Proposed Modification of Staging for Distal Cholangiocarcinoma Based on the Lymph Node Ratio Using Korean Multicenter Database
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Optimal Cutoff Value for the T Category
2.3. Optimal Cutoff Value for the TLNC, PLNC, and LNR
2.4. Multivariable Analysis for OSR
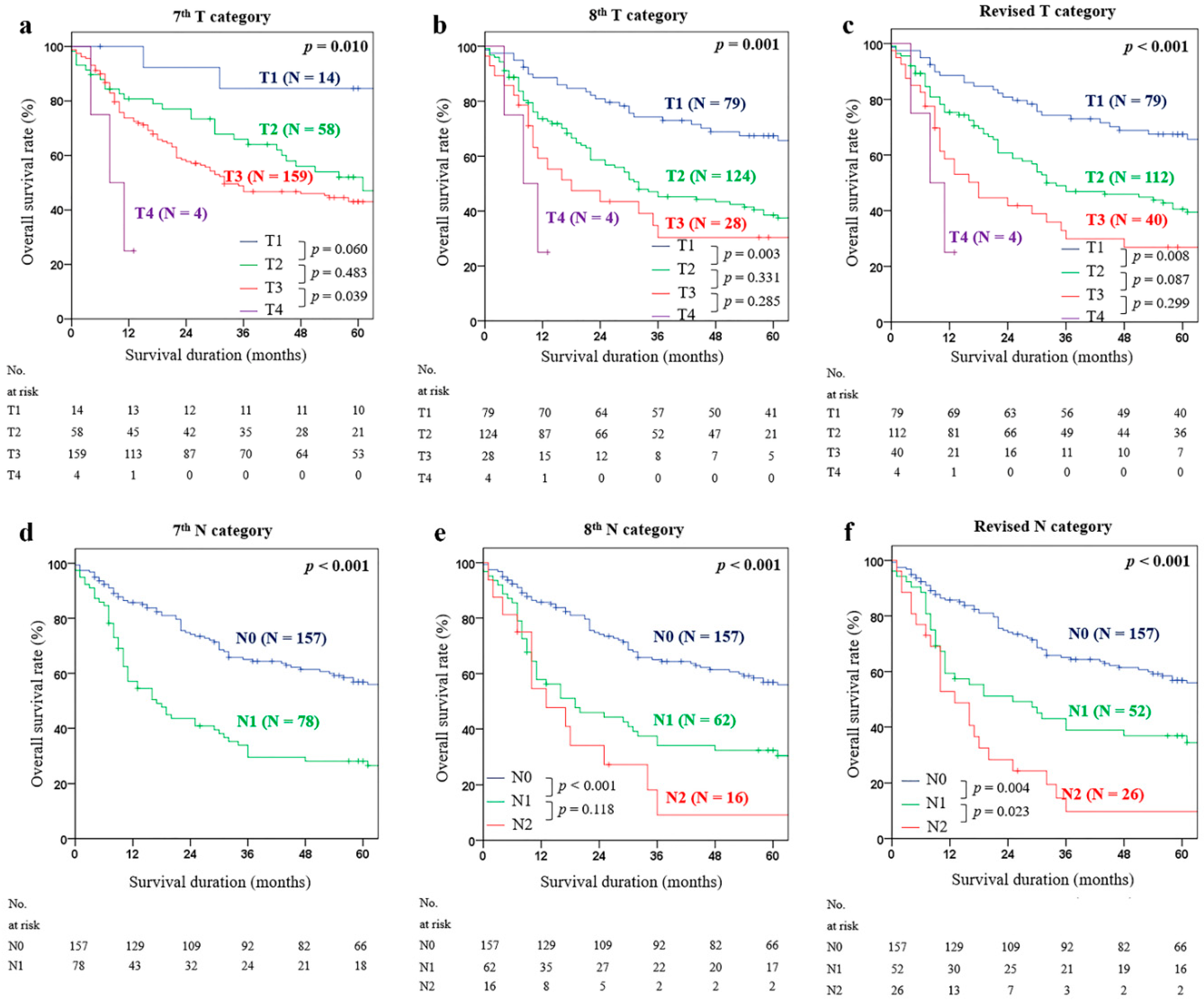
2.5. Comparison of Predictive Power of Each Staging Model (AJCC 7th TNM Staging, AJCC 8th TNM Staging, Revised TNM Staging, and Revised T(LNR-c)M Staging)
3. Discussion
4. Materials and Methods
4.1. Patients and Data Collection
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzalez, R.S.; Bagci, P.; Basturk, O.; Reid, M.D.; Balci, S.; Knight, J.H.; Kong, S.Y.; Memis, B.; Jang, K.-T.; Ohike, N.; et al. Intrapancreatic distal common bile duct carcinoma: Analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Mod. Pathol. 2016, 29, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.; Hess, K.R.; Sullivan, D.C.; et al. Organization of the AJCC Cancer Staging Manual; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 31–37. [Google Scholar]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Nakashima, A.; Kondo, N.; Sakabe, R.; Ohge, H.; Sueda, T. Prognostic Factors After Surgical Resection for Intrahepatic, Hilar, and Distal Cholangiocarcinoma. Ann. Surg. Oncol. 2010, 18, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kim, K.; Chie, E.K.; Kwon, J.; Jang, J.-Y.; Kim, S.W.; Oh, D.Y.; Bang, Y.-J. Long-Term Outcome of Distal Cholangiocarcinoma after Pancreaticoduodenectomy Followed by Adjuvant Chemoradiotherapy: A 15-Year Experience in a Single Institution. Cancer Res. Treat. 2017, 49, 473–483. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Los Angeles, CA, USA, 2017. [Google Scholar]
- Hong, S.-M.; Cho, H.; Moskaluk, C.A.; Yu, E. Measurement of the Invasion Depth of Extrahepatic Bile Duct Carcinoma. Am. J. Surg. Pathol. 2007, 31, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-Y.; Sung, Y.-N.; Lee, J.H.; Park, K.-M.; Lee, Y.-J.; Hong, S.-M. Validation of the Eighth American Joint Committee on Cancer Staging System for Distal Bile Duct Carcinoma. Cancer Res. Treat. 2018, 51, 98–111. [Google Scholar] [CrossRef]
