Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors
Abstract
:1. Introduction
2. Results
2.1. Association of OPD with Patient Survival
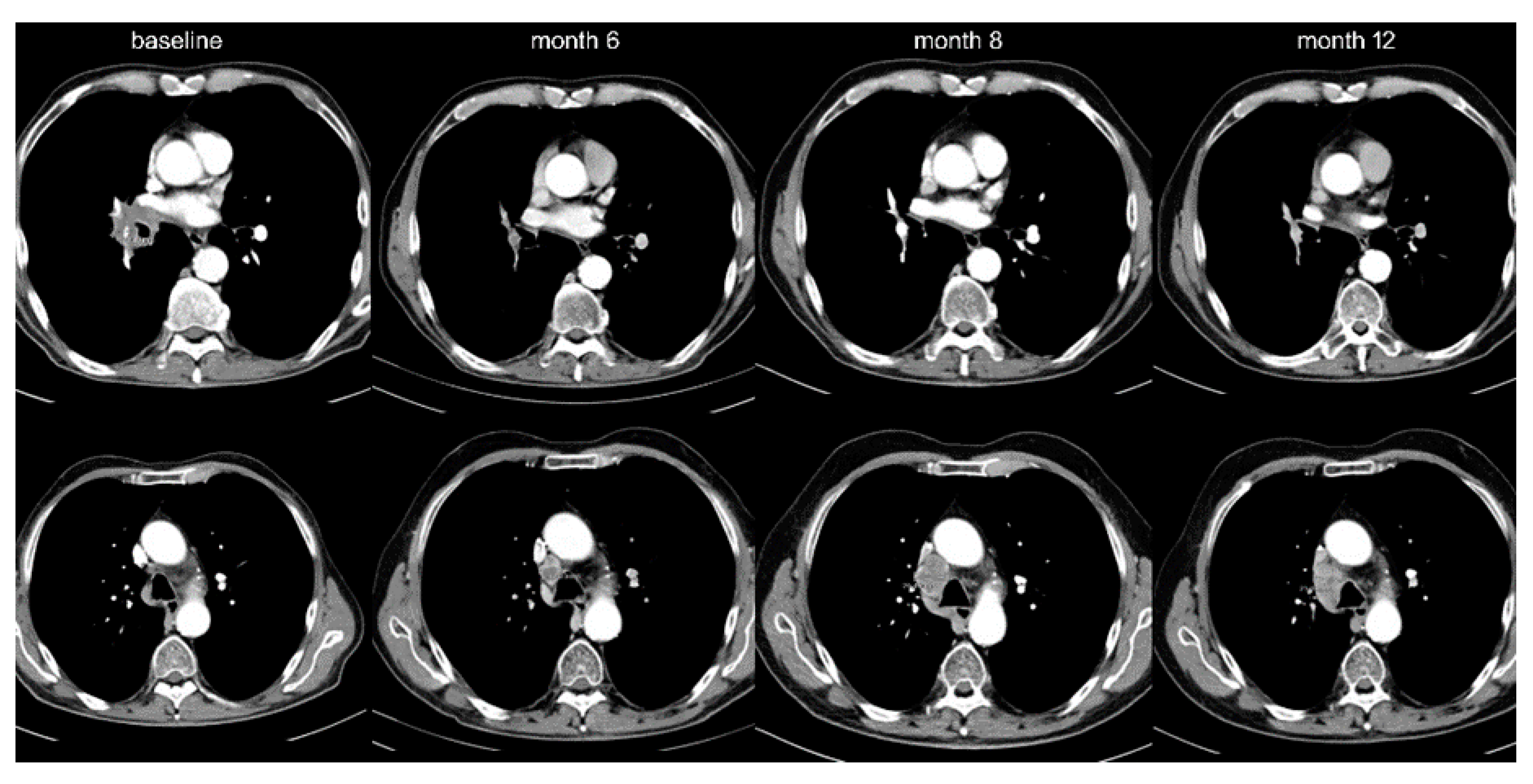
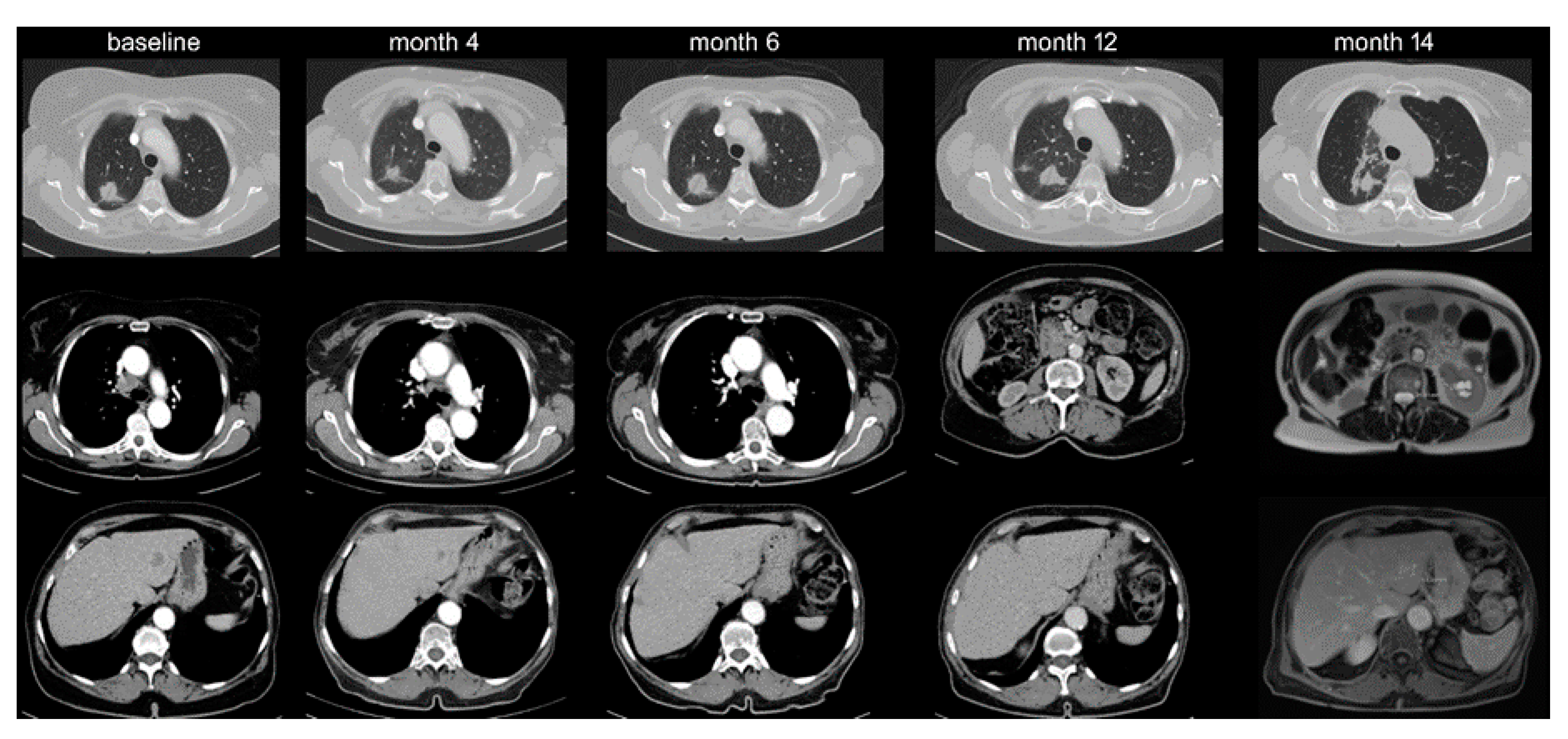
2.2. Therapeutic Relevance of Oligoprogression
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel, P.H.; Palma, D.; McDonald, F.; Tree, A.C. The Dandelion Dilemma Revisited for Oligoprogression: Treat the Whole Lawn or Weed Selectively? Clin. Oncol. 2019, 31, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, M.; Bartolomeo, N.; De Risi, I.; Fucci, L.; Armenio, A.; Filannino, R.; Ruggieri, E.; Macina, F.; Traversa, M.; Nardone, A.; et al. The Management of Oligoprogression in the Landscape of New Therapies for Metastatic Melanoma. Cancers 2019, 11, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumati, V.; Iyengar, P. The current state of oligometastatic and oligoprogressive non-small cell lung cancer. J. Thorac. Dis. 2018, 10, S2537–S2544. [Google Scholar] [CrossRef]
- Schmid, S.; Klingbiel, D.; Aeppli, S.; Britschgi, C.; Gautschi, O.; Pless, M.; Rothschild, S.I.; Wannesson, L.; Janthur, W.; Foerbs, D.; et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: A Swiss cohort study. Lung Cancer 2019, 130, 149–155. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Scheier, B.; Burke, J.M.; Gan, G.; Lu, X.; Bunn, P.A.; Aisner, D.L.; Gaspar, L.E.; Kavanagh, B.D.; Doebele, R.C.; et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Gan, G.N.; Weickhardt, A.J.; Scheier, B.; Doebele, R.C.; Gaspar, L.E.; Kavanagh, B.D.; Camidge, D.R. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int. J. Radiat. Oncol. 2014, 88, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Liu, H.; Meng, S.; Jiang, T.; Li, X.; Liang, S.; Ren, S.; Zhou, C. First-line continual EGFR-TKI plus local ablative therapy demonstrated survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. J. Cancer 2019, 10, 522–529. [Google Scholar] [CrossRef]
- Yu, H.A.; Sima, C.S.; Huang, J.; Solomon, S.B.; Rimner, A.; Paik, P.; Pietanza, M.C.; Azzoli, C.G.; Rizvi, N.A.; Krug, L.M.; et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 2013, 8, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Shukuya, T.; Takahashi, T.; Naito, T.; Kaira, R.; Ono, A.; Nakamura, Y.; Tsuya, A.; Kenmotsu, H.; Murakami, H.; Harada, H.; et al. Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure. Lung Cancer 2011, 74, 457–461. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef]
