Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer
Abstract
1. Introduction
2. Discussion
2.1. Biomarkers as Novel Diagnostic Tools
2.2. Alpha-Fetoprotein
2.3. β-subunit of Human Chorionic Gonadotropin
2.4. Lactate Dehydrogenase
2.5. Placental Alkaline Phosphatase
2.6. DNA Methylation and Histone Modification
2.7. MicroRNAs as Novel Emerging Biomarkers
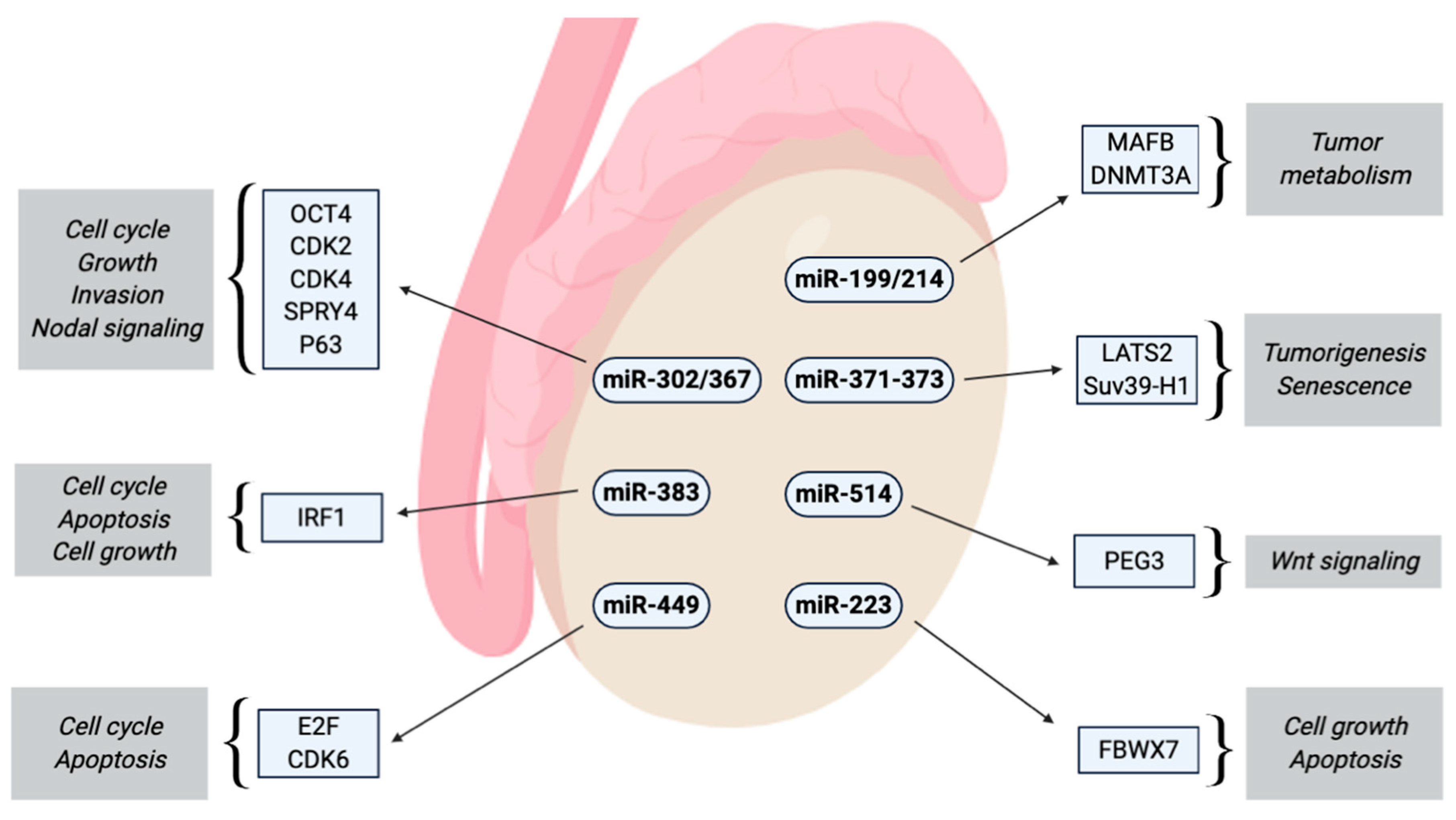
2.7.1. miR-302 Cluster
2.7.2. miR-367
2.7.3. miR-371-373 Cluster
2.7.4. miR-371a-3p
2.7.5. miR-372/373
2.7.6. miR-517/519
2.7.7. miR-223-3p
2.7.8. miR-449
2.7.9. miR-383
2.7.10. miR-514a-3p
2.7.11. miR-199a-3p/214
3. Conclusions
Funding
Conflicts of Interest
References
- Khan, O.; Protheroe, A. Testis cancer. Postgrad. Med. J. 2007, 83, 624–632. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Horwich, A.; Shipley, J.; Huddart, R. Testicular germ-cell cancer. Lancet 2006, 367, 754–765. [Google Scholar] [CrossRef]
- Bezan, A.; Posch, F.; Ploner, F.; Bauernhofer, T.; Pichler, M.; Szkandera, J.; Hutterer, G.C.; Pummer, K.; Gary, T.; Samonigg, H.; et al. Risk stratification for venous thromboembolism in patients with testicular germ cell tumors. PLoS ONE 2017, 12, e0176283. [Google Scholar] [CrossRef]
- Terbuch, A.; Posch, F.; Annerer, L.M.; Bauernhofer, T.; Pichler, M.; Szkandera, J.; Hutterer, G.C.; Pummer, K.; Partl, R.; Kapp, K.S.; et al. Long-term cardiovascular complications in stage I seminoma patients. Clin. Transl. Oncol. 2017, 19, 1400–1408. [Google Scholar] [CrossRef]
- Terbuch, A.; Posch, F.; Partl, R.; Zurl, B.; Bauernhofer, T.; Pichler, M.; Szkandera, J.; Hutterer, G.C.; Pummer, K.; Kapp, K.S.; et al. Risk stratification for febrile neutropenia in patients with testicular germ cell tumors. Cancer Med. 2018, 7, 508–514. [Google Scholar] [CrossRef]
