Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10
Abstract
1. Introduction
2. Results
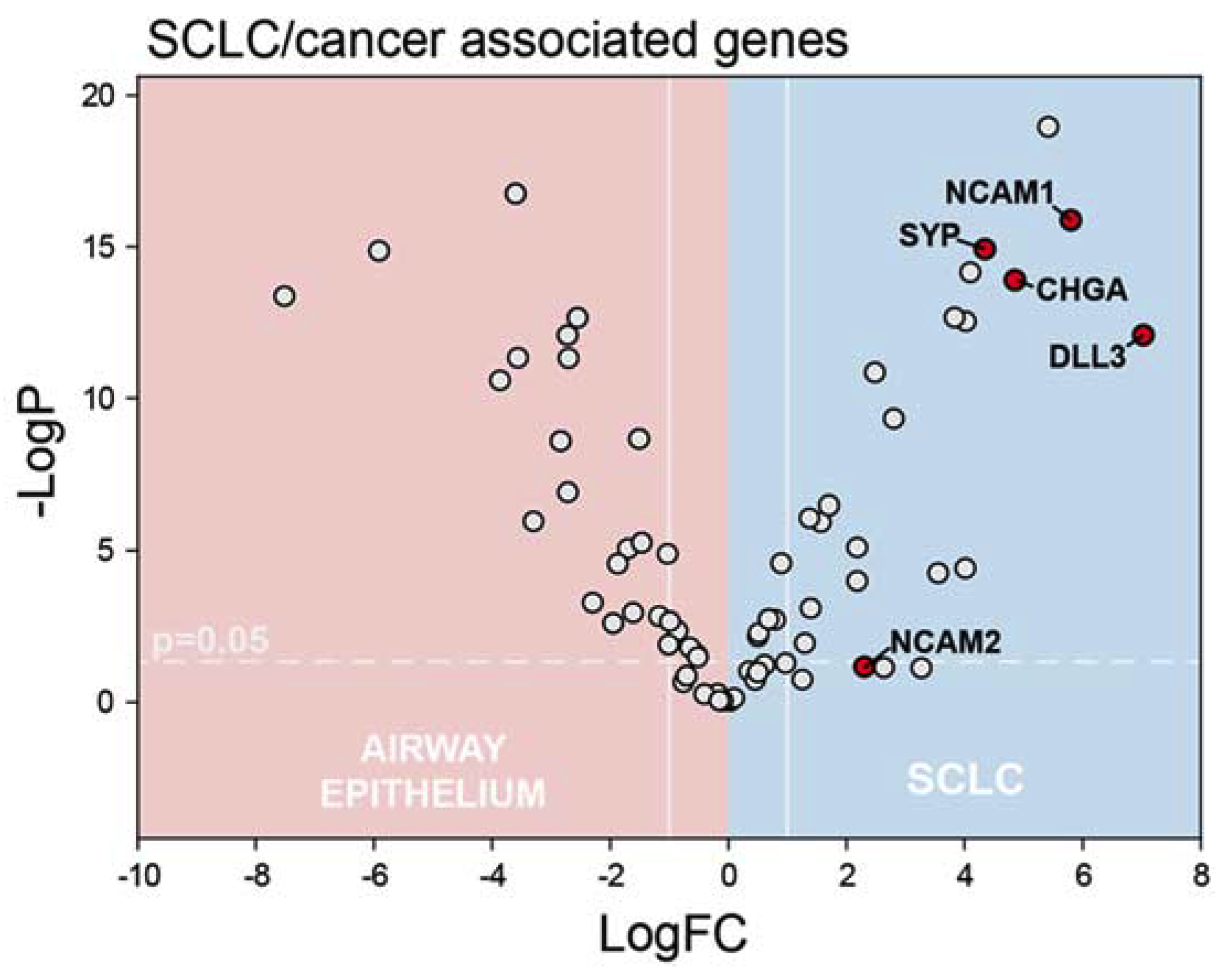
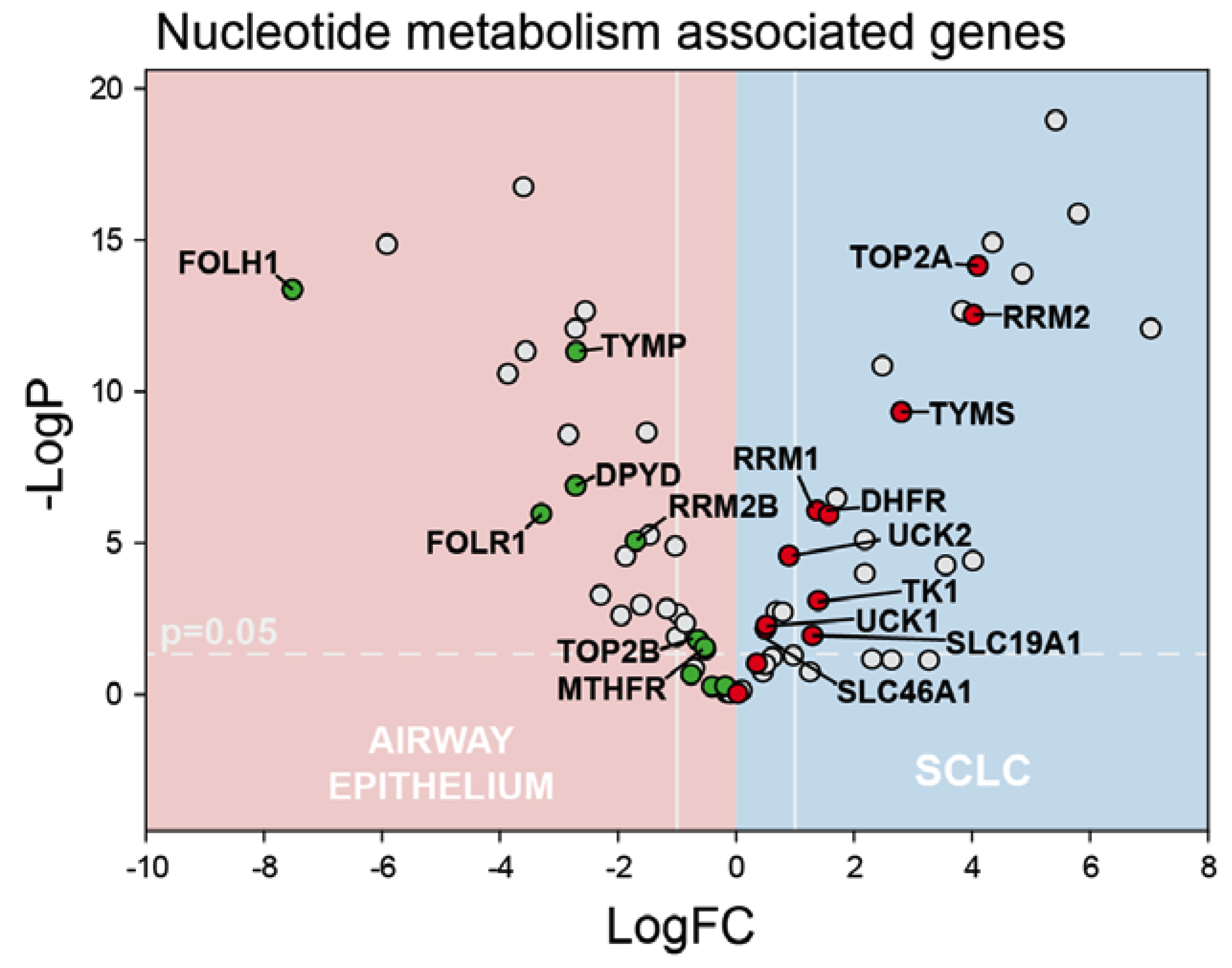
2.1. RNA-Seq Reveals Dysregulated Pyrimidine Biosynthesis in SCLC
2.2. Elevated De Novo Thy Biosynthesis Is Associated with E2F1-3 Upregulation
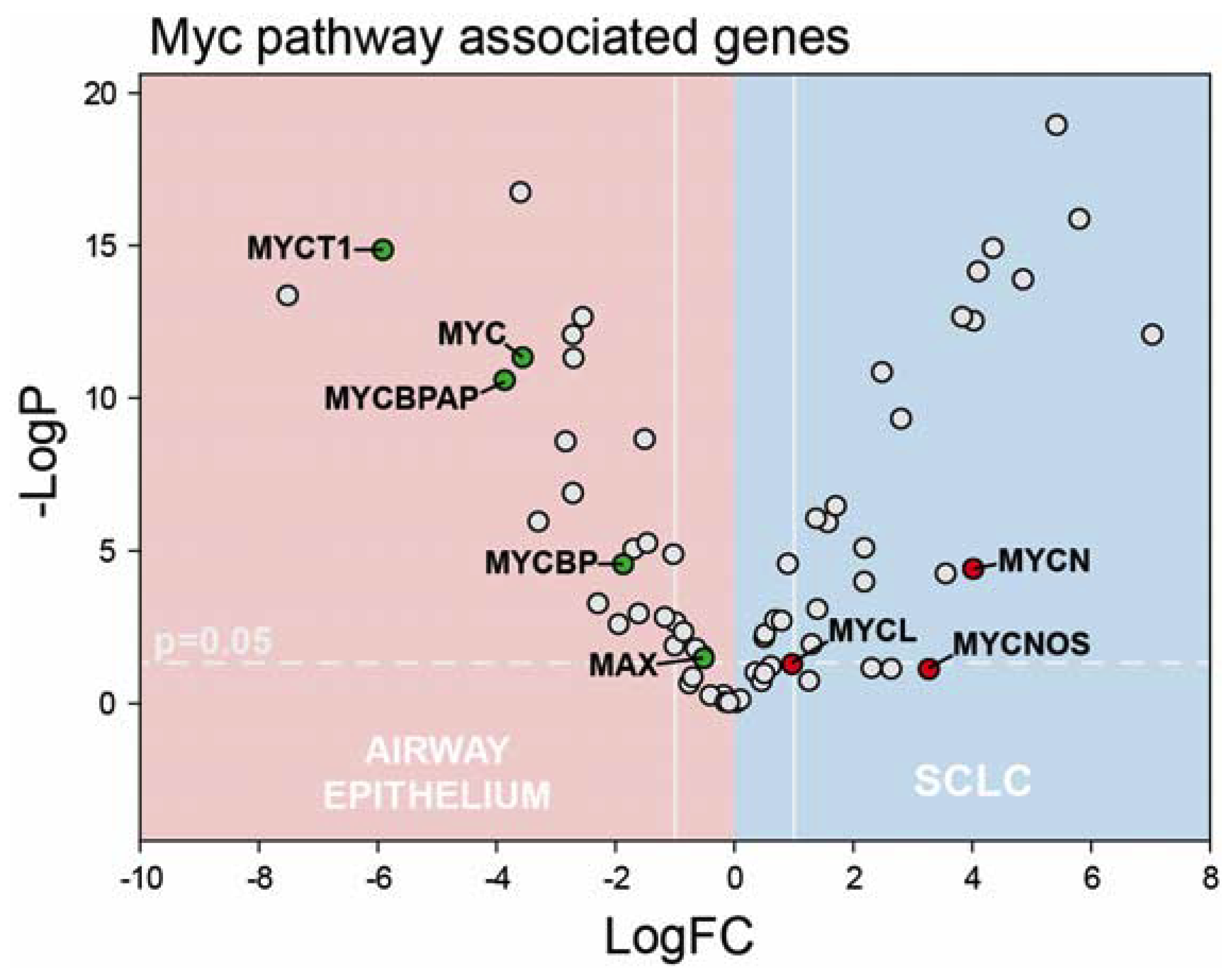
2.3. Myc-Family Expression and Gene Ontology Analysis
2.4. Dysregulated Pyrimidine Biosynthesis Is Associated with 5-FU Resistance, but CF10 Sensitivity
3. Discussion
4. Materials and Methods
4.1. Samples and Clinical Data
4.2. EBUS-TBNA
4.3. PDX Generation
4.4. PDO Formation and Drug Testing
4.5. RNA-Seq
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lally, B.E.; Urbanic, J.J.; Blackstock, A.W.; Miller, A.A.; Perry, M.C. Small cell lung cancer: Have we made any progress over the last 25 years? Oncologist 2007, 12, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Jackman, D.M.; Johnson, B.E. Small-cell lung cancer. Lancet 2005, 366, 1385–1396. [Google Scholar] [CrossRef]
- Alvarado-Luna, G.; Morales-Espinosa, D. Treatment for small cell lung cancer, where are we now?-a review. Transl. Lung Cancer Res. 2016, 5, 26–38. [Google Scholar] [CrossRef]
- Nesbit, E.G.; Leal, T.A.; Kruser, T.J. What is the role of radiotherapy for extensive-stage small cell lung cancer in the immunotherapy era? Transl. Lung Cancer Res. 2019, 8, S153–S162. [Google Scholar] [CrossRef]
- Le, T.; Gerber, D.E. Newer-Generation EGFR Inhibitors in Lung Cancer: How Are They Best Used? Cancers (Basel) 2019, 11, 366. [Google Scholar] [CrossRef]
- Karachaliou, N.; Sosa, A.E.; Rosell, R. Unraveling the genomic complexity of small cell lung cancer. Transl. Lung Cancer Res. 2016, 5, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Hsu, N.Y.; Hsu, W.H.; Tsai, C.H.; Lin, C.C.; Liang, J.A. Comparison of chemotherapy response with P-glycoprotein, multidrug resistance-related protein-1, and lung resistance-related protein expression in untreated small cell lung cancer. Lung 2005, 183, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Feng, B.; Chen, L.B. Update of research on drug resistance in small cell lung cancer chemotherapy. Asian Pac. J. Cancer Prev. 2012, 13, 3577–3581. [Google Scholar] [CrossRef][Green Version]