- Morgan, R.L.; Camidge, D.R. Reviewing RECIST in the Era of Prolonged and Targeted Therapy. J. Thorac. Oncol. 2018, 13, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harary, M.; Reardon, D.A.; Iorgulescu, J.B. Efficacy and safety of immune checkpoint blockade for brain metastases. CNS Oncol. 2019, 8, CNS33. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hoang, C.D.; Kesarwala, A.H.; Schrump, D.S.; Guha, U.; Rajan, A. Role of Local Ablative Therapy in Patients with Oligometastatic and Oligoprogressive Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mersiades, A.; Crumbaker, M.; Gao, B.; Nagrial, A.; Hui, R. P3.02c-033 Patterns of Progression and Management of Acquired Resistance to Anti-PD-1 Antibodies in Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, S1293. [Google Scholar] [CrossRef]
- Christopoulos, P.; Budczies, J.; Kirchner, M.; Dietz, S.; Sultmann, H.; Thomas, M.; Stenzinger, A. Defining molecular risk in ALK(+) NSCLC. Oncotarget 2019, 10, 3093–3103. [Google Scholar] [CrossRef]
- Berland, L.; Heeke, S.; Humbert, O.; Macocco, A.; Long-Mira, E.; Lassalle, S.; Lespinet-Fabre, V.; Lalvée, S.; Bordone, O.; Cohen, C.; et al. Current views on tumor mutational burden in patients with non-small cell lung cancer treated by immune checkpoint inhibitors. J. Thorac. Dis. 2019, 11, S71–S80. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, N.-W.; Coudert, B.; Perol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.; Han, J.-Y.; Lee, J.; Hochmair, M.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Dómine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Herbst, R.; Lopes, G.; Kowalski, D.; Nishio, M.; Wu, Y.-L.; Junior, G.D.C.; Baas, P.; Kim, D.-W.; Gubens, M.; Cristescu, R.; et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann. Oncol. 2019, 30, v916–v917. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Langer, C.; Novello, S.; Halmos, B.; Cheng, Y.; Gadgeel, S.; Hui, R.; Sugawara, S.; Borghaei, H.; Cristescu, R.; et al. Pembrolizumab (PEMBRO) plus platinium-based chemotherapy (CHEMO) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in keynote-021, 189, and 407. Ann. Oncol. 2019, 30 (Suppl. 5), v851–v934. [Google Scholar] [CrossRef]
