Loss of Y-Chromosome during Male Breast Carcinogenesis
Abstract
:1. Introduction
2. Results
2.1. General Patients and Tumor Characteristics
2.2. LOY in Male BC and Outcome
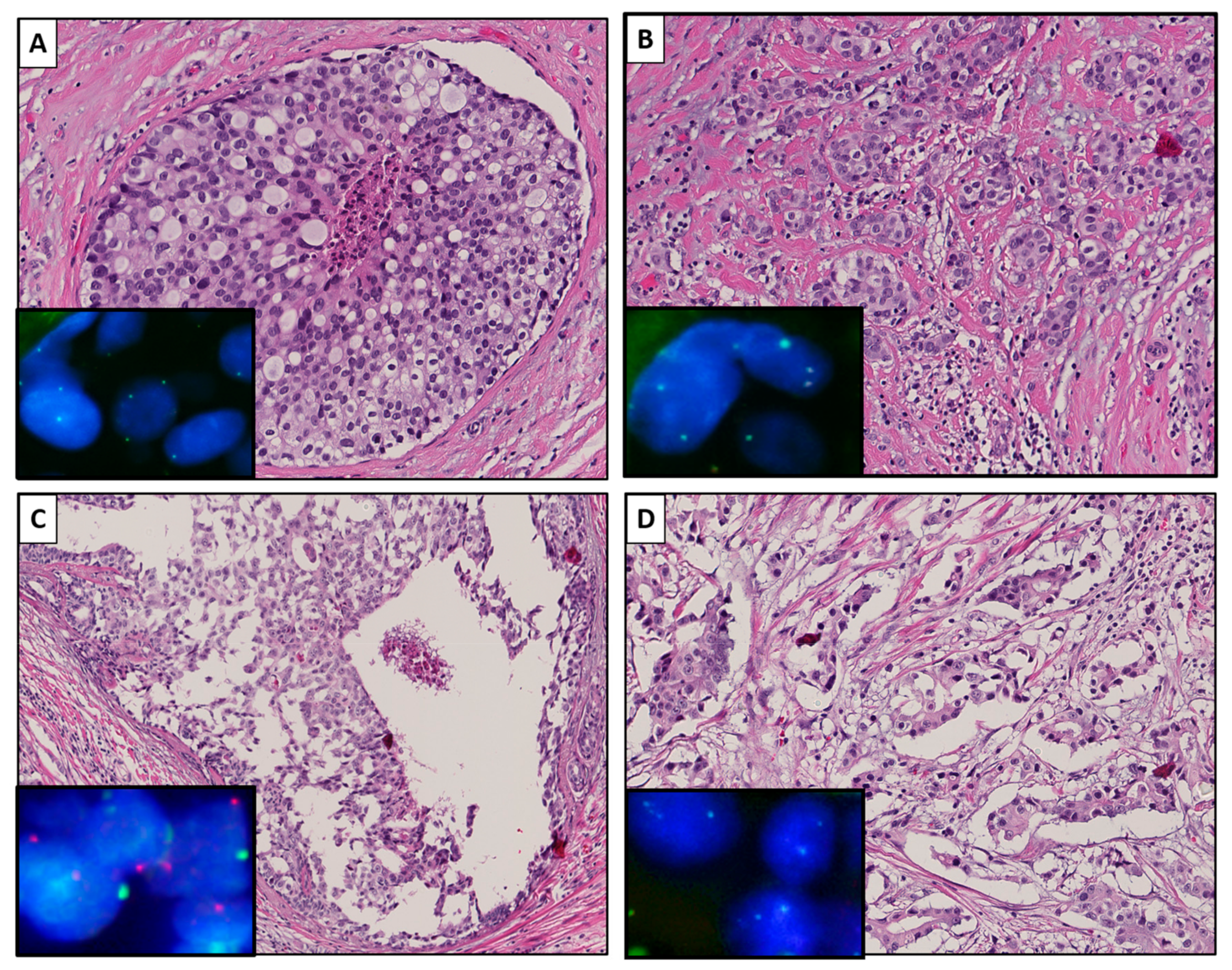
2.3. LOY during Progression from DCIS to Invasive BC
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry on Tissue Micro-Array
4.3. Fluorescent In Situ Hybridization on Invasive BC Using Tissue Micro-Array
4.4. Fluorescent In Situ Hybridization on Invasive BC and Adjacent DCIS Using Whole Tissue Slides
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fentiman, I.S. Male breast cancer is not congruent with the female disease. Crit. Rev. Oncol. Hematol. 2016, 101, 119–124. [Google Scholar] [CrossRef]
- Rudlowski, C. Male Breast Cancer. Breast Care 2008, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.H.; Afonso, N.; Abreu, P.H.; Menezes, F.; Lopes, P.; Henrique, R.; Pereira, D.; Lopes, C. Male breast cancer: Looking for better prognostic subgroups. Breast 2016, 26, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Johansson, I.; Ahlin, C.; Thorstenson, S.; Amini, R.-M.; Holmqvist, M.; Bergkvist, L.; Hedenfalk, I.; Fjällskog, M.-L. Molecular subtyping of male breast cancer using alternative definitions and its prognostic impact. Acta Oncol. 2012, 1, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Bartlett, J.M.S.; Slaets, L.; van Deurzen, C.H.M.; van Leeuwen-Stok, E.; Porter, P.; Linderholm, B.; Hedenfalk, I.; Schröder, C.; Martens, J.; et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 2017, 29, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Gramlich, T.; Abbott, K.; Varma, V. Y chromosome loss in esophageal carcinoma: An in situ hybridization study. Genes. Chromosomes Cancer 1993, 8, 172–177. [Google Scholar] [CrossRef]
- Wallrapp, C.; Hähnel, S.; Boeck, W.; Soder, A.; Mincheva, A.; Lichter, P.; Leder, G.; Gansauge, F.; Sorio, C.; Scarpa, A.; et al. Loss of the Y chromosome is a frequent chromosomal imbalance in pancreatic cancer and allows differentiation to chronic pancreatitis. Int. J. Cancer 2001, 91, 340–344. [Google Scholar] [CrossRef]
