Modelling Curved Contact Flexible Microstrip Applicators for Patient-Specific Superficial Hyperthermia Treatment Planning
Abstract
:1. Introduction
2. Materials and Methods
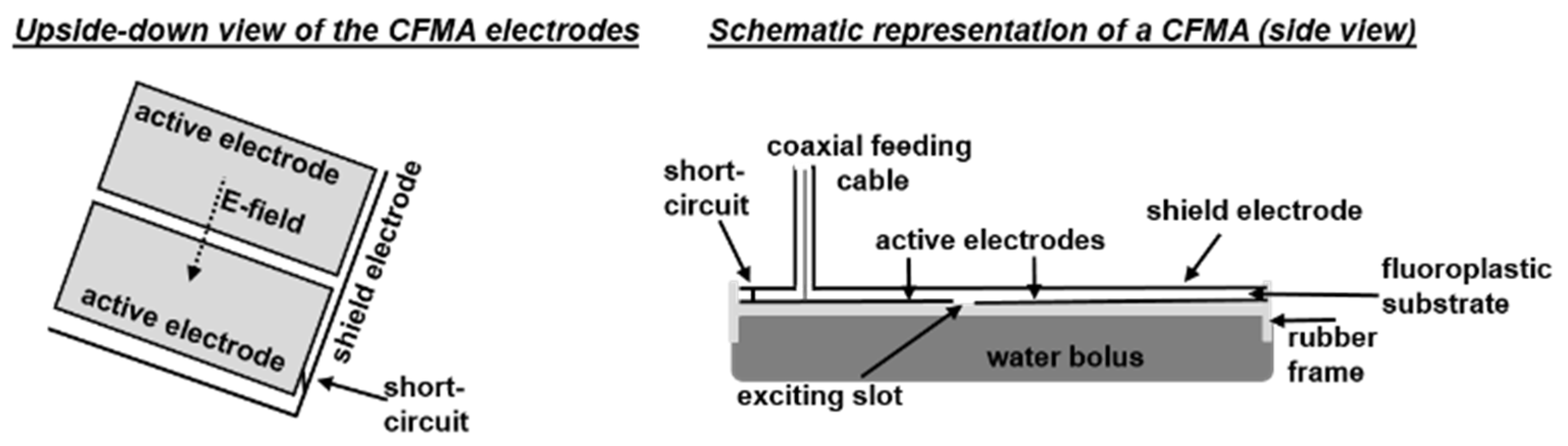
2.1. Contact Flexible Microstrip Applicators