- Terbuch, A.; Posch, F.; Bauernhofer, T.; Pichler, M.; Peinsith, H.; Szkandera, J.; Riedl, J.; Hutterer, G.C.; Pummer, K.; Partl, R.; et al. Age as a Predictor of Treatment Outcome in Metastatic Testicular Germ Cell Tumors. Anticancer Res. 2019, 39, 5589–5596. [Google Scholar] [CrossRef]
- Batool, A.; Karimi, N.; Wu, X.-N.; Chen, S.-R.; Liu, Y.-X. Testicular germ cell tumor: A comprehensive review. Cell Mol. Life Sci. CMLS 2019, 76, 1713–1727. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Simonsen-Richter, H.; Kulejewski, M.; Anheuser, P.; Zecha, H.; Isbarn, H.; Pichlmeier, U. Serum Tumour Markers in Testicular Germ Cell Tumours: Frequencies of Elevated Levels and Extents of Marker Elevation Are Significantly Associated with Clinical Parameters and with Response to Treatment. BioMed. Res. Int. 2019, 2019, 5030349. [Google Scholar] [CrossRef]
- Weissbach, L.; Bussar-Maatz, R.; Mann, K. The value of tumor markers in testicular seminomas. Results of a prospective multicenter study. Eur. Urol. 1997, 32, 16–22. [Google Scholar] [CrossRef]
- Bezan, A.; Gerger, A.; Pichler, M. MicroRNAs in Testicular Cancer: Implications for Pathogenesis, Diagnosis, Prognosis and Therapy. Anticancer Res. 2014, 34, 2709–2713. [Google Scholar]
- Pichler, M.; Calin, G.A. MicroRNAs in cancer: From developmental genes in worms to their clinical application in patients. Br. J. Cancer 2015, 113, 569–573. [Google Scholar] [CrossRef]
- TNM Classification of Malignant Tumours, 7th ed.; Available online: https://www.wiley.com/en-us/TNM+Classification+of+Malignant+Tumours%2C+7th+Edition-p-9781444358964 (accessed on 8 September 2019).