- Tanaka, F.; Wada, H.; Fukui, Y.; Fukushima, M. Thymidylate synthase (TS) gene expression in primary lung cancer patients: A large-scale study in Japanese population. Ann. Oncol. 2011, 22, 1791–1797. [Google Scholar] [CrossRef]
- Morere, J.F.; Duran, A.; Tcherakian, F.; Boaziz, C.; Valeyre, D.; Battesti, J.P.; Breu, J.L.; Israel, L. Cisplatin-5-fluorouracil in small cell lung cancer. A phase II study in 109 patients. Lung Cancer 1994, 11, 275–281. [Google Scholar] [CrossRef]
- Woll, P.J.; Basser, R.; Le Chevalier, T.; Drings, P.; Perez Manga, G.; Adenis, A.; Seymour, L.; Smith, F.; Thatcher, N. Phase II trial of raltitrexed (’Tomudex’) in advanced small-cell lung cancer. Br. J. Cancer 1997, 76, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Ferraldeschi, R.; Thatcher, N.; Lorigan, P. Pemetrexed in small-cell lung cancer: Background and review of the ongoing GALES pivotal trial. Expert Rev. Anticancer. Ther. 2007, 7, 635–640. [Google Scholar] [CrossRef]
- Salonga, D.; Danenberg, K.D.; Johnson, M.; Metzger, R.; Groshen, S.; Tsao-Wei, D.D.; Lenz, H.J.; Leichman, C.G.; Leichman, L.; Diasio, R.B.; et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. 2000, 6, 1322–1327. [Google Scholar] [PubMed]
- Takezawa, K.; Okamoto, I.; Okamoto, W.; Takeda, M.; Sakai, K.; Tsukioka, S.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br. J. Cancer 2011, 104, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H. Novel chemical strategies for thymidylate synthase inhibition. Curr. Med. Chem. 2005, 12, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ng, S.R.; Colon, C.I.; Drapkin, B.J.; Hsu, P.P.; Li, Z.; Nabel, C.S.; Lewis, C.A.; Romero, R.; Mercer, K.L.; et al. Identification of DHODH as a therapeutic target in small cell lung cancer. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Debinski, W.; Milligan, C.; Caudell, D.; Pardee, T.S. The applications of the novel polymeric fluoropyrimidine F10 in cancer treatment: Current evidence. Future Oncol. 2016, 12, 2009–2020. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Willingham, M.C.; Bourland, J.D.; Hatcher, H.C.; Smith, T.L.; D’Agostino, R.B., Jr.; Blackstock, W. F10 Inhibits Growth of PC3 Xenografts and Enhances the Effects of Radiation Therapy. J. Clin. Oncol. Res. 2014, 2, 1028. [Google Scholar]