- Min, K.-W.; Kim, N.-H.; Son, B.K.; Kim, E.-K.; Ahn, S.B.; Kim, S.H.; Jo, Y.J.; Park, Y.S.; Seo, J.; Oh, Y.H.; et al. Invasion Depth Measured in Millimeters is a Predictor of Survival in Patients with Distal Bile Duct Cancer: Decision Tree Approach. World J. Surg. 2016, 41, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-M.; Pawlik, T.M.; Cho, H.; Aggarwal, B.; Goggins, M.; Hruban, R.H.; Anders, R.A. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery 2009, 146, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Jang, K.-T. Validation of T Stage According to Depth of Invasion and N Stage Subclassification Based on Number of Metastatic Lymph Nodes for Distal Extrahepatic Bile Duct (EBD) Carcinoma. Medicine 2015, 94, e2064. [Google Scholar] [CrossRef]
- Oshiro, Y.; Sasaki, R.; Kobayashi, A.; Murata, S.; Fukunaga, K.; Kondo, T.; Oda, T.; Ohkohchi, N. Prognostic relevance of the lymph node ratio in surgical patients with extrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. (EJSO) 2011, 37, 60–64. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Sun, Y.; Gong, J.; Feng, H.; Tu, J. Prognostic Significance of the Lymph Node Ratio in Surgical Patients With Distal Cholangiocarcinoma. J. Surg. Res. 2018, 236, 2–11. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Chu, Y.-M.; Lan, Z.-M.; Tang, X.-L.; Chen, Y.-T.; Wang, C.-F.; Che, X. Correlation between metastatic lymph node ratio and prognosis in patients with extrahepatic cholangiocarcinoma. World J. Gastroenterol. 2015, 21, 4255–4260. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-M.; Presley, A.E.; Stelow, E.B.; Frierson, H.F.; Moskaluk, C.A. Reconsideration of the Histologic Definitions Used in the Pathologic Staging of Extrahepatic Bile Duct Carcinoma. Am. J. Surg. Pathol. 2006, 30, 744–749. [Google Scholar] [CrossRef]
- Berger, A.C.; Sigurdson, E.R.; Levoyer, T.; Hanlon, A.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon Cancer Survival Is Associated With Decreasing Ratio of Metastatic to Examined Lymph Nodes. J. Clin. Oncol. 2005, 23, 8706–8712. [Google Scholar] [CrossRef]
- Xu, D.-Z.; Geng, Q.-R.; Long, Z.-J.; Zhan, Y.-Q.; Li, W.; Zhou, Z.; Chen, Y.-B.; Sun, X.-W.; Chen, G.; Liu, Q. Positive Lymph Node Ratio Is an Independent Prognostic Factor in Gastric Cancer After D2 Resection Regardless of the Examined Number of Lymph Nodes. Ann. Surg. Oncol. 2008, 16, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-Y.; Yang, D.-H. Adjustment of N Stages of Gastric Cancer by the Ratio Between the Metastatic and Examined Lymph Nodes. Ann. Surg. Oncol. 