- Masterson, T.A.; Rice, K.R.; Beck, S.D.W. Current and future biologic markers for disease progression and relapse in testicular germ cell tumors: A review. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 261–271. [Google Scholar] [CrossRef]
- Costa, A.L.; Lobo, J.; Jerónimo, C.; Henrique, R. The epigenetics of testicular germ cell tumors: Looking for novel disease biomarkers. Epigenomics 2017, 9, 155–169. [Google Scholar] [CrossRef]
- Terbuch, A.; Adiprasito, J.B.; Stiegelbauer, V.; Seles, M.; Klec, C.; Pichler, G.P.; Resel, M.; Posch, F.; Lembeck, A.L.; Stöger, H.; et al. MiR-371a-3p Serum Levels Are Increased in Recurrence of Testicular Germ Cell Tumor Patients. Int. J. Mol. Sci. 2018, 12, 19. [Google Scholar] [CrossRef]
- AFP-Alpha-fetoprotein Precursor-Homo Sapiens (Human)-AFP Gene & Protein [Internet]. Available online: https://www.uniprot.org/uniprot/P02771 (accessed on 14 September 2019).
- Milose, J.C.; Filson, C.P.; Weizer, A.Z.; Hafez, K.S.; Montgomery, J.S. Role of biochemical markers in testicular cancer: Diagnosis, staging, and surveillance. Open Access J. Urol. 2011, 4, 1–8. [Google Scholar]
- Gitlin, D.; Boesman, M. Serum alpha-fetoprotein, albumin, and gamma-G-globulin in the human conceptus. J. Clin. Investig. 1966, 45, 1826–1838. [Google Scholar] [CrossRef]
- Klepp, O.; Olsson, A.M.; Henrikson, H.; Aass, N.; Dahl, O.; Stenwig, A.E.; Persson, B.E.; Cavallin-Ståhl, E.; Fosså, S.D.; Wahlqvist, L. Prognostic factors in clinical stage I nonseminomatous germ cell tumors of the testis: Multivariate analysis of a prospective multicenter study. Swedish-Norwegian Testicular Cancer Group. J. Clin. Oncol. 1990, 8, 509–518. [Google Scholar] [CrossRef]
- von Eyben, F.E. Laboratory markers and germ cell tumors. Crit. Rev. Clin. Lab. Sci. 2003, 40, 377–427. [Google Scholar] [CrossRef]
- CGB1-Choriogonadotropin Subunit Beta Variant 1 Precursor-Homo Sapiens (Human)-CGB1 Gene & Protein [Internet]. Available online: https://www.uniprot.org/uniprot/A6NKQ9 (accessed on 14 September 2019).
- Gilligan, T.D.; Seidenfeld, J.; Basch, E.M.; Einhorn, L.H.; Fancher, T.; Smith, D.C.; Stephenson, A.J.; Vaughn, D.J.; Cosby, R.; Hayes, D.F.; et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J. Clin. Oncol. 2010, 28, 3388–3404. [Google Scholar] [CrossRef]
- BRENDA-Information on EC 1.1.1.27-L-Lactate Dehydrogenase [Internet]. Available online: https://www.brenda-enzymes.org/enzyme.php?ecno=1.1.1.27# (accessed on 14 September 2019).
- Granchi, C.; Bertini, S.; Macchia, M.; Minutolo, F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr. Med. Chem. 2010, 17, 672–697. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Gilligan, T. Serum tumor markers and their utilization in the management of germ-cell tumors in adult males. Expert Rev. Anticancer Ther. 2011, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Huddart, R.A.; Coleman, N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat. Rev. Urol. 2016, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Favilla, V.; Cimino, S.; Madonia, M.; Morgia, G. New advances in clinical biomarkers in testis cancer. Front. Biosci. 2010, 2, 456–477. [Google Scholar]
- ALPP-Alkaline Phosphatase, Placental Type Precursor-Homo Sapiens (Human)-ALPP Gene & Protein [Internet]. Available online: https://www.uniprot.org/uniprot/P05187 (accessed on 16 September 2019).