- Liu, J.; Kolar, C.; Lawson, T.A.; Gmeiner, W.H. Targeted drug delivery to chemoresistant cells: Folic acid derivatization of FdUMP[10] enhances cytotoxicity toward 5-FU-resistant human colorectal tumor cells. J. Org. Chem. 2001, 66, 5655–5663. [Google Scholar] [CrossRef]
- Bunz, F.; Hwang, P.M.; Torrance, C.; Waldman, T.; Zhang, Y.; Dillehay, L.; Williams, J.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Investig. 1999, 104, 263–269. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Sordet, O.; Zhang, H.L.; Kohlhagen, G.; Antony, S.; Gmeiner, W.H.; Pommier, Y. A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes. Cancer Res. 2005, 65, 4844–4851. [Google Scholar] [CrossRef] [PubMed]
- Pardee, T.S.; Gomes, E.; Jennings-Gee, J.; Caudell, D.; Gmeiner, W.H. Unique dual targeting of thymidylate synthase and topoisomerase1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood 2012, 119, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, A.; Kalender, M.E.; Altinbas, M.; Ozkan, M.; Dikilitas, M.; Camci, C.; Anatolian Society of Medical, O. Irinotecan as a second-line monotherapy for small cell lung cancer. Asian Pac. J. Cancer Prev. 2011, 12, 1055–1059. [Google Scholar] [PubMed]
- Nagasaki, T.; Tsuchiya, T.; Tagawa, T.; Honda, S.; Yamasaki, N.; Miyazaki, T.; Hidaka, S.; Hayashi, T.; Nagayasu, T. Analysis of 5-fluorouracil-related enzymes in pulmonary neuroendocrine carcinoma: Differences in biological properties compared to epithelial carcinoma. Clin. Lung Cancer 2010, 11, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Monica, V.; Scagliotti, G.V.; Ceppi, P.; Righi, L.; Cambieri, A.; Lo Iacono, M.; Saviozzi, S.; Volante, M.; Novello, S.; Papotti, M. Differential Thymidylate Synthase Expression in Different Variants of Large-Cell Carcinoma of the Lung. Clin. Cancer Res. 2009, 15, 7547–7552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibe, T.; Shimizu, K.; Nakano, T.; Kakegawa, S.; Kamiyoshihara, M.; Nakajima, T.; Kaira, K.; Takeyoshi, I. High-grade neuroendocrine carcinoma of the lung shows increased thymidylate synthase expression compared to other histotypes. J. Surg. Oncol. 2010, 102, 11–17. [Google Scholar] [CrossRef]
