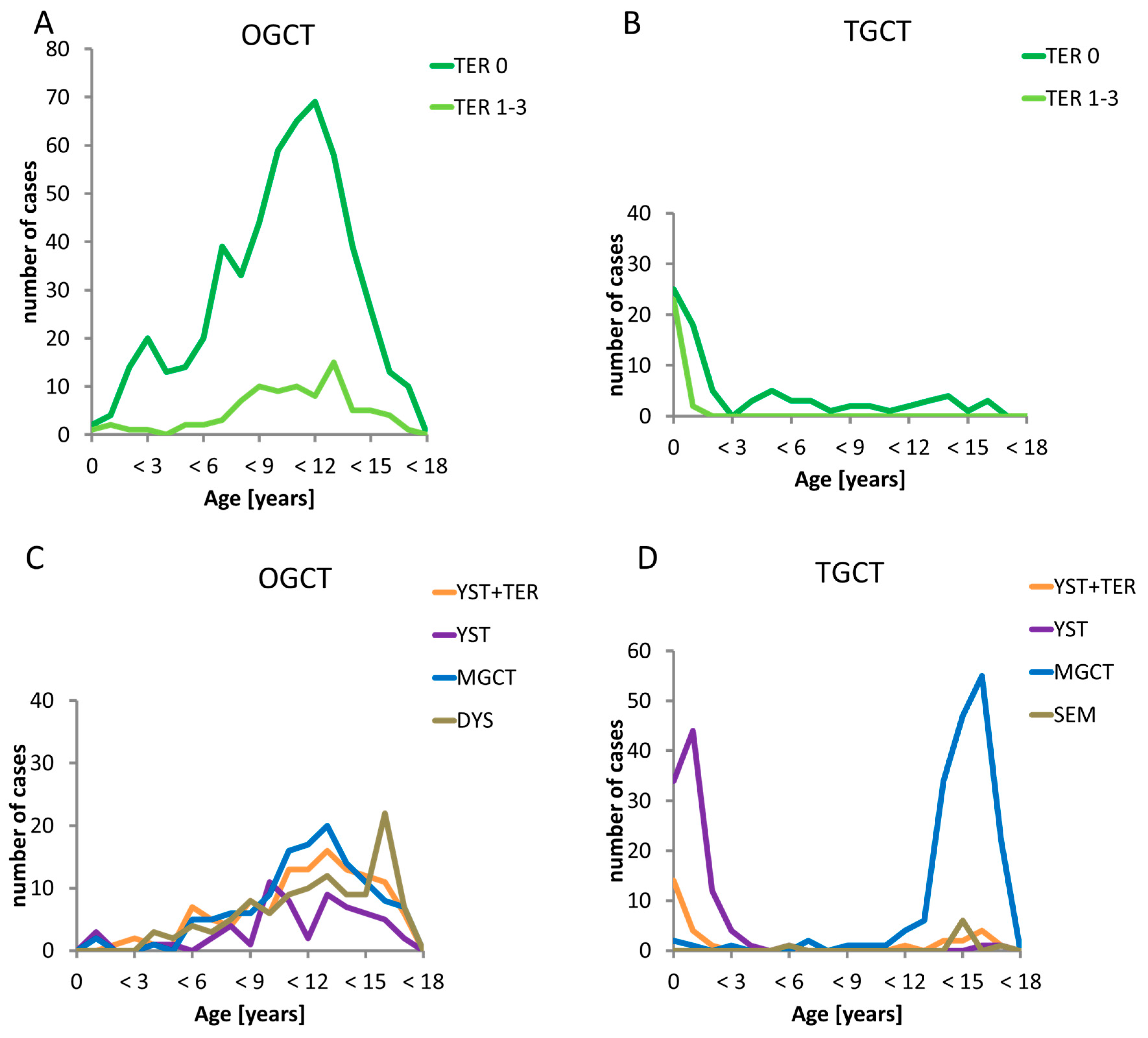
2.3. Histologic Subentities
2.4. Occurrence of Gonadal TER and MGCT with Respect to Sex, Age, and Histology
2.5. Stage of Disease in Respect to Age
2.6. Events in w&w Patients in Respect to Age and Histology
3. Discussion
3.1. Genomic Imprinting Decrypts Initiation of GCT
3.2. Proliferation of GCT
3.3. Teratoma (TER) and Malignant Germ Cell Tumors (MGCT)
3.4. Biologic Heterogeneity Despite Comparable Morphology
3.5. Patients with Best and Worst Prognosis
4. Methods
4.1. Protocol
4.2. Diagnosis and Staging
4.3. Tumor Resection
4.4. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha feto protein |
| ß-HCG | Beta-human chorionic gonadotropin |
| CHC | Choriocarcinomas |
| CNS | Central Nervous System |
| CR | Complete Remission |
| CT | Computer tomography |
| DOD | Dead of Disease |
| DOI | Dead of Lethal Infection |
| DOR | Dead of Other Reason |
| DYS | Dysgerminomas |
| GCT | Germ Cell Tumors |
| GCNIS | Germ Cell Neoplasia in situ of the Testis |
| EFS | Event Free Survival |
| EGGCT | Extragonadal Germ Cell Tumors |
| GER | Germinomas |
| IGCCC | International Germ Cell Classification Consensus |
| MEGCT | Malignant extragonadal Germ Cell Tumors |
| MGCT | Malignant Gonadal Germ Cell Tumors |
| MMGCT | Mixed malignant Germ Cell Tumors |
| MOGCT | Malignant ovarian Germ Cell Tumors |
| MRI | Magnet-resonance-imaging |
| MTGCT | Malignant testicular Germ Cell Tumors |
| OGCT | Ovarian Germ Cell Tumors |
| OS | Overall Survival |
| pts | Patients |
| OTER | Ovarian Teratoma |
| PGC | Primordial Germ Cells |
| RECIST | Response Evaluation Criteria in solid Tumors Guidelines |
| RTT | Rett syndrome is a genetic brain disorder, apparent after 6 to 18 months of age in females. Symptoms include problems with language, coordination, and repetitive movements, additionally slower growth, walking problems, and a smaller head size. |
| REL | Relapse |
| SEM | Seminomas |
| TER | Teratomas |
| TTER | Testicular Teratomas |
| TGCT | Testicular Germ Cell Tumors |
| w&w | Watchful Waiting |
| wpc | Weeks Post conception |
| YST | Yolk Sac Tumors |
References
- Arora, R.S.; Alston, R.D.; Eden, T.O.B.; Geraci, M.; Birch, J.M. Comparative incidence patterns and trends of gonadal and extragonadal germ cell tumors in England, 1979 to 2003. Cancer 2012, 118, 4290–4297. [Google Scholar] [CrossRef]
- Poynter, J.N.; Amatruda, J.F.; Ross, J.A. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer 2010, 116, 4882–4891. [Google Scholar] [CrossRef]
- Rusner, C.; Trabert, B.; Katalinic, A.; Kieschke, J.; Emrich, K.; Stang, A. Incidence patterns and trends of malignant gonadal and extragonadal germ cell tumors in Germany, 1998–2008. Cancer Epidemiol. 