Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates
Abstract
:1. Introduction
2. Results
2.1. Patients
2.2. MTCs and CSCs Frequencies in Peripheral Blood and LNs Aspirates
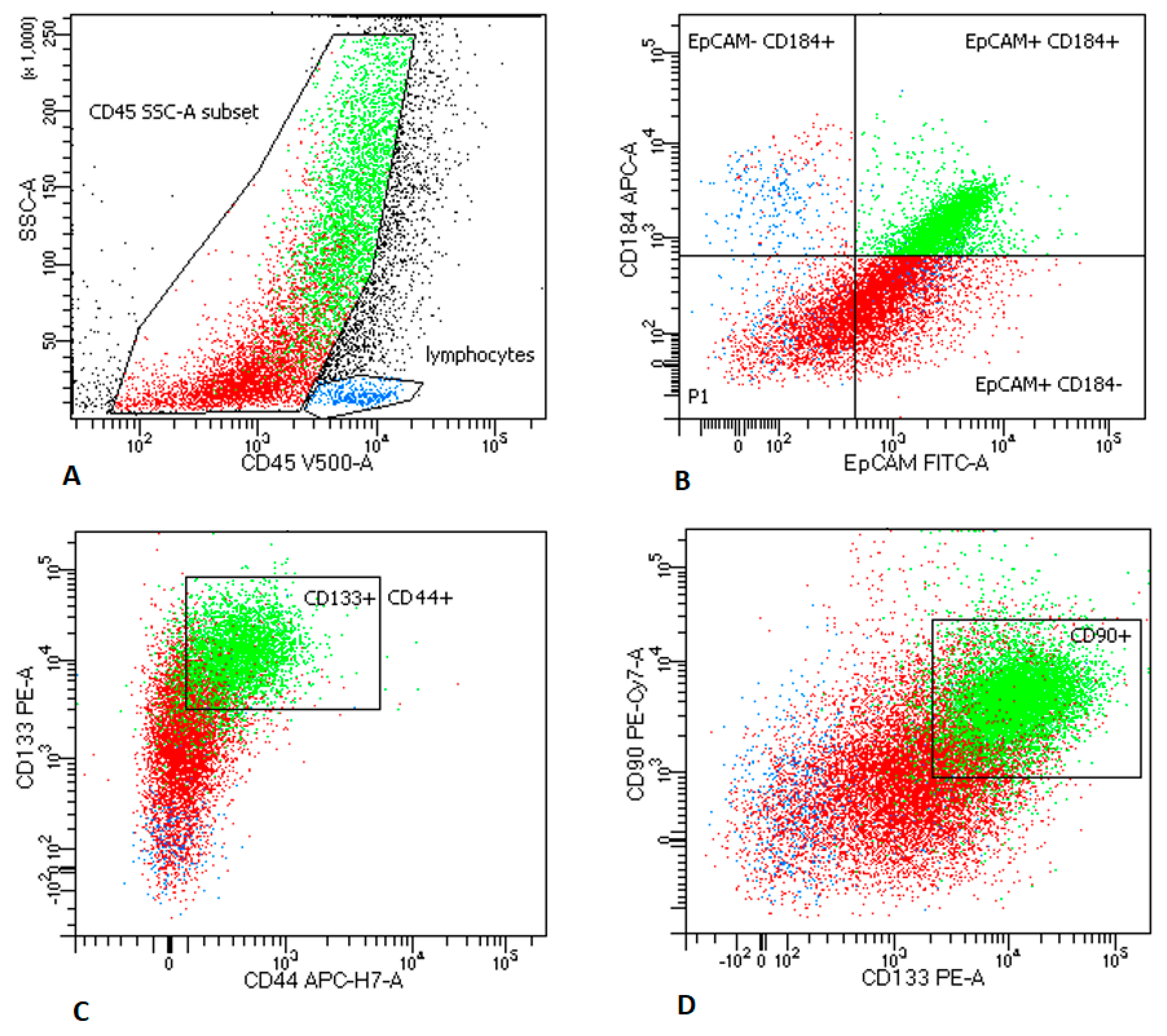
2.2.1. LNs Aspirates
2.2.2. Peripheral Blood Analysis
2.3. MTCs and CSCs Express Different Immunomodulatory Molecules on Their Surface
2.4. Frequencies of Lymphocyte Subsets in PB
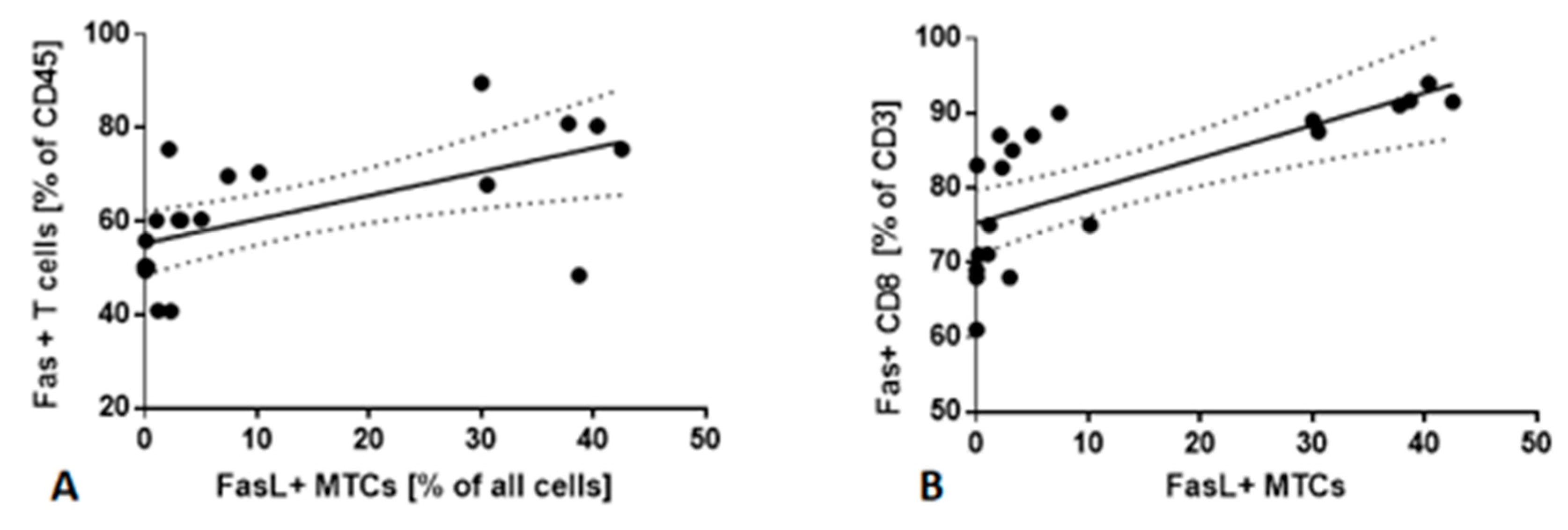
2.5. Fas/FasL Pathway
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Case | Investigated LN | Sex/age | Histological Subtype | EGFR/ALK/KRAS | Stage of Cancer | CSCs % of All Cells/Geomean | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD-L1+ | CD47+ | CD73+ | Fas+ | FasL+ | ||||||
| LNs with confirmed metastases | ||||||||||
| 1. | 11 | m/65 | ADC | -/+/- | IIIA | 7.58/4194 | 8.40/4200 | 1.70/1264 | 2.12/849 | 2.60/1600 |
| 2. | 4 | f/74 | LCNEC | IIIA | 3.83/2502 | 3.50/2246 | 0.42/1201 | 0.89/519 | 1.20/943 | |
| 3. | 4 | m/62 | NOS | +/-/- | IIIA | 2.20/565 | 1.99/687 | 0.02/128 | 0.98/34 | 1.62/113 |
| 4. | 7 | m/69 | ADC | -/-/- | IIIC | 6.70/2650 | 3.00/2831 | 2.60/762 | 3.20/480 | 3.74/745 |
| 5. | 4 | m/64 | ADC | -/-/+ | IV | 15.50/4133 | 14.50/5923 | 5.33/3155 | 0.69/209 | 5.90/2351 |
| 6. | 7 | f/67 | ADC | +/-/- | IIIB | 10.20/2091 | 9.50/934 | 2.41/1928 | 5.50/850 | 6.80/1900 |
| 7. | 4 | m/67 | SQCLC | IIIA | 5.90/2154 | 4.80/1900 | 3.54/637 | 1.18/134 | 5.20/1987 | |
| 8. | 7 | f/71 | ADC | +/-/- | IV | 9.84/4123 | 7.86/3724 | 5.54/2753 | 1.80/235 | 6.47/3291 |