2009, 16, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Tani, M.; Kobayashi, Y.; Tsuji, T.; Tabuse, K.; Horiuchi, T.; Oka, M.; Yamaguchi, K.; Sakata, Y.; Shimomura, T.; et al. The ratio between metastatic and examined lymph nodes is an independent prognostic factor for patients with resectable middle and distal bile duct carcinoma. Am. J. Surg. 2010, 199, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Gleisner, A.L.; Cameron, J.L.; Winter, J.M.; Assumpção, L.R.; Lillemoe, K.D.; Wolfgang, C.; Hruban, R.H.; Schulick, R.D.; Yeo, C.J.; et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007, 141, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Crippa, S.; Dominguez, I.; Barugola, G.; Capelli, P.; Marcucci, S.; Beghelli, S.; Scarpa, A.; Bassi, C.; Pederzoli, P. Prognostic Relevance of Lymph Node Ratio and Number of Resected Nodes after Curative Resection of Ampulla of Vater Carcinoma. Ann. Surg. Oncol. 2008, 15, 3178–3186. [Google Scholar] [CrossRef]
- Ito, K.; Ito, H.; Allen, P.J.; Gonen, M.; Klimstra, D.; D’Angelica, M.I.; Fong, Y.; DeMatteo, R.P.; Brennan, M.; Blumgart, L.H.; et al. Adequate Lymph Node Assessment for Extrahepatic Bile Duct Adenocarcinoma. Ann. Surg. 2010, 251, 675–681. [Google Scholar] [CrossRef]
- Schwarz, R.E.; Smith, D. Lymph Node Dissection Impact on Staging and Survival of Extrahepatic Cholangiocarcinomas, Based on U.S. Population Data. J. Gastrointest. Surg. 2007, 11, 158–165. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society of Biliary Surgery. General Rules for Surgical and Pathological Studies on Cancer of Biliary Tract, 3rd ed.; Kanehara: Tokyo, Japan, 1993. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Simonoff, J.; Tsai, C. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J. R. Stat. Soc. Ser. B Stat. Methodol. 1998, 60, 271–293. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Califf, R.M.; Pryor, D.B.; Rosati, R.A. Regression modelling strategies for improved prognostic prediction. Stat. Med. 1984, 3, 143–152. [Google Scholar] [CrossRef]
| Variable | No. of Valid Records | Patients (N = 235) | |
|---|---|---|---|
| No. | % | ||
| Age (years) | 235 | ||
| Median | 65 | ||
| Range | 31–88 | ||
| Gender | 235 | ||
| Male | 157 | 66.8 | |
| Female | 78 | 33.2 | |
| Preoperative bile drainage (n, %) | 235 | 219 | 93.2 |
| Type of operation | 235 | ||
| Pancreaticoduodenectomy | 232 | 98.7 | |
| Bile duct resection | 3 | 1.3 | |
| Operating time (min) | 235 | ||
| Median | 331 | ||