2013, 37, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.T.; Calaminus, G.; Koch, S.; Teske, C.; Schmidt, P.; Haas, R.J.; Harms, D.; Göbel, U. Epidemiologic analysis of 1,442 children and adolescents registered in the German germ cell tumor protocols. Pediatr. Blood Cancer 2004, 42, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Göbel, U.; Schneider, D.T.; Calaminus, G.; Jürgens, H.; Spaar, H.J.; Sternschulte, W.; Waag, K.; Harms, D. Multimodal treatment of malignant sacrococcygeal germ cell tumors: A prospective analysis of 66 patients of the German cooperative protocols MAKEI 83/86 and 89. J. Clin. Oncol 2001, 19, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Ablin, A.R.; Krailo, M.D.; Ramsay, N.K.; Malogolowkin, M.H.; Isaacs, H.; Raney, R.B.; Adkins, J.; Hays, D.M.; Benjamin, D.R.; Grosfeld, J.L. Results of treatment of malignant germ cell tumors in 93 children: A report from the Childrens Cancer Study Group. J. Clin. Oncol. 1991, 9, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- de Backer, A.; Madern, G.C.; Pieters, R.; Haentjens, P.; Hakvoort-Cammel, F.G.A.J.; Oosterhuis, J.W.; Hazebroek, F.W.J. Influence of tumor site and histology on long-term survival in 193 children with extracranial germ cell tumors. Eur. J. Pediatr. Surg. 2008, 18, 1–6. [Google Scholar] [CrossRef]
- Einhorn, L.H.; Donohue, J.P. Improved chemotherapy in disseminated testicular cancer. J. Urol. 1977, 117, 65–69. [Google Scholar] [CrossRef]
- Frazier, A.L.; Rumcheva, P.; Olson, T.; Giller, R.; Cushing, B.; Cullen, J.; Marina, N.; London, W.B. Application of the adult international germ cell classification system to pediatric malignant non-seminomatous germ cell tumors: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 50, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Frazier, A.L.; Hale, J.P.; Rodriguez-Galindo, C.; Dang, H.; Olson, T.; Murray, M.J.; Amatruda, J.F.; Thornton, C.; Arul, G.S.; Billmire, D.; et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J. Clin. Oncol. 2015, 33, 195–201. [Google Scholar] [CrossRef]
- Teilum, G. Classification of endodermal sinus tumour (mesoblatoma vitellinum) and so-called “embryonal carcinoma” of the ovary. Acta Pathol. Microbiol. Scand. 1965, 64, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Bussey, K.J.; Lawce, H.J.; Olson, S.B.; Arthur, D.C.; Kalousek, D.K.; Krailo, M.; Giller, R.; Heifetz, S.; Womer, R.; Magenis, R.E. Chromosome abnormalities of eighty-one pediatric germ cell tumors: Sex-, age-, site-, and histopathology-related differences—A Children’s Cancer Group study. Genes Chromosomes Cancer 1999, 25, 134–146. [Google Scholar] [CrossRef]
- Mostert, M.C.; Verkerk, A.J.; van de Pol, M.; Heighway, J.; Marynen, P.; Rosenberg, C.; van Kessel, A.G.; van Echten, J.; de Jong, B.; Oosterhuis, J.W.; et al. Identification of the critical region of 12p over-representation in testicular germ cell tumors of adolescents and adults. Oncogene 1998, 16, 2617–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosterhuis, J.W.; Looijenga, L.H.J. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 2005, 5, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Göbel, U.; Schneider, D.T.; Calaminus, G.; Haas, R.J.; Schmidt, P.; Harms, D. Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann. Oncol. 2000, 11, 263–271. [Google Scholar] [CrossRef]