- Minner, S.; Kilgué, A.; Stahl, P.; Weikert, S.; Rink, M.; Dahlem, R.; Fisch, M.; Höppner, W.; Wagner, W.; Bokemeyer, C.; et al. Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology 2010, 42, 356–359. [Google Scholar] [CrossRef]
- Wong, A.K.; Fang, B.; Zhang, L.; Guo, X.; Lee, S.; Schreck, R. Loss of the Y chromosome: An age-related or clonal phenomenon in acute myelogenous leukemia/myelodysplastic syndrome? Arch. Pathol. Lab. Med. 2008, 132, 1329–1332. [Google Scholar]
- Adeyinka, A.; Mertens, F.; Bondeson, L.; Garne, J.P.; Borg, Å.; Baldetorp, B.; Pandis, N. Cytogenetic Heterogeneity and Clonal Evolution in Synchronous Bilateral Breast Carcinomas and their Lymph Node Metastases from a Male Patient without Any Detectable BRCA2 Germline Mutation. Cancer Genet. Cytogenet. 2000, 118, 42–47. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Maloney, V.; Cooke, R.; Crolla, J.A.; Ashworth, A.; Swerdlow, A.J. Male breast cancer, age and sex chromosome aneuploidy. Br. J. Cancer 2013, 108, 959–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudas, M.; Schmidinger, M.; Wenzel, C.; Okamoto, I.; Budinsky, A.; Fazeny, B.; Marosi, C. Karyotypic Findings in Two Cases of Male Breast Cancer. Cancer Genet. Cytogenet. 2000, 121, 190–193. [Google Scholar] [CrossRef]
- Wong, H.Y.; Wang, G.M.; Croessmann, S.; Zabransky, D.J.; Chu, D.; Garay, J.P.; Cidado, J.; Cochran, R.L.; Beaver, J.A.; Aggarwal, A.; et al. TMSB4Y is a candidate tumor suppressor on the Y chromosome and is deleted in male breast cancer. Oncotarget 2015, 6, 44927–44940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsberg, L.A.; Rasi, C.; Malmqvist, N.; Davies, H.; Pasupulati, S.; Pakalapati, G.; Sandgren, J.; Diaz de Ståhl, T.; Zaghlool, A.; Giedraitis, V.; et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat. Genet. 2014, 46, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-F.; Feng, W.-L.; Miao, L.-L.; Chen, B.; Yang, H.-J. The prognostic significance of molecular subtype for male breast cancer: A 10-year retrospective study. The Breast 2013, 22, 824–827. [Google Scholar] [CrossRef]