- Bertino, J.R.; Banerjee, D. Thymidylate synthase as an oncogene? Cancer Cell 2004, 5, 301–302. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretic, L.; Kong, G.; Leenders, F.; Lu, X.; Fernandez-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Chen, H.Z.; Tsai, S.Y.; Leone, G. Emerging roles of E2Fs in cancer: An exit from cell cycle control. Nat. Rev. Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef]
- Drivsholm, L.; Paloheimo, L.I.; Osterlind, K. Chromogranin A, a significant prognostic factor in small cell lung cancer. Br. J. Cancer 1999, 81, 667–671. [Google Scholar] [CrossRef]
- Kontogianni, K.; Nicholson, A.G.; Butcher, D.; Sheppard, M.N. CD56: A useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artefact. J. Clin. Pathol. 2005, 58, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Taneja, T.K.; Sharma, S.K. Markers of small cell lung cancer. World J. Surg. Oncol. 2004, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef]
- Johnston, P.G.; Lenz, H.J.; Leichman, C.G.; Danenberg, K.D.; Allegra, C.J.; Danenberg, P.V.; Leichman, L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995, 55, 1407–1412. [Google Scholar]
- Peters, G.J.; Backus, H.H.; Freemantle, S.; van Triest, B.; Codacci-Pisanelli, G.; van der Wilt, C.L.; Smid, K.; Lunec, J.; Calvert, A.H.; Marsh, S.; et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim. Biophys. Acta 2002, 1587, 194–205. [Google Scholar] [CrossRef]
- Semenova, E.A.; Nagel, R.; Berns, A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev. 2015, 29, 1447–1462. [Google Scholar] [CrossRef]
- Poppy Roworth, A.; Ghari, F.; La Thangue, N.B. To live or let die—Complexity within the E2F1 pathway. Mol. Cell. Oncol. 2015, 2, e970480. [Google Scholar] [CrossRef]
- Engelmann, D.; Putzer, B.M. The dark side of E2F1: In transit beyond apoptosis. Cancer Res. 2012, 72, 571–575. [Google Scholar] [CrossRef]