- Göbel, U.; Calaminus, G.; Haas, R.; Teske, C.; Schönberger, S.; Schneider, D.T.; Leuschner, I.; Harms, D. Testicular germ cell tumors in adolescents—Results of the protocol MAHO 98 and the identification of good risk patients. Klin. Padiatr. 2014, 226, 316–322. [Google Scholar] [CrossRef]
- Witschi, E. Migration of the Germ Cells of Human Embryos from the Yolk Sac to the Primitive Gonadal Folds. Contrib. Embryol. Carnegie Inst. 1948, 32, 67–80. [Google Scholar]
- Mamsen, L.S.; Brøchner, C.B.; Byskov, A.G.; Møllgard, K. The migration and loss of human primordial germ stem cells from the hind gut epithelium towards the gonadal ridge. Int. J. Dev. Biol. 2012, 56, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Runyan, C.; Gu, Y.; Shoemaker, A.; Looijenga, L.; Wylie, C. The distribution and behavior of extragonadal primordial germ cells in Bax mutant mice suggest a novel origin for sacrococcygeal germ cell tumors. Int. J. Dev. Biol. 2008, 52, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.T.; Schuster, A.E.; Fritsch, M.K.; Calaminus, G.; Harms, D.; Göbel, U.; Perlman, E.J. Genetic analysis of childhood germ cell tumors with comparative genomic hybridization. Klin. Padiatr 2001, 213, 204–211. [Google Scholar] [CrossRef]
- Frazier, A.L.; Weldon, C.; Amatruda, J. Fetal and neonatal germ cell tumors. Semin. Fetal Neonatal Med. 2012, 17, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kraggerud, S.M.; Hoei-Hansen, C.E.; Alagaratnam, S.; Skotheim, R.I.; Abeler, V.M.; Rajpert-De Meyts, E.; Lothe, R.A. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: Implications for pathogenesis. Endocr. Rev. 2013, 34, 339–376. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.L.; Frazier, A.L.; Amatruda, J.F. Pediatric Germ Cell Tumors: A Developmental Perspective. Adv. Urol. 2018, 2018, 9059382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, S.; Alemazkour, K.; Zahn, S.; Perlman, E.J.; Gillis, A.J.; Looijenga, L.H.; Göbel, U.; Schneider, D.T. IGF2/H19 imprinting analysis of human germ cell tumors (GCTs) using the methylation-sensitive single-nucleotide primer extension method reflects the origin of GCTs in different stages of primordial germ cell development. Genes Chromosomes Cancer 2005, 44, 256–264. [Google Scholar] [CrossRef]
- Schneider, D.T.; Schuster, A.E.; Fritsch, M.K.; Hu, J.; Olson, T.; Lauer, S.; Göbel, U.; Perlman, E.J. Multipoint imprinting analysis indicates a common precursor cell for gonadal and nongonadal pediatric germ cell tumors. Cancer Res. 2001, 61, 7268–7276. [Google Scholar]
- Zhang, C.; Berney, D.M.; Hirsch, M.S.; Cheng, L.; Ulbright, T.M. Evidence supporting the existence of benign teratomas of the postpubertal testis: A clinical, histopathologic, and molecular genetic analysis of 25 cases. Am. J. Surg. Pathol. 2013, 37, 827–835. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Stoop, J.A.; Rijlaarsdam, M.A.; Biermann, K.; Smit, V.T.H.B.M.; Hersmus, R.; Looijenga, L.H.J. Pediatric germ cell tumors presenting beyond childhood? Andrology 2015, 3, 70–77. [Google Scholar] [CrossRef]
- Looijenga, L.H.J.; Kao, C.-S.; Idrees, M.T. Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology. Int. J. Mol. Sci. 2019, 20, 5017. [Google Scholar] [CrossRef] [Green Version]