- Thompson, D.J.; Genovese, G.; Halvardson, J.; Ulirsch, J.C.; Wright, D.J.; Terao, C.; Davidsson, O.B.; Day, F.R.; Sulem, P.; Jiang, Y.; et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 2019, 575, 652–657. [Google Scholar] [CrossRef]
- Vermeulen, M.A.; Doebar, S.C.; Van Deurzen, C.H.M.; Martens, J.W.M.; Van Diest, P.J.; Moelans, C.B. Copy number profiling of oncogenes in ductal carcinoma in situ of the male breast. Endocr. Relat. Cancer 2018, 25, 173–184. [Google Scholar] [CrossRef]
- Vermeulen, M.A.; van Deurzen, C.; Doebar, S.C.; de Leng, W.; Martens, J.; van Diest, P.J.; Moelans, C.B. Promoter hypermethylation in ductal carcinoma in situ of the male breast. Endocr. Relat. Cancer 2019, 26, 575–584. [Google Scholar] [CrossRef]
- Vermeulen, M.A.; Slaets, L.; Cardoso, F.; Giordano, S.H.; Tryfonidis, K.; Van Diest, P.J.; Dijkstra, N.H.; Schrö Der, C.P.; Van Asperen, C.J.; Linderholm, B.; et al. Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program ScienceDirect. Eur. J. Cancer 2017, 82, 219–227. [Google Scholar] [CrossRef]
- Doebar, S.C.; Slaets, L.; Cardoso, F.; Giordano, S.H.; Bartlett, J.M.; Tryfonidis, K.; Dijkstra, N.H.; Schröder, C.P.; van Asperen, C.J.; Linderholm, B.; et al. Male breast cancer precursor lesions: Analysis of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Mod. Pathol. 2017, 30, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Dutch Institute for Clinical Auditing Leiden, Factsheet Indicatoren NABON Breast Cancer Audit (NBCA). 2017. Available online: https://dica.nl/media/1567/NBCA%202019.6%20Factstheet%20indicatoren%20extern%202019.pdf (accessed on 15 February 2017).
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federa.org. Human Tissue and Medical Research: Code of conduct for responsible use. 2011. Available online: https://www.federa.org/sites/default/files/digital_version_first_part_code_of_conduct_in_uk_2011_12092012.pdf (accessed on 15 February 2017).