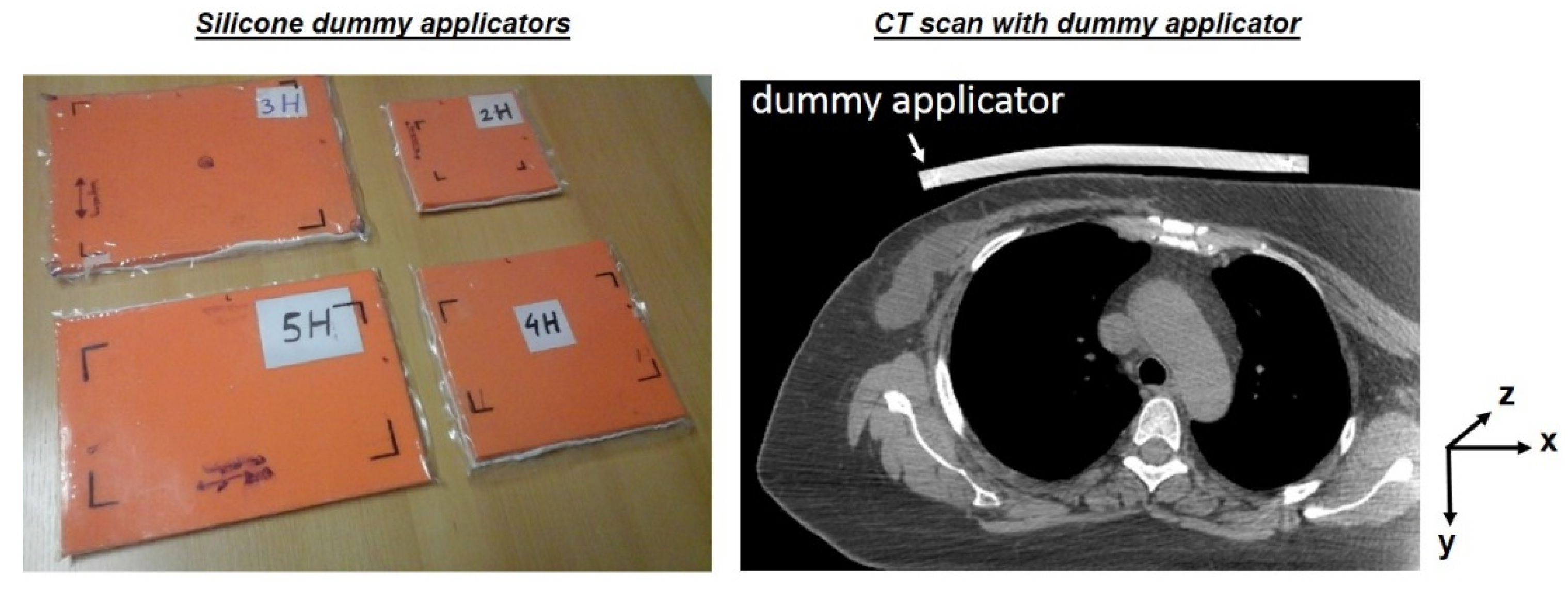
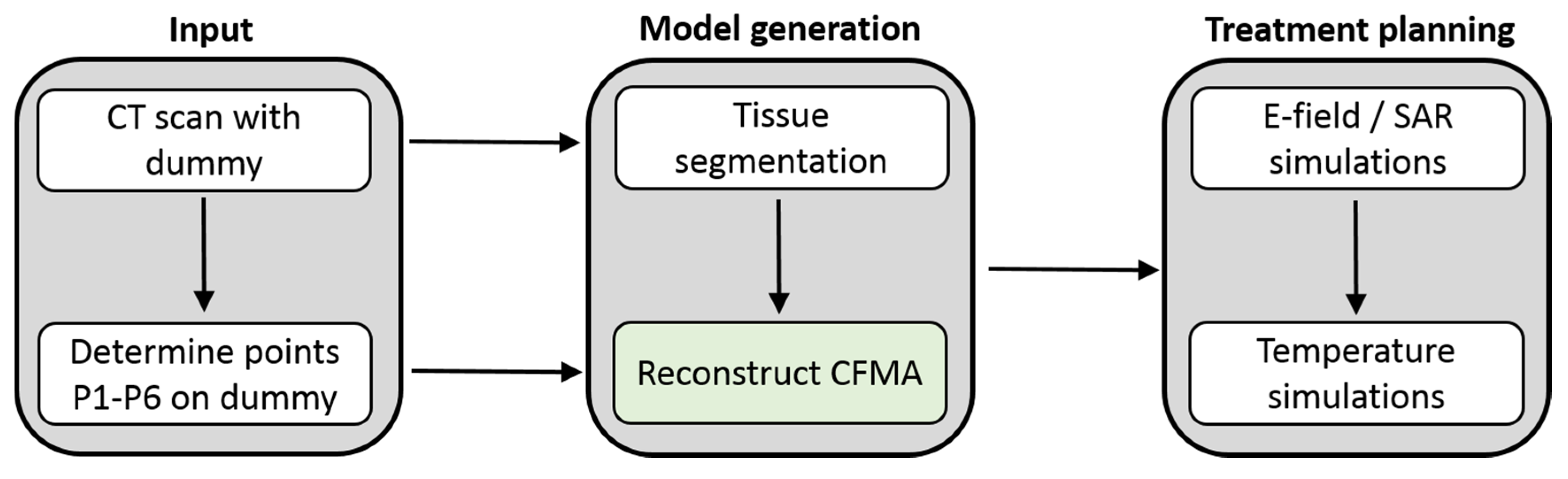
2.2. Generation of a Patient and Applicator Model
2.3. Applicator Curvature Estimation
- The slope is approximately zero near the rigid coaxial cable connector on top of the applicator.