| Range | 195–840 | ||
| Tumor size (cm) | 234 | ||
| Median | 2.5 | ||
| Range | 0.8–8.5 | ||
| LVI | 135 | 90 | 66.7 |
| PNI | 176 | 136 | 77.3 |
| Tumor differentiation | 235 | ||
| Well/moderate | 165 | 70.2 | |
| Poorly | 70 | 29.8 | |
| Resection margin status | 235 | ||
| R0 | 220 | 93.6 | |
| R1 | 12 | 5.1 | |
| R2 | 3 | 1.3 | |
| Recurrence | 235 | 133 | 56.6 |
| Tumor invasion depth (mm) | 230 | ||
| Median | 6.0 | ||
| Range | 0.2–25.0 | ||
| TLNC | 235 | ||
| Median | 18 | ||
| Range | 1–64 | ||
| PLNC | 235 | ||
| Median | 2 | ||
| Range | 1–17 | ||
| LNR | 235 | ||
| Median | 0.11 | ||
| Range | 0.02–1.00 | ||
| AJCC 7th T category | 235 | ||
| T1 | 14 | 5.9 | |
| T2 | 58 | 24.7 | |
| T3 | 159 | 67.7 | |
| T4 | 4 | 1.7 | |
| AJCC 8th T category | 235 | ||
| T1 | 79 | 33.6 | |
| T2 | 124 | 52.8 | |
| T3 | 28 | 11.9 | |
| T4 | 4 | 1.7 | |
| AJCC 7th N category | 235 | ||
| N0 | 157 | 66.8 | |
| N1 | 78 | 33.2 | |
| AJCC 8th N category | 235 | ||
| N0 | 157 | 66.8 | |
| N1 | 62 | 26.4 | |
| N2 | 16 | 6.8 | |
| AJCC 7th TNM staging | 235 | ||
| IA | 13 | 5.5 | |
| IB | 45 | 19.1 | |
| IIA | 96 | 40.9 | |
| IIB | 77 | 32.8 | |
| III | 4 | 1.7 | |
| AJCC 8th TNM staging | 235 | ||
| I | 74 | 31.5 | |
| IIA | 77 | 32.8 | |
| IIB | 64 | 27.2 | |
| IIIA | 16 | 6.8 | |
| IIIB | 4 | 1.7 | |
| Tumor Invasion Depth (mm) | Number of Cases (%) | Median Survival (months) | p-Value | χ2 Score |
|---|---|---|---|---|
| T category-a | < 0.001 | 18.125 | ||
| T1 (<5) | 79 (33.6) | 131.0 | ||
| T2 (5–10) | 112 (47.7) | 32.0 | ||
| T3 (>10) | 40 (17.0) | 18.0 | ||
| T4 | 4 (1.7) | 8.0 | ||
| T category-b | 0.001 | 17.441 | ||
| T1 (<5) | 79 (33.6) | 131.0 | ||
| T2 (5–11) | 114 (48.5) | 32.0 | ||
| T3 (>11) | 38 (16.2) | 16.0 | ||
| T4 | 4 (1.7) | 8.0 | ||
| T category-c | 0.001 | 16.059 | ||
| T1 (<5) | 79 (33.6) | 131.0 | ||
| T2 (5–12) | 114 (52.8) | 32.0 | ||
| T3 (>12) | 38 (11.9) | 18.0 | ||
| T4 | 4 (1.7) | 8.0 | ||
| T category-d | 0.001 | 15.844 | ||
| T1 (<5) | 79 (33.6) | 131.0 | ||
| T2 (5–13) | 114 (53.2) | 32.0 | ||
| T3 (>13) | 38 (11.5) | 16.0 | ||
| T4 | 4 (1.7) | 8.0 | ||
| T category-e | 0.002 | 15.263 | ||
| T1 (<5) | 79 (33.6) | 131.0 | ||
| T2 (5–14) | 114 (54.5) | 31.0 | ||
| T3 (>14) | 38 (10.2) | 18.0 | ||
| T4 | 4 (1.7) | 8.0 | ||
| T category-f | 0.001 | 15.447 | ||
| T1 (<5) | 79 (33.6) | 131.0 | ||
| T2 (5–15) | 114 (59.6) | 30.0 | ||
| T3 (>15) | 38 (5.1) | - | ||