- Herbst, R.; Lopes, G.; Kowalski, D.; Kasahara, K.; Wu, Y.-L.; De Castro, G.; Cho, B.; Turna, H.; Cristescu, R.; Aurora-Garg, D.; et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann. Oncol. 2019, 30, xi63–xi64. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodriguez-Abreu, D.; Felip, E.; Esteban, E.; Speranza, G.; Reck, M.; Hui, R.; Boyer, M.; Garon, E.; Horinouchi, H.; et al. KRAS mutational status and efficacy in KEYNOTE-189: Pembrolizumab (pembro) plus chemotherapy (chemo) vs. placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann. Oncol. 2019, 30, xi64–xi65. [Google Scholar] [CrossRef]
- Wang, Z.; Zhan, P.; Lv, Y.; Shen, K.; Wei, Y.; Liu, H.; Song, Y. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: A meta-analysis. Transl. Lung Cancer Res. 2019, 8, 214–226. [Google Scholar] [CrossRef]
- Christopoulos, P.; Schneider, M.A.; Bozorgmehr, F.; Kuon, J.; Engel-Riedel, W.; Kollmeier, J.; Baum, V.; Muley, T.R.; Schnabel, P.A.; Bischoff, H.; et al. Large cell neuroendocrine lung carcinoma induces peripheral T-cell repertoire alterations with predictive and prognostic significance. Lung Cancer 2018, 119, 48–55. [Google Scholar] [CrossRef]
- Stanta, G.; Bonin, S. Overview on Clinical Relevance of Intra-Tumor Heterogeneity. Front. Med. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Christopoulos, P.; Dietz, S.; Kirchner, M.; Volckmar, A.-L.; Endris, V.; Neumann, O.; Ogrodnik, S.; Heussel, C.P.; Herth, F.J.; Eichhorn, M.E.; et al. Detection of TP53 Mutations in Tissue or Liquid Rebiopsies at Progression Identifies ALK+ Lung Cancer Patients with Poor Survival. Cancers 2019, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Christopoulos, P.; Kohlhäufl, J.; Bozorgmehr, F.; Kuon, J.; Schneider, M.; Neumann, O.; Liersch, S.; Heussel, C.; Winter, H.; Herth, F.; et al. Clinical and laboratory predictors of immune checkpoint inhibitor efficacy in non-small cell lung cancer. Ann. Oncol. 2018, 29, x3–x4. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2013, 94, 107–116. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Diem, S.; Li, Q.; Krapf, M.; Flatz, L.; Leschka, S.; Desbiolles, L.; Klingbiel, D.; Jochum, W.; Früh, M. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol. Immunother. 2018, 67, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Dai, C.; Zheng, H.; Zhou, F.; She, Y.; Jiang, G.; Fei, K.; Yang, P.; Xie, N.; Chen, C. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 2016, 7, 53245–53253. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Kissel, M.; Martel-Lafay, I.; LeQuesne, J.; Faivre, J.-C.; Le Pechoux, C.; Stefan, D.; Barraux, V.; Loiseau, C.; Grellard, J.-M.; Danhier, S.; et al. Stereotactic ablative radiotherapy and systemic treatments for extracerebral oligometastases, oligorecurrence, oligopersistence and oligoprogression from lung cancer. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetzlaff, M.T.; Nelson, K.; Diab, A.; Staerkel, G.A.; Nagarajan, P.; Torres-Cabala, C.A.; Chasen, B.A.; Wargo, J.A.; Prieto, V.G.; Amaria, R.N.; et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J. Immunother. Cancer 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Gkiozos, I.; Kopitopoulou, A.; Kalkanis, A.; Vamvakaris, I.N.; Judson, M.A.; Syrigos, K.N.; Ioannis, V.N.; Syrigos, K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol. 2018, 13, 1076–1082. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]


| IO Monotherapy-Treated Stage IV NSCLC Patients | IO+CHT-Treated NSCLC Patients | p-Value 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| OPD (13%, n = 38) | Diffuse PD (n = 259) | p-Value 1 | OPD (13%, n = 10) | Diffuse PD (n = 65) | ||||
| Age (Median; SD) | 63 (10) | 64 (9) | ns | 64 (11) | 65 (10) | ns | ||
| Gender (% male) | 58 | 58 | ns | 40 | 58 | ns | ||
| Smoking Status (%) 2 | never smokers | 0 | 10 | p < 0.05 | 20 | 22 | ns | |
| ex-smokers | 61 | 53 | ns | 40 | 48 | ns | ||
| current smokers | 39 | 37 | ns | 40 | 30 | ns | ||
| ECOG PS (%) 3 | 0 | 47 | 41 | ns | 50 | 40 | ns | |
| 1 | 47 | 58 | ns | 50 | 59 | ns | ||
| 2 | 5 | 2 | ns | 0 | 1 | ns | ||
| Histology (%) | adenocarcinoma | 68 | 63 | ns | 90 | 88 | ns | |
| squamous cell carcinoma | 29 | 31 | ns | 1 | 7 | ns | ||
| other (LCNEC, NOS, mixed) | 3 | 6 | ns | 0 | 6 | ns | ||
| No. of Metastatic Sites at IO Start (Mean; SD) | 2.4 (1.2) | 2.5 (1.4) | ns | 1.1 (2.5) | 1.8 (2.7) | ns | ||
| PD-L1 IHC 4 (Average % of Positive Cells; SD) | 65 (33) | 41 (36) | p < 0.001 | 17 (22) | 18 (30) | ns | ||
| LNR (Mean; SD) | 0.24 (0.11) | 0.23 (0.46) | ns | 0.21 (0.09) | 0.17 (0.14) | ns | ||
| IO Treatment | first line | 18 | 71 | p < 0.05 | 10 | 65 | ||
| second-and-beyond line | 20 | 188 | p < 0.05 | |||||
| TTP from IO Treatment Start in Months, Median | 9 | 2 | p < 0.001 | |||||
| first-line patients | 11 | 2 | p < 0.001 | 4 | 4 | ns | ||
| second-and-beyond-line patients | 5 | 2 | p = 0.015 | |||||