- The reconstructed curve can consist of two segments, described by a different function.
- The areas with the strongest changes in slope are near the coaxial cable connector.
2.4. Curvature Recognition
2.5. Applicator Position and Orientation
2.6. Integration in Treatment Planning
2.7. Validation of the Curvature Approximation
2.8. Superficial Hyperthermia Treatment Planning
3. Results
3.1. Curvature Reconstruction
3.2. Treatment Planning Example
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; Van der Zee, J.; van Putten, W.L.J.; Van Rhoon, G.C.; van Dijk, J.D.P.; González, D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [PubMed] [Green Version]
- Bakker, A.; Holman, R.; Rodrigues, D.B.; Dobsicek Trefna, H.; Stauffer, P.R.; van Tienhoven, G.; Rasch, C.R.N.; Crezee, H. Analysis of clinical data to determine the minimum number of sensors required for adequate skin temperature monitoring of superficial hyperthermia treatments. Int. J. Hyperth. 2018, 34, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.; Van der Zee, J.; van tienhoven, G.; Kok, H.P.; Rasch, C.R.N.; Crezee, H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019, 36, 1024–1039. [Google Scholar] [CrossRef] [Green Version]
- Trefna, H.D.; Crezee, H.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Hartmann, J.; Nadobny, J.; Gellermann, J.; van Holthe, N.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int. J. Hyperth. 2017, 33, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Kim, J.K.; Kim, S. Role of Simulations in the Treatment Planning of Radiofrequency Hyperthermia Therapy in Clinics. J. Oncol. 2019, 2019, 9685476. [Google Scholar] [CrossRef] [Green Version]
- Kok, H.P.; Crezee, J. A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int. J. Hyperth. 2017, 33, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Kok, H.P.; De Greef, M.; Correia, D.; Zum Vörde Sive Vörding, P.J.; Van Stam, G.; Gelvich, E.A.; Bel, A.; Crezee, J. FDTD simulations to assess the performance of CFMA-434 applicators for superficial hyperthermia. Int. J. Hyperth. 2009, 25, 462–476. [Google Scholar] [CrossRef]
- De Bruijne, M.; Wielheesen, D.H.; Van der Zee, J.; Chavannes, N.; Van Rhoon, G.C. Benefits of superficial hyperthermia treatment planning: Five case studies. Int. J. Hyperth. 2007, 23, 417–429. [Google Scholar] [CrossRef]
- Trujillo-Romero, C.J.; Paulides, M.M.; Drizdal, T.; van Rhoon, G.C. Impact of silicone and metal port-a-cath implants on superficial hyperthermia treatment quality. Int. J. Hyperth. 2015, 31, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, A.; Kolff, M.W.; Holman, R.; van Leeuwen, C.M.; Korshuize-van Straten, L.; de Kroon-Oldenhof, R.; Rasch, C.R.N.; van Tienhoven, G.; Crezee, H. Thermal Skin Damage during Reirradiation and Hyperthermia Is Time-Temperature Dependent. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, P.J.; van Putten, W.L.; Van der Zee, J.; Van Rhoon, G.C. Comparison of the clinical effectiveness of the 433 MHz Lucite cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 681–687. [Google Scholar] [CrossRef]
- Van Rhoon, G.C.; Rietveld, P.J.; van der Zee, J. A 433 MHz Lucite cone waveguide applicator for superficial hyperthermia. Int. J. Hyperth. 1998, 14, 13–27. [Google Scholar] [CrossRef]