| T4 | 4 (1.7) | 8.0 |
| Number of Cases (%) | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | p-Value | χ2 Score | HR | 95% CI | p-Value | ||
| TLNC | |||||||
| ≥2 | 233 (99.1) | 0.249 | 0.171 | 2.194 | 0.266 | 0.035–2.014 | 0.200 |
| ≥3 | 227 (96.6) | 1.032 | 0.951 | 0.004 | 0.809 | 0.295–2.223 | 0.682 |
| ≥4 | 222 (94.5) | 0.703 | 0.336 | 0.935 | 0.325 | 0.336–1.436 | 0.325 |
| ≥5 | 219 (93.2) | 0.729 | 0.337 | 0.928 | 0.660 | 0.343–1.271 | 0.214 |
| ≥6 | 214 (91.1) | 0.726 | 0.274 | 1.205 | 0.671 | 0.447–0.897 | 0.177 |
| ≥7 | 205 (87.2) | 0.683 | 0.117 | 2.481 | 0.598 | 0.369–0.971 | 0.038 |
| ≥8 | 204 (86.8) | 0.663 | 0.084 | 3.018 | 0.584 | 0.363–0.940 | 0.027 |
| ≥9 | 199 (84.7) | 0.693 | 0.105 | 2.657 | 0.639 | 0.408–1.000 | 0.050 |
| ≥10 | 192 (81.7) | 0.653 | 0.043 | 4.159 | 0.608 | 0.400–0.923 | 0.020 |
| ≥11 | 183 (77.9) | 0.630 | 0.020 | 5.484 | 0.594 | 0.400–0.884 | 0.010 |
| ≥12 | 175 (74.5) | 0.648 | 0.024 | 5.164 | 0.601 | 0.409–0.884 | 0.010 |
| ≥ 13 | 167 (71.1) | 0.644 | 0.018 | 5.704 | 0.597 | 0.411–0.866 | 0.007 |
| ≥14 | 156 (66.4) | 0.687 | 0.037 | 4.389 | 0.649 | 0.454–0.927 | 0.017 |
| ≥15 | 148 (63.0) | 0.714 | 0.057 | 3.669 | 0.656 | 0.462–0.933 | 0.019 |
| PLNC-a | <0.001 | 23.747 | 0.007 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1 | 34 (14.5) | 2.017 | 0.003 | 1.680 | 1.025–2.752 | 0.040 | |
| ≥2 | 44 (18.7) | 2.501 | <0.001 | 2.070 | 1.284–3.337 | 0.003 | |
| PLNC-b | <0.001 | 33.963 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1–2 | 52 (22.1) | 1.820 | 0.004 | 1.536 | 0.980–2.408 | 0.062 | |
| ≥3 | 26 (11.1) | 3.654 | <0.001 | 2.968 | 1.742–5.057 | <0.001 | |
| PLNC-c | <0.001 | 28.769 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1–3 | 62 (26.4) | 2.030 | <0.001 | 1.667 | 1.089–2.550 | 0.019 | |
| ≥4 | 16 (6.8) | 3.654 | <0.001 | 3.144 | 1.689–5.854 | <0.001 | |
| PLNC-d | <0.001 | 24.628 | 0.005 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1–4 | 66 (28.1) | 2.148 | <0.001 | 1.749 | 1.157–2.657 | 0.008 | |
| ≥5 | 12 (5.1) | 3.138 | 0.001 | 2.657 | 1.315–5.371 | 0.006 | |
| PLNC-e | <0.001 | 23.470 | 0.008 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1–5 | 70 (29.8) | 2.203 | <0.001 | 1.793 | 1.189–2.706 | 0.005 | |
| ≥6 | 8 (3.4) | 2.909 | 0.007 | 2.425 | 1.082–5.434 | 0.031 | |
| PLNC-f | <0.001 | 23.648 | 0.004 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1–6 | 74 (31.5) | 2.219 | <0.001 | 1.793 | 1.195–2.691 | 0.005 | |