- Wermann, H.; Stoop, H.; Gillis, A.J.M.; Honecker, F.; van Gurp, R.J.H.L.M.; Ammerpohl, O.; Richter, J.; Oosterhuis, J.W.; Bokemeyer, C.; Looijenga, L.H. Global DNA methylation in fetal human germ cells and germ cell tumours: Association with differentiation and cisplatin resistance. J. Pathol. 2010, 221, 433–442. [Google Scholar] [CrossRef]
- Boccellino, M.; Vanacore, D.; Zappavigna, S.; Cavaliere, C.; Rossetti, S.; D’Aniello, C.; Chieffi, P.; Amler, E.; Buonerba, C.; Di Lorenzo, G.; et al. Testicular cancer from diagnosis to epigenetic factors. Oncotarget 2017, 8, 104654–104663. [Google Scholar] [CrossRef]
- Ushida, H.; Kawakami, T.; Minami, K.; Chano, T.; Okabe, H.; Okada, Y.; Okamoto, K. Methylation profile of DNA repetitive elements in human testicular germ cell tumor. Mol. Carcinog. 2012, 51, 711–722. [Google Scholar] [CrossRef]
- Ellinger, J.; Albers, P.; Perabo, F.G.; Müller, S.C.; von Ruecker, A.; Bastian, P.J. CpG island hypermethylation of cell-free circulating serum DNA in patients with testicular cancer. J. Urol. 2009, 182, 324–329. [Google Scholar] [CrossRef]
- Rajpert-De Meyts, E.; Nielsen, J.E.; Skakkebaek, N.E.; Almstrup, K. Diagnostic markers for germ cell neoplasms: From placental-like alkaline phosphatase to micro-RNAs. Folia Histochem. Cytobiol. 2015, 53, 177–188. [Google Scholar] [CrossRef]
- Füllgrabe, J.; Kavanagh, E.; Joseph, B. Histone onco-modifications. Oncogene 2011, 30, 3391–3403. [Google Scholar] [CrossRef]
- Lambrot, R.; Kimmins, S. Histone methylation is a critical regulator of the abnormal expression of POU5F1 and RASSF1A in testis cancer cell lines. Int. J. Androl. 2011, 34, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.A.; Slaby, O.; Klec, C.; Juracek, J.; Drula, R.; Calin, G.A.; Pichler, M. Current Concepts of Non-Coding RNAs in the Pathogenesis of Non-Clear Cell Renal Cell Carcinoma. Cancers 2019, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Klec, C.; Prinz, F.; Pichler, M. Involvement of the long noncoding RNA NEAT1 in carcinogenesis. Mol. Oncol. 2019, 13, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Schanza, L.-M.; Seles, M.; Stotz, M.; Fosselteder, J.; Hutterer, G.C.; Pichler, M.; Stiegelbauer, V. MicroRNAs Associated with Von Hippel-Lindau Pathway in Renal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2017, 22, 18. [Google Scholar] [CrossRef]
- Gutschner, T.; Richtig, G.; Haemmerle, M.; Pichler, M. From biomarkers to therapeutic targets-the promises and perils of long non-coding RNAs in cancer. Cancer Metast. Rev. 2018, 37, 83–105. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Wagner, A.; Mayr, C.; Bach, D.; Illig, R.; Plaetzer, K.; Berr, F.; Pichler, M.; Neureiter, D.; Kiesslich, T. MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines. Int. J. Mol. Sci. 2014, 15, 20134–20157. [Google Scholar] [CrossRef]
- Pehserl, A.-M.; Ress, A.L.; Stanzer, S.; Resel, M.; Karbiener, M.; Stadelmeyer, E.; Stiegelbauer, V.; Gerger, A.; Mayr, C.; Scheideler, M.; et al. Comprehensive Analysis of miRNome Alterations in Response to Sorafenib Treatment in Colorectal Cancer Cells. Int. J. Mol. Sci. 2016, 1, 17. [Google Scholar]
- Smolle, M.A.; Prinz, F.; Calin, G.A.; Pichler, M. Current concepts of non-coding RNA regulation of immune checkpoints in cancer. Mol. Aspects Med. 2019, 70, 117–126. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Iftikhar, H.; Carney, G.E. Evidence and potential in vivo functions for biofluid miRNAs: From expression profiling to functional testing: Potential roles of extracellular miRNAs as indicators of physiological change and as agents of intercellular information exchange. BioEssays News Rev. Mol. Cell Dev. Biol. 2016, 38, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbacher, D.; Klec, C.; Pasculli, B.; Cerk, S.; Rinner, B.; Karbiener, M.; Ivan, C.; Barbano, R.; Ling, H.; Wulf-Goldenberg, A.; et al. MiR-1287-5p inhibits triple negative breast cancer growth by interaction with phosphoinositide 3-kinase CB, thereby sensitizing cells for PI3Kinase inhibitors. Breast Cancer Res. 2019, 21, 20. [Google Scholar] [CrossRef]