- McNair, C.; Xu, K.; Mandigo, A.C.; Benelli, M.; Leiby, B.; Rodrigues, D.; Lindberg, J.; Gronberg, H.; Crespo, M.; De Laere, B.; et al. Differential impact of RB status on E2F1 reprogramming in human cancer. J. Clin. Investig. 2018, 128, 341–358. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Qiao, W.; Kong, L.; Sun, D.; Li, Z. Transcription factor E2F1 promotes EMT by regulating ZEB2 in small cell lung cancer. BMC Cancer 2017, 17, 719. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Y.; Jiang, H.; Zhang, T.; Jin, C.; Young, C.Y.; Yuan, H. Differential regulation of MMPs by E2F1, Sp1 and NF-kappa B controls the small cell lung cancer invasive phenotype. BMC Cancer 2014, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Mannava, S.; Grachtchouk, V.; Wheeler, L.J.; Im, M.; Zhuang, D.; Slavina, E.G.; Mathews, C.K.; Shewach, D.S.; Nikiforov, M.A. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle 2008, 7, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Mollaoglu, G.; Guthrie, M.R.; Bohm, S.; Bragelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef]
- Nau, M.M.; Brooks, B.J., Jr.; Carney, D.N.; Gazdar, A.F.; Battey, J.F.; Sausville, E.A.; Minna, J.D. Human small-cell lung cancers show amplification and expression of the N-myc gene. Proc. Natl. Acad. Sci. USA 1986, 83, 1092–1096. [Google Scholar] [CrossRef]
- Funa, K.; Steinholtz, L.; Nou, E.; Bergh, J. Increased expression of N-myc in human small cell lung cancer biopsies predicts lack of response to chemotherapy and poor prognosis. Am. J. Clin. Pathol. 1987, 88, 216–220. [Google Scholar] [CrossRef]
- Vadie, N.; Saayman, S.; Lenox, A.; Ackley, A.; Clemson, M.; Burdach, J.; Hart, J.; Vogt, P.K.; Morris, K.V. MYCNOS functions as an antisense RNA regulating MYCN. RNA Biol. 2015, 12, 893–899. [Google Scholar] [CrossRef]
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 4. [Google Scholar] [CrossRef]
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012, 2, 798–811. [Google Scholar] [CrossRef]
- Aimiuwu, J.; Wang, H.; Chen, P.; Xie, Z.; Wang, J.; Liu, S.; Klisovic, R.; Mims, A.; Blum, W.; Marcucci, G.; et al. RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood 2012, 119, 5229–5238. [Google Scholar] [CrossRef]