| ≥7 | 4 (1.7) | 3.275 | 0.021 | 3.439 | 1.206–9.811 | 0.021 | |
| PLNC-g | <0.001 | 27.934 | 0.002 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| 1–7 | 75 (31.9) | 2.199 | <0.001 | 1.749 | 1.210–2.711 | 0.004 | |
| ≥8 | 3 (1.3) | 6.177 | 0.002 | 2.657 | 1.600–19.170 | 0.007 | |
| LNR-a | <0.001 | 30.916 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.05 | 9 (3.8) | 0.870 | 0.786 | 0.925 | 0.338–2.534 | 0.879 | |
| ≥ 0.05 | 96 (29.7) | 2.594 | <0.001 | 2.530 | 1.772–3.612 | <0.001 | |
| LNR-b | <0.001 | 33.373 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.07 | 22 (9.4) | 1.288 | 0.416 | 0.969 | 0.508–1.847 | 0.924 | |
| ≥0.07 | 56 (23.8) | 2.862 | <0.001 | 2.638 | 1.734–4.014 | <0.001 | |
| LNR-c | <0.001 | 40.812 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.1 | 33 (14.0) | 1.382 | 0.208 | 1.099 | 0.642–1.881 | 0.731 | |
| ≥0.1 | 45 (19.2) | 3.400 | 3.400 | 3.254 | 2.078–5.095 | <0.001 | |
| LNR-d | <0.001 | 36.183 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.2 | 59 (25.1) | 1.888 | 0.00 | 1.518 | 1.015–2.410 | 0.061 | |
| ≥0.2 | 19 (8.1) | 4.209 | <0.001 | 3.912 | 2.303–7.091 | <0.001 | |
| LNR-e | <0.001 | 33.438 | <0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.3 | 69 (28.9) | 2.068 | <0.001 | 1.166 | 1.100–2.523 | 0.016 | |
| ≥0.3 | 9 (4.3) | 5.416 | <0.001 | 5.184 | 2.479–10.854 | <0.001 | |
| LNR-f | < 0.001 | 25.466 | 0.001 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.4 | 72 (30.6) | 2.172 | <0.001 | 1.756 | 1.165–2.645 | 0.007 | |
| ≥0.4 | 6 (2.6) | 3.810 | 0.002 | 3.878 | 1.627–9.248 | 0.002 | |
| LNR-g | <0.001 | 24.847 | 0.002 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.5 | 73 (31.1) | 2.191 | 0.001 | 1.780 | 1.184–2.676 | 0.006 | |
| ≥0.5 | 5 (2.1) | 3.746 | 0.005 | 3.702 | 1.448–9.462 | 0.006 | |
| LNR-e | <0.001 | 25.853 | 0.002 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.6 | 74 (31.5) | 2.194 | <0.001 | 1.795 | 1.197–2.693 | 0.005 | |
| ≥0.6 | 4 (1.7) | 4.490 | 0.004 | 4.156 | 1.465–11.792 | 0.007 | |
| LNR-f | <0.001 | 23.606 | 0.004 | ||||
| 0 | 157 (66.8) | 1 | 1 | ||||
| >0 to 0.7 | 76 (32.3) | 2.238 | <0.001 | 1.812 | 1.209–2.716 | 0.004 | |
| ≥0.7 | 2 (0.9) | 3.827 | 0.062 | 4.26 | 1.032–17.620 | 0.045 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (years) | 0.015 | 0.003 | ||||
| <65 | 1 | 1 | ||||
| ≥65 | 1.905 | 1.129–3.213 | 1.692 | 1.195–2.396 | ||