| OS from IO Treatment Start in Months, Median (Mean) | n.r. (26) | 10 (13) | p < 0.001 | |||||
| first-line patients | n.r. (39) | 14 (15) | p < 0.001 | n.r. | n.r. | ns | ||
| second-and-beyond-line patients | 16 | 10 | p < 0.05 | |||||
| Location of OPD, no. (%) 1 | IO monotherapy | 1L IO + CHT 1 | TTP of OPD | |||
|---|---|---|---|---|---|---|
| All OPD 1 (n = 38, 13%) | 1L IO OPD (n = 18, 20%) | 2+L IO OPD (n = 20, 10%) | All OPD Cases (n = 10, 13%) | in Months, Median (IQR) | ||
| Lymph Nodes | All | 16 (5%) | 8 (9%) | 8 (4%) | 0 (0%) | 8 (4–14) |
| mediastinal | 10 | 6 | 4 | |||
| axillary, cervical | 5 | 2 | 3 | |||
| abdominal | 2 | 1 | 2 | |||
| Brain | 15 (5%) | 3 (3%) | 10 (5%) | 3 (4%) | 4 (2–11) | |
| Lung | All | 9 (3%) | 5 (6%) | 5 (2%) | 2 (2%) | 7 (3–15) |
| primary tumor | 4 | 1 | 3 | 2 | ||
| lung metastasis | 5 | 4 | 2 | 0 | ||
| Adrenal Gland | 6 (2%) | 1 (1%) | 4 (2%) | 2 (2%) | 8 (5–13) | |
| Bone | 3 (1%) | 1 (1%) | 0 | 3 (4%) | 4 (3–27) | |
| Liver | 2 (< 1%) | 1 (1%) | 1 (< 1%) | 1 (2%) | 12 (7–15) | |
| Skin/Soft Tissue | 1 (< 1%) | 0 | 1 (< 1%) | 0 | 9 (n/a) | |
| No. of Anatomic Sites, Average (SD)2 | 1.4 (0.5) | 1.1 (0.3) * | 1.5 (0.6) | 1.1 (0.3) | ||
| No. of Lesions, Average (SD) | 1.8 (1.2) | 1.4 (0.8) * | 2.3 (1.4) | 1.6 (0.8) | ||
| TTP in Months, Median | 11 | 11 ** | 5 | 6 | ||
| IO monotherapy | 1L IO + CHT | |||||
| All Patients with PD (no.) | all (297) | 1L (89) | 2+L (208) | all (85) | ||
| FU, Median (IQR) | 15 (9–21) | 13 (7–21) | 15 (9–22) | 7 (3–10) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rheinheimer, S.; Heussel, C.-P.; Mayer, P.; Gaissmaier, L.; Bozorgmehr, F.; Winter, H.; Herth, F.J.; Muley, T.; Liersch, S.; Bischoff, H.; et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers 2020, 12, 1046. https://doi.org/10.3390/cancers12041046
Rheinheimer S, Heussel C-P, Mayer P, Gaissmaier L, Bozorgmehr F, Winter H, Herth FJ, Muley T, Liersch S, Bischoff H, et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers. 2020; 12(4):1046. https://doi.org/10.3390/cancers12041046
Chicago/Turabian StyleRheinheimer, Stephan, Claus-Peter Heussel, Philipp Mayer, Lena Gaissmaier, Farastuk Bozorgmehr, Hauke Winter, Felix J. Herth, Thomas Muley, Stephan Liersch, Helge Bischoff, and et al. 2020. "Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors" Cancers 12, no. 4: 1046. https://doi.org/10.3390/cancers12041046