- Pichler, M.; Stiegelbauer, V.; Vychytilova-Faltejskova, P.; Ivan, C.; Ling, H.; Winter, E.; Zhang, X.; Goblirsch, M.; Wulf-Goldenberg, A.; Ohtsuka, M.; et al. Genome-Wide miRNA Analysis Identifies miR-188-3p as a Novel Prognostic Marker and Molecular Factor Involved in Colorectal Carcinogenesis. Clin. Cancer Res. 2017, 23, 1323–1333. [Google Scholar] [CrossRef]
- Li, H.-L.; Wei, J.-F.; Fan, L.-Y.; Wang, S.-H.; Zhu, L.; Li, T.-P.; Lin, G.; Sun, Y.; Sun, Z.J.; Ding, J.; et al. miR-302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4-dependent manner. Cell Death Dis. 2016, 7, e2078. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, X.; Dou, Y. The MiR-302/367 Cluster: A Comprehensive Update on Its Evolution and Functions. Open Biol. 2015, 5, 150138. [Google Scholar] [CrossRef]
- Das, M.K.; Evensen, H.S.F.; Furu, K.; Haugen, T.B. MiRNA-302s may act as oncogenes in human testicular germ cell tumours. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Lee, J.; Go, Y.; Kang, I.; Han, Y.-M.; Kim, J. Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem. J. 2010, 426, 171–181. [Google Scholar] [CrossRef]
- Ma, G.; Li, Q.; Dai, W.; Yang, X.; Sang, A. Prognostic Implications of miR-302a/b/c/d in Human Gastric Cancer. Pathol. Oncol. Res. POR 2017, 23, 899–905. [Google Scholar] [CrossRef]
- Wang, L.; Yao, J.; Shi, X.; Hu, L.; Li, Z.; Song, T.; Huang, C. MicroRNA-302b suppresses cell proliferation by targeting EGFR in human hepatocellular carcinoma SMMC-7721 cells. BMC Cancer 2013, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Wu, X.; Kawamoto, K.; Nishida, N.; Konno, M.; Koseki, J.; Matsui, H.; Noguchi, K.; Gotoh, N.; Yamamoto, T.; et al. MicroRNAs Induce Epigenetic Reprogramming and Suppress Malignant Phenotypes of Human Colon Cancer Cells. PLoS ONE 2015, 10, e0127119. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Furu, K.; Evensen, H.F.; Haugen, Ø.P.; Haugen, T.B. Knockdown of SPRY4 and SPRY4-IT1 inhibits cell growth and phosphorylation of Akt in human testicular germ cell tumours. Sci. Rep. 2018, 8, 2462. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Halsall, D.J.; Hook, C.E.; Williams, D.M.; Nicholson, J.C.; Coleman, N. Identification of microRNAs From the miR-371~373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am. J. Clin. Pathol. 2011, 135, 119–125. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Deng, J.H.; Deng, Q.; Ying, S.-Y. A novel role of miR-302/367 in reprogramming. Biochem. Biophys. Res. Commun. 2012, 417, 11–16. [Google Scholar] [CrossRef]
- Syring, I.; Bartels, J.; Holdenrieder, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Circulating serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers in patients with testicular germ cell cancer. J. Urol. 2015, 193, 331–337. [Google Scholar] [CrossRef]
- Murray, M.J.; Bell, E.; Raby, K.L.; Rijlaarsdam, M.A.; Gillis, A.J.M.; Looijenga, L.H.J.; Brown, H.; Destenaves, B.; Nicholson, J.C.; Coleman, N. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br. J. Cancer 2016, 114, 151–162. [Google Scholar] [CrossRef]
- van Agthoven, T.; Looijenga, L.H.J. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 2016, 8, 58037–58049. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Radtke, A.; Spiekermann, M.; Balks, T.; Matthies, C.; Becker, P.; Ruf, C.; Oing, C.; Oechsle, K.; Bokemeyer, C.; et al. Serum Levels of MicroRNA miR-371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumours. Eur. Urol. 2017, 71, 213–220. [Google Scholar] [CrossRef]