| Gender | 0.425 | |||||
| Male | 1 | |||||
| Female | 1.252 | 0.721–2.173 | ||||
| Preoperative bile drainage | 0.581 | |||||
| No | 1 | |||||
| Yes | 1.330 | 0.482–3.676 | ||||
| Operation type | 0.413 | |||||
| Pancreaticoduodenectomy | 1 | |||||
| Bile duct resection | 0.379 | 0.034–4.236 | ||||
| Operating time (min) | 0.957 | |||||
| <350 | 1 | |||||
| ≥350 | 0.986 | 0.582–1.669 | ||||
| Tumor size (cm) | 0.874 | |||||
| <3 | 1 | |||||
| ≥3 | 0.958 | 0.562–1.631 | ||||
| LVI | 0.014 | 0.401 | ||||
| No | 1 | |||||
| Yes | 1.973 | 1.143–3.405 | ||||
| PNI | 0.419 | |||||
| No | 1 | |||||
| Yes | 1.240 | 0.736–2.090 | ||||
| Tumor differentiation | 0.034 | 0.221 | ||||
| Well/moderate | 1 | |||||
| Poorly | 1.877 | 1.046–3.369 | ||||
| Resection margin status | 0.416 | |||||
| R0 | 1 | |||||
| R1/R2 | 1.577 | 0.522–4.767 | ||||
| Revised T category | <0.001 | 0.494 | ||||
| T1 (<5) | 1 | |||||
| T2 (5–10) | 1.716 | 1.144–2.575 | 0.009 | |||
| T3 (>10) | 2.591 | 1.567–4.282 | <0.001 | |||
| T4 | 6.193 | 1.863–20.588 | 0.003 | |||
| Optimal TLNC | 0.018 | 0.033 | ||||
| <13 | 1 | 1 | ||||
| ≥13 | 0.644 | 0.447–0.927 | 0.668 | 0.461–0.969 | ||
| Revised N category | <0.001 | 0.731 | ||||
| 0 | 1 | |||||
| 1–2 | 1.820 | 1.213–2.732 | 0.004 | |||
| ≥3 | 3.654 | 2.271–5.880 | <0.001 | |||
| LNR-c | <0.001 | 0.553 | ||||
| 0 | 1 | |||||
| >0 to < 0.17 | 1.731 | 1.151–2.597 | 0.008 | |||
| ≥0.17 | 4.408 | 2.727–7.126 | <0.001 | |||
| AJCC 7th T category | 0.019 | 0.160 | ||||
| T1 | 1 | |||||
| T2 | 2.550 | 0.903–7.202 | 0.077 | |||
| T3 | 2.929 | 1.076–7.976 | 0.035 | |||
| T4 | 10.542 | 2.314–48.031 | 0.002 | |||
| AJCC 8th T category | 0.001 | 0.783 | ||||
| T1 | 1 | |||||
| T2 | 1.813 | 1.218–2.698 | 0.003 | |||
| T3 | 2.369 | 1.342–4.181 | 0.003 | |||
| T4 | 6.316 | 1.846–20.393 | 0.003 | |||
| AJCC 7th N category | <0.001 | 0.553 | ||||
| N0 | 1 | |||||
| N1 | 2.270 | 1.605–3.210 | ||||
| AJCC 8th N category | <0.001 | 0.670 | ||||
| N0 | 1 | |||||
| N1 | 2.030 | 1.394–2.956 | <0.001 | |||
| N2 | 3.654 | 2.047–6.522 | <0.001 | |||
| AJCC 7th TNM staging | <0.001 | 0.307 | ||||
| IA | 1 | |||||
| IB | 2.517 | 0.756–8.416 | 0.134 | |||
| IIA | 2.816 | 0.876–2.816 | 0.082 | |||
| IIB | 5.940 | 1.855–19.023 | 0.003 | |||
| III | 14.035 | 2.777–70.966 | 0.001 | |||
| AJCC 8th TNM staging | <0.001 | 0.244 | ||||
| IA | 1 | |||||
| IIA | 1.752 | 1.101–2.789 | 0.018 | |||