| Patients and Tumor Characteristics | n | Range/% |
|---|---|---|
| Age at diagnosis (in years) | ||
| Median-range | 67 | 25–98 |
| Tumor size (in mm) (missing; n = 413) | ||
| Median-range | 20 | 1–110 |
| Grade (%) (missing; n = 30) | ||
| Low | 190 | 24.8 |
| Intermediate | 401 | 52.3 |
| High | 175 | 22.8 |
| Precursor lesion (missing; n = 27) | ||
| None | 403 | 52.4 |
| DCIS | 360 | 46.8 |
| Other | 6 | 0.8 |
| TIL density (missing; n = 32) | ||
| Minimal-Mild | 652 | 81.9 |
| Moderate-Severe | 112 | 14.1 |
| ER status (missing; n = 54) | ||
| ER+ | 694 | 93.5 |
| ER− | 48 | 6.5 |
| PR status (missing; n = 42) | ||
| PR+ | 548 | 72.7 |
| PR− | 206 | 27.3 |
| AR status (missing; n = 45) | ||
| AR+ | 533 | 71.0 |
| AR− | 218 | 29.0 |
| HER2 status (missing; n = 24) | ||
| HER2+ | 34 | 4.4 |
| HER2− | 738 | 95.6 |
| IHC subtype (Undermined; n = 77) | ||
| ER+PR+/-HER2− | 649 | 90.3 |
| ER+PR+HER2+ | 32 | 4.5 |
| ER-PR-HER2+ | 1 | 0.1 |
| ER-PR-HER2− | 37 | 5.1 |
| Loss of Y (Undetermined; n = 74) | ||
| XY | 630 | 87.3 |
| X_ | 92 | 12.7 |
| Distant metastasis (missing; n = 494) | ||
| Yes | 66 | 21.9 |
| No | 236 | 78.1 |
| Survival (missing; n = 6) | ||
| Alive | 296 | 37.5 |
| Dead | 494 | 62.5 |
| Patient and Tumor Characteristics | XY Status | p-Value | |
|---|---|---|---|
| XY (Range/%) | X_ (Range/%) | ||
| Age at diagnosis | 0.311 | ||
| Median (years) | 67.0 (25–95) | 64.5 (34–98) | |
| Tumor size (missing; n = 362) | 0.093 | ||
| Median (mm) | 20.0 (1–110) | 21.0 (0–90) | |
| Grade (missing; n = 26) | 0.056 | ||
| Low | 161 (26.5) | 13 (14.7) | |
| Intermediate | 306 (50.3) | 53 (60.2) | |
| High | 141 (23.2) | 22 (25.0) | |
| Precursor lesion (missing; n = 24) | 0.605 | ||
| None | 317 (52.0) | 48 (54.5) | |
| DCIS | 287 (47.0) | 40 (45.5) | |
| Other | 6 (1.0) | 0 (0.0) | |
| TIL density (missing; n = 28) | 0.268 | ||
| Minimal-Mild | 515 (85.0) | 13 (54.2) | |
| Moderate-Severe | 91 (15.0) | 11 (45.8) | |
| ER status (missing; n = 41) | 0.017 | ||
| ER+ | 561 (94.4) | 76 (87.4) | |
| ER− | 33 (5.6) | 11 (12.6) | |
| PR status (missing; n = 34) | 0.01 | ||
| PR+ | 448 (74.4) | 53 (61.6) | |
| PR− | 154 (24.6) | 33 (38.4) | |
| AR status (missing; n = 32) | 0.327 | ||
| AR+ | 428 (67.9) | 67 (72.8) | |
| AR− | 174 (27.6) | 21 (22.8) | |
| HER2 status (missing; n = 17) | 0.542 | ||
| HER2+ | 29 (4.7) | 3 (3.3) | |
| HER2− | 585 (95.3) | 88 (96.7) | |
| IHC subtype (undetermined; n = 60) | 0.242 | ||
| ER+/-PR+/-HER2− | 525 (90.7) | 72 (86.7) | |
| ER+PR+/-HER2+ | 27 (4.6) | 3 (3.6) | |
| ER-PR-HER2+ | 1 (0.2) | 0 (0.0) | |
| ER-PR-HER2− | 26 (4.5) | 8 (9.6) | |
| Distant metastasis (missing; n = 451) | 0.157 | ||
| Yes | 54 (22.6) | 3 (10.8) | |
| No | 189 (79.4) | 25 (89.2) | |