- Cook, G.J.; Caudell, D.L.; Elford, H.L.; Pardee, T.S. The efficacy of the ribonucleotide reductase inhibitor Didox in preclinical models of AML. PLoS ONE 2014, 9, e112619. [Google Scholar] [CrossRef] [PubMed]
- Komori, S.; Osada, S.; Mori, R.; Matsui, S.; Sanada, Y.; Tomita, H.; Tokuyama, Y.; Takahashi, T.; Yamaguchi, K.; Yoshida, K. Contribution of thymidylate synthase to gemcitabine therapy for advanced pancreatic cancer. Pancreas 2010, 39, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Pardee, T.S.; Stadelman, K.; Jennings-Gee, J.; Caudell, D.L.; Gmeiner, W.H. The poison oligonucleotide F10 is highly effective against acute lymphoblastic leukemia while sparing normal hematopoietic cells. Oncotarget 2014, 5, 4170–4179. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H.; Lema-Tome, C.; Gibo, D.; Jennings-Gee, J.; Milligan, C.; Debinski, W. Selective anti-tumor activity of the novel fluoropyrimidine polymer F10 towards G48a orthotopic GBM tumors. J. Neurooncol. 2014, 116, 447–454. [Google Scholar] [CrossRef][Green Version]
- Gmeiner, W.H.; Reinhold, W.C.; Pommier, Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol. Cancer Ther. 2010, 9, 3105–3114. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Gearhart, P.J.; Pommier, Y.; Nakamura, J. F10 cytotoxicity via topoisomerase I cleavage complex repair consistent with a unique mechanism for thymineless death. Future Oncol. 2016, 12, 2183–2188. [Google Scholar] [CrossRef]
- Gmeiner, W.H. Entrapment of DNA topoisomerase-DNA complexes by nucleotide/nucleoside analogs. Cancer Drug Resist. 2019, 2, 994–1001. [Google Scholar] [CrossRef]
- Huang, S.Y.; Murai, J.; Dalla Rosa, I.; Dexheimer, T.S.; Naumova, A.; Gmeiner, W.H.; Pommier, Y. TDP1 repairs nuclear and mitochondrial DNA damage induced by chain-terminating anticancer and antiviral nucleoside analogs. Nucleic Acids Res. 2013, 41, 7793–7803. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Dominijanni, A.; Caudell, D.; D’Agostino, R., Jr.; Smith, T.L.; Deng, Z.; Mani, C.; Palle, K.; Haber, A.; Brody, J. Efficacy of the fluoropyrimidine polymer CF10 in colorectal cancer thru increased replication stress. 2020. In preparation. [Google Scholar]