- Oosterhuis, J.W.; Looijenga, L.H.J. Current views on the pathogenesis of testicular germ cell tumours and perspectives for future research: Highlights of the 5th Copenhagen Workshop on Carcinoma in situ and Cancer of the Testis. APMIS 2003, 111, 280–289. [Google Scholar] [CrossRef]
- Michala, L.; Goswami, D.; Creighton, S.M.; Conway, G.S. Swyer syndrome: Presentation and outcomes. BJOG 2008, 115, 737–741. [Google Scholar] [CrossRef]
- Cools, M.; Wolffenbuttel, K.P.; Hersmus, R.; Mendonca, B.B.; Kaprová, J.; Drop, S.L.S.; Stoop, H.; Gillis, A.J.M.; Oosterhuis, J.W.; Costa, E.M.F.; et al. Malignant testicular germ cell tumors in postpubertal individuals with androgen insensitivity: Prevalence, pathology and relevance of single nucleotide polymorphism-based susceptibility profiling. Hum. Reprod. 2017, 32, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Hennes, E.; Zahn, S.; Lopes, L.F.; Schönberger, S.; Leuschner, I.; Göbel, U.; Calaminus, G.; Schneider, D.T. Molecular genetic analysis of bilateral ovarian germ cell tumors. Klin. Padiatr. 2012, 224, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Harms, D.; Zahn, S.; Göbel, U.; Schneider, D.T. Pathology and molecular biology of teratomas in childhood and adolescence. Klin. Padiatr 2006, 218, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zahn, S.; Sievers, S.; Alemazkour, K.; Orb, S.; Harms, D.; Schulz, W.A.; Calaminus, G.; Göbel, U.; Schneider, D.T. Imbalances of chromosome arm 1p in pediatric and adult germ cell tumors are caused by true allelic loss: A combined comparative genomic hybridization and microsatellite analysis. Genes Chromosomes Cancer 2006, 45, 995–1006. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Human germ cell tumours from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef]
- Pashankar, F.; Hale, J.P.; Dang, H.; Krailo, M.; Brady, W.E.; Rodriguez-Galindo, C.; Nicholson, J.C.; Murray, M.J.; Bilmire, D.F.; Stoneham, S.; et al. Is adjuvant chemotherapy indicated in ovarian immature teratomas? A combined data analysis from the Malignant Germ Cell Tumor International Collaborative. Cancer 2016, 122, 230–237. [Google Scholar] [CrossRef]
- Biskup, W.; Calaminus, G.; Schneider, D.T.; Leuschner, I.; Göbel, U. Teratoma with malignant transformation: Experiences of the cooperative GPOH protocols MAKEI 83/86/89/96. Klin. Padiatr. 2006, 218, 303–308. [Google Scholar] [CrossRef]
- Greimelmaier, K.; Calaminus, G.; Kristiansen, G.; Vokuhl, C.; Klapper, W. Chromosomal gains of 12p and 1q are not associated with inferior outcome of pediatric and adolescent germ cell tumors. Pediatr. Blood Cancer 2019, 66, e27777. [Google Scholar] [CrossRef]
- Kottmeir, H.L. Classification and Staging of Malignant Tumours in the Female Pelvis. Acta Obstet. Gynecol. Scand. 1971, 50, 1–7. [Google Scholar]
- Cavalli, F.; Monfardini, S.; Pizzocaro, G. Report on the International Workshop on Staging and Treatment of Testicular Cancer. Eur. J. Cancer 1980, 16, 1367–1372. [Google Scholar] [CrossRef]