| IIB | 2.400 | 1.506–3.824 | <0.001 | |||
| IIIA | 4.964 | 2.612–9.434 | <0.001 | |||
| IIIB | 9.830 | 3.371–28.662 | <0.001 | |||
| Revised TNM staging | <0.001 | 0.806 | ||||
| IA | 1 | |||||
| IIA | 1.761 | 1.104–2.810 | 0.018 | |||
| IIB | 2.140 | 1.318–3.476 | 0.002 | |||
| IIIA | 4.940 | 2.851–8.560 | <0.001 | |||
| IIIB | 7.382 | 2.201–24.765 | 0.001 | |||
| Revised T(LNR-c)M staging | 3.810 | <0.001 | <0.001 | |||
| IA | 1 | 1 | ||||
| IIA | 1.683 | 1.049–2.698 | 0.031 | 1.559 | 0.968–2.511 | 0.068 |
| IIB | 2.346 | 1.472–3.738 | <0.001 | 1.723 | 1.000–2.969 | 0.050 |
| IIIA | 5.707 | 3.153–10.333 | <0.001 | 4.606 | 2.835–7.481 | <0.001 |
| IIIB | 7.420 | 2.211–24.897 | 0.001 | 8.575 | 2.535–29.002 | 0.001 |
| Model | AIC | BIC | AICC | Harrell’s C-Statistics |
|---|---|---|---|---|
| AJCC 7th staging | 1298.281 | 1309.842 | 1300.753 | 0.562 |
| AJCC 8th staging | 1297.589 | 1312.041 | 1300.256 | 0.658 |
| Revised TNM staging | 1294.025 | 1308.477 | 1296.692 | 0.662 |
| Revised T(LNR-c)M staging | 1288.925 | 1303.377 | 1291.592 | 0.667 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Shin, Y.C.; Choi, D.W.; Heo, J.S.; Shin, S.H.; Kim, N.; Jang, K.-T.; Kim, H.; Lim, C.-S.; Chang, S.H.; et al. Proposed Modification of Staging for Distal Cholangiocarcinoma Based on the Lymph Node Ratio Using Korean Multicenter Database. Cancers 2020, 12, 762. https://doi.org/10.3390/cancers12030762
You Y, Shin YC, Choi DW, Heo JS, Shin SH, Kim N, Jang K-T, Kim H, Lim C-S, Chang SH, et al. Proposed Modification of Staging for Distal Cholangiocarcinoma Based on the Lymph Node Ratio Using Korean Multicenter Database. Cancers. 2020; 12(3):762. https://doi.org/10.3390/cancers12030762
Chicago/Turabian StyleYou, Yunghun, Yong Chan Shin, Dong Wook Choi, Jin Seok Heo, Sang Hyun Shin, Naru Kim, Kee-Taek Jang, Hongbeom Kim, Chang-Sup Lim, Sun Hee Chang, and et al. 2020. "Proposed Modification of Staging for Distal Cholangiocarcinoma Based on the Lymph Node Ratio Using Korean Multicenter Database" Cancers 12, no. 3: 762. https://doi.org/10.3390/cancers12030762
APA StyleYou, Y., Shin, Y. C., Choi, D. W., Heo, J. S., Shin, S. H., Kim, N., Jang, K.-T., Kim, H., Lim, C.-S., Chang, S. H., Han, K. M., & Han, I. W. (2020). Proposed Modification of Staging for Distal Cholangiocarcinoma Based on the Lymph Node Ratio Using Korean Multicenter Database. Cancers, 12(3), 762. https://doi.org/10.3390/cancers12030762