- Pritchard, D.M.; Watson, A.J.; Potten, C.S.; Jackman, A.L.; Hickman, J.A. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: Evidence for the involvement of RNA perturbation. Proc. Natl. Acad. Sci. USA 1997, 94, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Saif, M.W.; El-Rayes, B.F.; Fakih, M.G.; Cartwright, T.H.; Posey, J.A.; King, T.R.; von Borstel, R.W.; Bamat, M.K. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017, 123, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, C.R.; Chatterjee, A.B.; Chin, R., Jr.; Conforti, J.; Adair, N.; Haponik, E. Conventional and endobronchial ultrasound-guided transbronchial needle aspiration: Complementary procedures. South Med. J. 2012, 105, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, S.; Mehta, N.; Devarasetty, M.; Sivakumar, H.; Gmeiner, W.; Soker, S.; Votanopoulos, K.; Skardal, A. Development of a Colorectal Cancer 3D Micro-tumor Construct Platform From Cell Lines and Patient Tumor Biospecimens for Standard-of-Care and Experimental Drug Screening. Ann. Biomed. Eng. 2019, 48, 940–952. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gmeiner, W.H.; Miller, L.D.; Chou, J.W.; Dominijanni, A.; Mutkus, L.; Marini, F.; Ruiz, J.; Dotson, T.; Thomas, K.W.; Parks, G.; et al. Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10. Cancers 2020, 12, 788. https://doi.org/10.3390/cancers12040788
Gmeiner WH, Miller LD, Chou JW, Dominijanni A, Mutkus L, Marini F, Ruiz J, Dotson T, Thomas KW, Parks G, et al. Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10. Cancers. 2020; 12(4):788. https://doi.org/10.3390/cancers12040788
Chicago/Turabian StyleGmeiner, William H., Lance D. Miller, Jeff W. Chou, Anthony Dominijanni, Lysette Mutkus, Frank Marini, Jimmy Ruiz, Travis Dotson, Karl W. Thomas, Graham Parks, and et al. 2020. "Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10" Cancers 12, no. 4: 788. https://doi.org/10.3390/cancers12040788
APA StyleGmeiner, W. H., Miller, L. D., Chou, J. W., Dominijanni, A., Mutkus, L., Marini, F., Ruiz, J., Dotson, T., Thomas, K. W., Parks, G., & Bellinger, C. R. (2020). Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10. Cancers, 12(4), 788. https://doi.org/10.3390/cancers12040788