- Leão, R.; van Agthoven, T.; Figueiredo, A.; Jewett, M.A.S.; Fadaak, K.; Sweet, J.; Ahmad, A.E.; Anson-Cartwright, L.; Chung, P.; Hansen, A.; et al. Serum miRNA Predicts Viable Disease after Chemotherapy in Patients with Testicular Nonseminoma Germ Cell Tumor. J. Urol. 2018, 200, 126–135. [Google Scholar] [CrossRef]
- Rosas Plaza, X.; van Agthoven, T.; Meijer, C.; van Vugt, M.A.T.M.; de Jong, S.; Gietema, J.A.; Looijenga, L.H.J. miR-371a-3p, miR-373-3p and miR-367-3p as Serum Biomarkers in Metastatic Testicular Germ Cell Cancers Before, During and After Chemotherapy. Cells 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.M.; Stoop, H.J.; Hersmus, R.; Oosterhuis, J.W.; Sun, Y.; Chen, C.; Guenther, S.; Sherlock, J.; Veltman, I.; Baeten, J.; et al. High-throughput microRNAome analysis in human germ cell tumours. J. Pathol. 2007, 213, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cao, C.; Xu, X.; Wang, J. Diverse functions of miR-373 in cancer. J. Transl. Med. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.D.; Murray, M.J.; Saini, H.K.; van Dongen, S.; Abreu-Goodger, C.; Muralidhar, B.; Pett, M.R.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 2010, 70, 2911–2923. [Google Scholar] [CrossRef]
- Voorhoeve, P.M.; le Sage, C.; Schrier, M.; Gillis, A.J.M.; Stoop, H.; Nagel, R.; Liu, Y.P.; van Duijse, J.; Drost, J.; Griekspoor, A.; et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 2006, 124, 1169–1181. [Google Scholar] [CrossRef]
- Pan, B.; He, B.; Xu, X.; Liu, X.; Xu, T.; Xu, M.; Chen, X.; Zeng, K.; Lin, K.; Hu, X.; et al. MicroRNA-371-3 cluster as biomarkers for the diagnosis and prognosis of cancers. Cancer Manag. Res. 2019, 11, 5437–5457. [Google Scholar] [CrossRef]
- Takwi, A.; Li, Y. The p53 Pathway Encounters the MicroRNA World. Curr. Genom. 2009, 10, 194–197. [Google Scholar] [CrossRef]
- Vilela-Salgueiro, B.; Barros-Silva, D.; Lobo, J.; Costa, A.L.; Guimarães, R.; Cantante, M.; Lopes, P.; Braga, I.; Oliveira, J.; Henrique, R.; et al. Germ cell tumour subtypes display differential expression of microRNA371a-3p. Philos. Trans. R. Soc. B Biol. Sci. 2018, 5, 373. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Spiekermann, M.; Balks, T.; Flor, I.; Löning, T.; Bullerdiek, J.; Belge, G. MicroRNAs miR-371-3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br. J. Cancer 2012, 107, 1754–1760. [Google Scholar] [CrossRef]
- Pelloni, M.; Coltrinari, G.; Paoli, D.; Pallotti, F.; Lombardo, F.; Lenzi, A.; Gandini, L. Differential expression of miRNAs in the seminal plasma and serum of testicular cancer patients. Endocrine 2017, 57, 518–527. [Google Scholar] [CrossRef]
- Radtke, A.; Cremers, J.-F.; Kliesch, S.; Riek, S.; Junker, K.; Mohamed, S.A.; Anheuser, P.; Belge, G.; Dieckmann, K.P. Can germ cell neoplasia in situ be diagnosed by measuring serum levels of microRNA371a-3p? J. Cancer Res. Clin. Oncol. 2017, 143, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Lembeck, A.L.; Puchas, P.; Hutterer, G.; Barth, D.A.; Terbuch, A.; Bauernhofer, T.; Pichler, M. MicroRNAs as Appropriate Discriminators in Non-Specific Alpha-Fetoprotein (AFP) Elevation in Testicular Germ Cell Tumor Patients. Non Coding RNA 2020, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, M.; Belge, G.; Winter, N.; Ikogho, R.; Balks, T.; Bullerdiek, J.; Dieckmann, K.P. MicroRNA miR-371a-3p in serum of patients with germ cell tumours: Evaluations for establishing a serum biomarker. Andrology 2015, 3, 78–84. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Spiekermann, M.; Balks, T.; Ikogho, R.; Anheuser, P.; Wosniok, W.; Loening, T.; Bullerdiek, J.; Belge, G. MicroRNA miR-371a-3p-A Novel Serum Biomarker of Testicular Germ Cell Tumors: Evidence for Specificity from Measurements in Testicular Vein Blood and in Neoplastic Hydrocele Fluid. Urol. Int. 2016, 97, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Hennig, F.; Ikogho, R.; Hammel, J.; Anheuser, P.; Wülfing, C.; Belge, G.; Dieckmann, K.P. The Novel Biomarker of Germ Cell Tumours, Micro-RNA-371a-3p, Has a Very Rapid Decay in Patients with Clinical Stage 1. Urol. Int. 2018, 100, 470–475. [Google Scholar] [CrossRef]