- Puric, E.; Heuberger, J.; Lomax, N.; Timm, O.; Bodis, S. The Benefit of Thermoradiotherapy in the Treatment of Superficially Localized Tumors: Experience with Bsd 500 Microwave Hyperthermia System. Strahlenther. Onkol. 2009, 185, 648. [Google Scholar]
- Johnson, J.E.; Neuman, D.G.; Maccarini, P.F.; Juang, T.; Stauffer, P.R.; Turner, P. Evaluation of a dual-arm Archimedean spiral array for microwave hyperthermia. Int. J. Hyperth. 2006, 22, 475–490. [Google Scholar] [CrossRef]
- Gelvich, E.A.; Mazokhin, V.N. Contact flexible microstrip applicators (CFMA) in a range from microwaves up to short waves. IEEE Trans. Biomed. Eng. 2002, 49, 1015–1023. [Google Scholar] [CrossRef]
- Gabriele, P.; Ferrara, T.; Baiotto, B.; Garibaldi, E.; Marini, P.G.; Penduzzu, G.; Giovannini, V.; Bardati, F.; Guiot, C. Radio hyperthermia for re-treatment of superficial tumours. Int. J. Hyperth. 2009, 25, 189–198. [Google Scholar] [CrossRef]
- Lamaitre, G.; Van Dijk, J.D.P.; Gelvich, E.A.; Wiersma, J.; Schneider, C.J. SAR characteristics of three types of Contact Flexible Microstrip Applicators for superficial hyperthermia. Int. J. Hyperth. 1996, 12, 255–269. [Google Scholar] [CrossRef]
- Florack, L.M.J.; Ter Haar Romeney, B.M.; Koenderink, J.J.; Viergever, M.A. Cartesian Differential Invariants in Scale-Space. J. Math. Imaging Vis. 1993, 3, 327–348. [Google Scholar] [CrossRef]
- Kok, H.P.; Correia, D.; De Greef, M.; Van Stam, G.; Bel, A.; Crezee, J. SAR deposition by curved CFMA-434 applicators for superficial hyperthermia: Measurements and simulations. Int. J. Hyperth. 2010, 26, 171–184. [Google Scholar]
- Linthorst, M.; Drizdal, T.; Joosten, H.; van Rhoon, G.C.; van der Zee, J. Procedure for creating a three-dimensional (3D) model for superficial hyperthermia treatment planning. Strahlenther. Onkol. 2011, 187, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Drizdal, T.; Paulides, M.M.; Linthorst, M.; van Rhoon, G.C. Reconstruction of applicator positions from multiple-view images for accurate superficial hyperthermia treatment planning. Phys. Med. Biol. 2012, 57, 2491–2503. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Kotte, A.N.T.J.; Crezee, J. Planning, optimisation and evaluation of hyperthermia treatments. Int. J. Hyperth. 2017, 33, 593–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornsleth, S.N.; Mella, O.; Dahl, O. A new segmentation algorithm for finite difference based treatment planning systems. In Hyperthermic Oncology; Franconi, C., Arcangeli, G., Cavaliere, R., Eds.; Tor Vergata: Rome, Italy, 1996; Volume 2, pp. 521–523. [Google Scholar]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ESHO Taskgroup Committee. Treatment Planning and Modelling in Hyperthermia, a Task Group Report of the European Society for Hyperthermic Oncology; Tor Vergata: Rome, Italy, 1992. [Google Scholar]
- Taflove, A.; Hagness, S.C. Computational Electrodynamics, 2nd ed.; Artech House: Boston, MA, USA; London, UK, 2000. [Google Scholar]
- Berenger, J.P. A Perfectly Matched Layer for the Absorption of Electromagnetic-Waves. J. Comput. Phys. 1994, 114, 185–200. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Canters, R.A.; Franckena, M.; Paulides, M.M.; Van Rhoon, G.C. Patient positioning in deep hyperthermia: Influences of inaccuracies, signal correction possibilities and optimization potential. Phys. Med. Biol. 2009, 54, 3923–3936. [Google Scholar] [CrossRef]