- Göbel, U.; Haas, R.; Calaminus, G.; Botorek, P.; Schmidt, P.; Teske, C.; Schoenberger, S.; Schneider, D.T.; Harms, D. Testicular germ cell tumors in boys <10 years: Results of the protocol MAHO 98 in respect to surgery and watch & wait strategy. Klin. Padiatr. 2013, 225, 296–302. [Google Scholar]
- Harms, D.; Gottschalk, I.; Jänig, U. Pathologische Anatomie der Keimzelltumoren (besonders Hodentumoren) bei Kindern. Klin. Padiatr. 1983, 195, 181–189. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; Mostofi, F.K., Sesterhenn, I.A., Eds.; IARC Press: Lyon, France, 2004; ISBN 92 832 2412 4. [Google Scholar]
- Gonzalez-Crussi, F. Extragonadal Teratomas, Atlas of Tumor Pathology, Second Series, Fascicle 18 ed.; AFIP: Washington, DC, USA, 1982. [Google Scholar]
- Vaysse, C.; Delsol, M.; Carfagna, L.; Bouali, O.; Combelles, S.; Lemasson, F.; Le Mandat, A.; Castex, M.-P.; Pasquet, M.; Moscovici, J.; et al. Ovarian germ cell tumors in children. Management, survival and ovarian prognosis. A report of 75 cases. J. Pediatr. Surg. 2010, 45, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Chabaud-Williamson, M.; Netchine, I.; Fasola, S.; Larroquet, M.; Lenoir, M.; Patte, C.; Bénifla, J.L.; Coulomb-L’herminé, A.; Grapin, C.; Audry, G.; et al. Ovarian-sparing surgery for ovarian teratoma in children. Pediatr. Blood Cancer 2011, 57, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Göbel, U.; von Kries, R.; Teske, C.; Schneider, D.T.; Beyerlein, A.; Bernbeck, B.; Calaminus, G. Brain metastases during follow-up of children and adolescents with extracranial malignant germ cell tumors: Risk adapted management decision tree analysis based on data of the MAHO/MAKEI-registry. Pediatr. Blood Cancer. 2013, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.T.; Wessalowski, R.; Calaminus, G.; Pape, H.; Bamberg, M.; Engert, J.; Waag, K.; Gadner, H.; Göbel, U. Treatment of recurrent malignant sacrococcygeal germ cell tumors: Analysis of 22 patients registered in the German protocols MAKEI 83/86, 89, and 96. J. Clin. Oncol. 2001, 19, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Wessalowski, R.; Schneider, D.T.; Göbel, U.; Calaminus, G.; MAKEI study group. Salvage treatment of relapsed or refractory germ-cell tumours—Authors’ reply. Lancet Oncol. 2013, 14, 843–852. [Google Scholar] [CrossRef]
- Shen, D.-W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, T.A.; Murray, M.J.; Rodriguez-Galindo, C.; Nicholson, J.C.; Billmire, D.F.; Krailo, M.D.; Dang, H.M.; Amatruda, J.F.; Thornton, C.M.; Arul, G.S.; et al. Pediatric and Adolescent Extracranial Germ Cell Tumors: The Road to Collaboration. J. Clin. Oncol. 2015, 33, 3018–3028. [Google Scholar] [CrossRef] [Green Version]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef]



| Tumor Site | Treatment Strategy | Patients | Progression/Relapse and Alive | Died |
|---|---|---|---|---|
| MOGCT | FIGO I w&w | 137 | 46 (33.6%) | 2 (1.5%) |
| FIGO I + chemo | 108 | 11 (10.2%) | 0 (0%) | |
| FIGO II−IV + chemo | 172 | 11 (6.4%) | 8 (4.7%) | |
| MTGCT | Lugano I A/C w&w | 103 | 12 (11.7%) | 0 (0 ) |
| Lugano I A/C + chemo | 67 | 2 (3.0%) | 1 (1.5%) | |