- Radtke, A.; Dieckmann, K.-P.; Grobelny, F.; Salzbrunn, A.; Oing, C.; Schulze, W.; Belge, G. Expression of miRNA-371a-3p in seminal plasma and ejaculate is associated with sperm concentration. Andrology 2019, 7, 469–474. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef]
- Mego, M.; Agthoven, T.; Gronesova, P.; Chovanec, M.; Miskovska, V.; Mardiak, J.; Looijenga, L.H.J. Clinical utility of plasma miR-371a-3p in germ cell tumors. J. Cell Mol. Med. 2019, 23, 1128–1136. [Google Scholar]
- Gillis, A.J.M.; Rijlaarsdam, M.A.; Eini, R.; Dorssers, L.C.J.; Biermann, K.; Murray, M.J.; Nicholson, J.C.; Coleman, N.; Dieckmann, K.P.; Belge, G.; et al. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: A proof of principle. Mol. Oncol. 2013, 7, 1083–1092. [Google Scholar] [CrossRef]
- Myklebust, M.P.; Rosenlund, B.; Gjengstø, P.; Bercea, B.S.; Karlsdottir, Á.; Brydøy, M.; Dahl, O. Quantitative PCR Measurement of miR-371a-3p and miR-372-p Is Influenced by Hemolysis. Front. Genet. 2019, 10, 463. [Google Scholar] [CrossRef]
- Henrique, R.; Jerónimo, C. Testicular Germ Cell Tumors Go Epigenetics: Will miR-371a-3p Replace Classical Serum Biomarkers? Eur. Urol. 2017, 71, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E.; et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Toffanin, S.; Hoshida, Y.; Lachenmayer, A.; Villanueva, A.; Cabellos, L.; Minguez, B.; Savic, R.; Ward, S.C.; Thung, S.; Chiang, D.Y.; et al. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastroenterology 2011, 140, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Shukla, K.; Balwierz, A.; Soons, Z.; König, R.; Sahin, O.; Wiemann, S. MicroRNA-519a is a novel oncomir conferring tamoxifen resistance by targeting a network of tumour-suppressor genes in ER+ breast cancer. J. Pathol. 2014, 233, 368–379. [Google Scholar] [CrossRef]
- Flor, I.; Spiekermann, M.; Löning, T.; Dieckmann, K.-P.; Belge, G.; Bullerdiek, J. Expression of microRNAs of C19MC in Different Histological Types of Testicular Germ Cell Tumour. Cancer Genom. Proteom. 2016, 13, 281–289. [Google Scholar]
- Liu, J.; Shi, H.; Li, X.; Chen, G.; Larsson, C.; Lui, W.-O. MiR-223-3p regulates cell growth and apoptosis via FBXW7 suggesting an oncogenic role in human testicular germ cell tumors. Int. J. Oncol. 2016, 50, 356–364. [Google Scholar] [CrossRef]
- Welcker, M.; Clurman, B.E. FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 2008, 8, 83–93. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Liang, X.; Sun, M.; Wang, G.; De, W.; Wu, W. MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J. Cancer Res. Clin. Oncol. 2012, 138, 763–774. [Google Scholar] [CrossRef]
- Kurashige, J.; Watanabe, M.; Iwatsuki, M.; Kinoshita, K.; Saito, S.; Hiyoshi, Y.; Kamohara, H.; Baba, Y.; Mimori, K.; Baba, H. Overexpression of microRNA-223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinoma. Br. J. Cancer 2012, 106, 182–188. [Google Scholar] [CrossRef]
- Mavrakis, K.J.; Van Der Meulen, J.; Wolfe, A.L.; Liu, X.; Mets, E.; Taghon, T.; Khan, A.A.; Setty, M.; Rondou, P.; Vandenberghe, P.; et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat. Genet. 2011, 43, 673–678. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, W.; Jia, H.; Yan, J.; Zhang, G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J. Exp. Clin. Cancer Res. CR 2015, 34, 28. [Google Scholar] [CrossRef] [PubMed]