| Survival (missing; n = 5) | 0.469 | ||
| Alive | 240 (38.4) | 30 (33.0) | |
| Dead | 386 (61.6) | 61 (67.0) | |
| Patient | DCIS | Invasive BC |
|---|---|---|
| A: Patients with LOY in the Invasive Component (n = 22) | ||
| 1 | X_ | X_ |
| 2 | X_ | X_ |
| 3 | X_ | X_ |
| 4 | X_ | X_ |
| 5 | XY | X_ |
| 6 | X_ | X_ |
| 7 | X_ | X_ |
| 8 | X_ | X_ |
| 9 | X_ | X_ |
| 10 | XY | X_ |
| 11 | X_ | X_ |
| 12 | XY | X_ |
| 13 | X_ | X_ |
| 14 | Undetermined | X_ |
| 15 | X_ | X_ |
| 16 | X_ | X_ |
| 17 | X_ | X_ |
| 18 | X_ | X_ |
| 19 | X_ | X_ |
| 20 | X_ | X_ |
| 21 | X_ | X_ |
| 22 | XY | X_ |
| B: Patients without LOY in the invasive component (n = 20) | ||
| 1 | XY | XY |
| 2 | XY | XY |
| 3 | XY | XY |
| 4 | XY | XY |
| 5 | XY | XY |
| 6 | XY | XY |
| 7 | XY | XY |
| 8 | XY | XY |
| 9 | XY | XY |
| 10 | XY | XY |
| 11 | XY | XY |
| 12 | XY | XY |
| 13 | XY | XY |
| 14 | XY | XY |
| 15 | XY | XY |
| 16 | XY | XY |
| 17 | XY | XY |
| 18 | XY | XY |
| 19 | XY | XY |
| 20 | XY | XY |
| Antibody | Type | Company | Clone | Lot Number | Dilution | Antigen Retrieval pH | Incubation Time |
|---|---|---|---|---|---|---|---|
| ER | Anti-mouse | Dako | 1D5 | M7047 | 1:40 | 9 | 60 min |
| PR | Anti-mouse | Dako | PgR 636 | M3569 | 1:50 | 9 | 60 min |
| AR | Anti-mouse | ErasmusMC | F39.4 | Trapman | 1:50 | 9 | Overnight |
| HER2neu | Anti-Rabbit | Dako | Herceptest | K5204 | ready to use | ready to use | 60 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agahozo, M.C.; Timmermans, M.A.M.; Sleddens, H.F.B.M.; Foekens, R.; Trapman-Jansen, A.M.A.C.; Schröder, C.P.; van Leeuwen-Stok, E.; Martens, J.W.M.; N. M. Dinjens, W.; van Deurzen, C.H.M. Loss of Y-Chromosome during Male Breast Carcinogenesis. Cancers 2020, 12, 631. https://doi.org/10.3390/cancers12030631
Agahozo MC, Timmermans MAM, Sleddens HFBM, Foekens R, Trapman-Jansen AMAC, Schröder CP, van Leeuwen-Stok E, Martens JWM, N. M. Dinjens W, van Deurzen CHM. Loss of Y-Chromosome during Male Breast Carcinogenesis. Cancers. 2020; 12(3):631. https://doi.org/10.3390/cancers12030631
Chicago/Turabian StyleAgahozo, Marie Colombe, Mieke A. M. Timmermans, Hein F. B. M. Sleddens, Renée Foekens, Anita M. A. C. Trapman-Jansen, Carolien P. Schröder, Elise van Leeuwen-Stok, John W. M. Martens, Winand N. M. Dinjens, and Carolien H. M. van Deurzen. 2020. "Loss of Y-Chromosome during Male Breast Carcinogenesis" Cancers 12, no. 3: 631. https://doi.org/10.3390/cancers12030631
APA StyleAgahozo, M. C., Timmermans, M. A. M., Sleddens, H. F. B. M., Foekens, R., Trapman-Jansen, A. M. A. C., Schröder, C. P., van Leeuwen-Stok, E., Martens, J. W. M., N. M. Dinjens, W., & van Deurzen, C. H. M. (2020). Loss of Y-Chromosome during Male Breast Carcinogenesis. Cancers, 12(3), 631. https://doi.org/10.3390/cancers12030631