- Van der Gaag, M.L.; de Bruijne, M.; Samaras, T.; Van der Zee, J.; Van Rhoon, G.C. Development of a guideline for the water bolus temperature in superficial hyperthermia. Int. J. Hyperth. 2006, 22, 637–656. [Google Scholar] [CrossRef]
- Arunachalam, K.; Maccarini, P.F.; Craciunescu, O.I.; Schlorff, J.L.; Stauffer, P.R. Thermal characteristics of thermobrachytherapy surface applicators for treating chest wall recurrence. Phys. Med. Biol. 2010, 55, 1949–1969. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.; Kok, H.P.; De Greef, M.; Bel, A.; Van Wieringen, N.; Crezee, J. Body conformal antennas for superficial hyperthermia: The impact of bending Contact Flexible Microstrip Applicators on their electromagnetic behavior. IEEE Trans. Biomed. Eng. 2009, 56, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Van Wieringen, N.; Wiersma, J.; Zum Vörde Sive Vörding, P.J.; Oldenborg, S.; Gelvich, E.A.; Mazokhin, V.N.; Van Dijk, J.D.P.; Crezee, H. Characteristics and performance evaluation of the capacitive Contact Flexible Microstrip Applicator operating at 70 MHz for external hyperthermia. Int. J. Hyperth. 2009, 25, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Van Stam, G.; Kok, H.P.; Hulshof, M.; Kolff, M.W.; van Tienhoven, G.; Sijbrands, J.; Bakker, A.; Zum Vorde Sive Vording, P.J.; Oldenborg, S.; de Greef, M.; et al. A flexible 70 MHz phase-controlled double waveguide system for hyperthermia treatment of superficial tumours with deep infiltration. Int. J. Hyperth. 2017, 33, 796–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunachalam, K.; Craciunescu, O.I.; Markewitz, E.J.; Maccarini, P.F.; Schlorff, J.L.; Stauffer, P.R. Preclinical assessment of comfort and secure fit of thermobrachytherapy surface applicator (TBSA) on volunteer subjects. J. Appl. Clin. Med. Phys. 2012, 13, 3845. [Google Scholar] [CrossRef]
- Laaksomaa, M.; Sarudis, S.; Rossi, M.; Lehtonen, T.; Pehkonen, J.; Remes, J.; Luukkanen, H.; Skytta, T.; Kapanen, M. AlignRT® and CatalystTM in whole-breast radiotherapy with DIBH: Is IGRT still needed? J. Appl. Clin. Med. Phys. 2019, 20, 97–104. [Google Scholar] [CrossRef] [Green Version]




| Session | Avg. Deviation (mm) | Max. Deviation (mm) |
|---|---|---|
| 1 | 1.47 ± 1.45 | 4.12 |
| 2 | 1.53 ± 1.01 | 3.25 |
| 3 | 0.81 ± 0.85 | 2.47 |
| 4 | 1.10 ± 0.99 | 3.43 |
| Applicator | Avg. Deviation (mm) | Max. Deviation (mm) |
|---|---|---|
| 1H | 0.99 ± 0.43 | 1.5 |
| 2H | 0.28 ± 0.19 | 0.48 |
| 3H | 0.96 ± 0.68 | 1.98 |
| 4H | 0.59 ± 0.37 | 1.21 |
| 5H | 0.68 ± 0.46 | 1.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kok, H.P.; Groen, J.; Bakker, A.; Crezee, J. Modelling Curved Contact Flexible Microstrip Applicators for Patient-Specific Superficial Hyperthermia Treatment Planning. Cancers 2020, 12, 656. https://doi.org/10.3390/cancers12030656
Kok HP, Groen J, Bakker A, Crezee J. Modelling Curved Contact Flexible Microstrip Applicators for Patient-Specific Superficial Hyperthermia Treatment Planning. Cancers. 2020; 12(3):656. https://doi.org/10.3390/cancers12030656
Chicago/Turabian StyleKok, H. Petra, Jort Groen, Akke Bakker, and Johannes Crezee. 2020. "Modelling Curved Contact Flexible Microstrip Applicators for Patient-Specific Superficial Hyperthermia Treatment Planning" Cancers 12, no. 3: 656. https://doi.org/10.3390/cancers12030656