- Lizé, M.; Pilarski, S.; Dobbelstein, M. E2F1-inducible microRNA 449a/b suppresses cell proliferation and promotes apoptosis. Cell Death Differ. 2010, 17, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Polager, S.; Ginsberg, D. p53 and E2f: Partners in life and death. Nat. Rev. Cancer 2009, 9, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, M.; Jiang, X.; Wu, Z.; Li, Z.; Aau, M.; Yu, Q. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes. Dev. 2009, 23, 2388–2393. [Google Scholar] [CrossRef]
- Yong-Ming, H.; Ai-Jun, J.; Xiao-Yue, X.; Jian-Wei, L.; Chen, Y.; Ye, C. MiR-449a: A potential therapeutic agent for cancer. Anticancer Drugs 2017, 28, 1067–1078. [Google Scholar] [CrossRef]
- Lian, J.; Tian, H.; Liu, L.; Zhang, X.-S.; Li, W.-Q.; Deng, Y.-M.; Yao, G.-D.; Yin, M.-M.; Sun, F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death Dis. 2010, 1, e94. [Google Scholar] [CrossRef]
- Huang, H.; Tian, H.; Duan, Z.; Cao, Y.; Zhang, X.-S.; Sun, F. microRNA-383 impairs phosphorylation of H2AX by targeting PNUTS and inducing cell cycle arrest in testicular embryonal carcinoma cells. Cell. Signal. 2014, 26, 903–911. [Google Scholar] [CrossRef]
- Relaix, F.; Wei, X.; Li, W.; Pan, J.; Lin, Y.; Bowtell, D.D.; Sassoon, D.A.; Wu, X. Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 2105–2110. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, Y.; Yang, H.W.; Agar, N.Y.R.; Frado, L.; Johnson, M.D. The imprinted gene PEG3 inhibits Wnt signaling and regulates glioma growth. J. Biol. Chem. 2010, 285, 8472–8480. [Google Scholar] [CrossRef]
- Özata, D.M.; Li, X.; Lee, L.; Liu, J.; Warsito, D.; Hajeri, P.; Hultman, I.; Fotouhi, O.; Marklund, S.; Ährlund-Richter, L.; et al. Loss of miR-514a-3p regulation of PEG3 activates the NF-kappa B pathway in human testicular germ cell tumors. Cell Death Dis. 2017, 8, e2759. [Google Scholar] [CrossRef]
- Liu, X.; Duan, H.; Zhou, S.; Liu, Z.; Wu, D.; Zhao, T.; Xu, S.; Yang, L.; Li, D. microRNA-199a-3p functions as tumor suppressor by regulating glucose metabolism in testicular germ cell tumors. Mol. Med. Rep. 2016, 14, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Cheung, H.H.; Lee, T.L.; Lu, G.; Poon, W.S.; Chan, W.Y. Molecular mechanisms of regulation and action of microRNA-199a in testicular germ cell tumor and glioblastomas. PLoS ONE 2013, 8, e83980. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-F.; Gu, S.; Suen, Y.-K.; Li, L.; Chan, W.-Y. MicroRNA-199a-3p, DNMT3A, and aberrant DNA methylation in testicular cancer. Epigenetics 2014, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-F.; Suen, Y.-K.; Gu, S.; Li, L.; Chan, W.-Y. A miR-199a/miR-214 self-regulatory network via PSMD10, TP53 and DNMT1 in testicular germ cell tumor. Sci. Rep. 2014, 4, 6413. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regouc, M.; Belge, G.; Lorch, A.; Dieckmann, K.-P.; Pichler, M. Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers 2020, 12, 749. https://doi.org/10.3390/cancers12030749
Regouc M, Belge G, Lorch A, Dieckmann K-P, Pichler M. Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers. 2020; 12(3):749. https://doi.org/10.3390/cancers12030749
Chicago/Turabian StyleRegouc, Manuel, Gazanfer Belge, Anja Lorch, Klaus-Peter Dieckmann, and Martin Pichler. 2020. "Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer" Cancers 12, no. 3: 749. https://doi.org/10.3390/cancers12030749
APA StyleRegouc, M., Belge, G., Lorch, A., Dieckmann, K.-P., & Pichler, M. (2020). Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers, 12(3), 749. https://doi.org/10.3390/cancers12030749