| Lugano II−IIIC + chemo | 142 | 13 (9.2%) | 9(6.3%) |
| Tumor Site | Age | 0 to <3 | 3 to <6 | 6 to <9 | 9 to <12 | 12 to <15 | 15 to <18 |
|---|---|---|---|---|---|---|---|
| MOGCT | all pts | 6 | 12 | 50 | 101 | 142 | 106 |
| DOD | - | - | - | 4 | 3 ¹ | 2 ¹ | |
| DOR | - | - | - | - | 1 2 | - | |
| MTGCT | all pts | 112 | 6 | 4 | 3 | 47 | 140 |
| DOD | - | - | - | - | 3 3 | 5 | |
| DOI | - | - | - | - | - | 2 |
| Histology | OGCT n (%) | TGCT n (%) | p | ||
|---|---|---|---|---|---|
| TER Σ | 630 | 106 | |||
| mature TER (grade 0) | 544 | (86.3) | 81 | (76.4) | <0.001 |
| immature TER grade 1 | 60 | (9.5) | 14 | (13.2) | 0.24 |
| immature TER grade 2 | 22 | (3.5) | 8 | (7.5) | 0.05 |
| immature TER grade 3 | 4 | (0.6) | 3 | (2.8) | 0.03 |
| MGCT Σ | 417 | 312 | |||
| YST + TER | 119 | (28.5) | 29 | (9.3) | <0.001 |
| pure YST | 62 | (9.2) | 98 | (31.4) | <0.001 |
| pure EC | 1 | (0.2) | 9 | (2.9) | <0.002 |
| pure CC | 11 | (2.6) | 5 | (1.6) | 0.360 |
| MMGCT | 115 | (27.6) | 163 | (52.2) | <0.001 |
| DYS respectively SEM | 109 | (26.1) | 8 | (2.6) | <0.001 |
| TER + MGCT Σ | 1047 | 418 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calaminus, G.; Schneider, D.T.; von Schweinitz, D.; Jürgens, H.; Infed, N.; Schönberger, S.; Olson, T.A.; Albers, P.; Vokuhl, C.; Stein, R.; et al. Age-Dependent Presentation and Clinical Course of 1465 Patients Aged 0 to Less than 18 Years with Ovarian or Testicular Germ Cell Tumors; Data of the MAKEI 96 Protocol Revisited in the Light of Prenatal Germ Cell Biology. Cancers 2020, 12, 611. https://doi.org/10.3390/cancers12030611
Calaminus G, Schneider DT, von Schweinitz D, Jürgens H, Infed N, Schönberger S, Olson TA, Albers P, Vokuhl C, Stein R, et al. Age-Dependent Presentation and Clinical Course of 1465 Patients Aged 0 to Less than 18 Years with Ovarian or Testicular Germ Cell Tumors; Data of the MAKEI 96 Protocol Revisited in the Light of Prenatal Germ Cell Biology. Cancers. 2020; 12(3):611. https://doi.org/10.3390/cancers12030611
Chicago/Turabian StyleCalaminus, Gabriele, Dominik T. Schneider, Dietrich von Schweinitz, Heribert Jürgens, Nacera Infed, Stefan Schönberger, Thomas A. Olson, Peter Albers, Christian Vokuhl, Raimund Stein, and et al. 2020. "Age-Dependent Presentation and Clinical Course of 1465 Patients Aged 0 to Less than 18 Years with Ovarian or Testicular Germ Cell Tumors; Data of the MAKEI 96 Protocol Revisited in the Light of Prenatal Germ Cell Biology" Cancers 12, no. 3: 611. https://doi.org/10.3390/cancers12030611
APA StyleCalaminus, G., Schneider, D. T., von Schweinitz, D., Jürgens, H., Infed, N., Schönberger, S., Olson, T. A., Albers, P., Vokuhl, C., Stein, R., Looijenga, L., Sehouli, J., Metzelder, M., Claviez, A., Dworzak, M., Eggert, A., Fröhlich, B., Gerber, N. U., Kratz, C. P., ... Göbel, U. (2020). Age-Dependent Presentation and Clinical Course of 1465 Patients Aged 0 to Less than 18 Years with Ovarian or Testicular Germ Cell Tumors; Data of the MAKEI 96 Protocol Revisited in the Light of Prenatal Germ Cell Biology. Cancers, 12(3), 611. https://doi.org/10.3390/cancers12030611