| 9. | 7 | m/58 | ADC | +/-/- | IV | 6.60/3109 | 4.90/2523 | 2.60/823 | 1.10/198 | 4.08/765 |
| 10. | 11 | m/64 | SQCLC | IIIB | 2.87/1123 | 2.54/987 | 0.98/346 | 0.40/90 | 1.10/680 | |
| 11. | 4 | m/72 | ADC | -/-/+ | IV | 12.20/4581 | 10.89/5201 | 6.70/1932 | 2.20/847 | 5.44/830 |
| 12. | 4 | M/66 | SQCLC | IV | 10.20/2454 | 8.74/3954 | 4.87/3645 | 1.90/658 | 2.15/745 | |
| 13. | 7 | F/67 | ADC | -/+/- | IIIC | 9.40/3654 | 6.53/4380 | 2.98/1245 | 2.10/487 | 3.65/2300 |
| LNs without confirmed metastases | ||||||||||
| 1. | 4 | m/66 | SQCLC | IB | 0.50/723 | 0.48/748 | 0.27/532 | 0.20/75 | 0.30/180 | |
| 2. | 4 | m/69 | ASC | IB | 0.09/222 | 0.08/123 | 0.02/143 | 0.07/60 | 0.02/90 | |
| 3. | 11 | f/69 | ASC | IIB | 0.01/250 | 0.05/99 | 0.00/160 | 0.03/50 | 0.15/164 | |
| 4. | 4 | f/60 | NOS | IIB | 0.00/0 | 0.00/0 | 0.00/0 | 0.00/0 | 0.00/0 | |
| 5. | 11 | f/64 | SQCLC | IIIA | 0.00/0 | 0.00/0 | 0.00/0 | 0.00/0 | 0.00/0 | |
| 6. | 11 | f/68 | ADC | +/-/- | IIIA | 2.50/524 | 2.10/490 | 1.89/410 | 1.96/75 | 2.30/179 |
| 7. | 4 | f/59 | ADC | -/-/- | IIB | 0.01/50 | 0.00/0 | 0.01/0 | 0.00/0 | 0.01/25 |


References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. GLOBOCAN 2018 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 15. 2018. Available online: https://gco.iarc.fr (accessed on 16 January 2020).
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, A.; Grynovska, M.; Lucibello, F.; Moisés, J.; Pagès, F.; Tsao, M.S.; Shepherd, F.A.; Bouchaab, H.; Garassino, M.; Aerts, J.G.J.V.; et al. Immunotherapy: A new standard of care in thoracic malignancies? A summary of the European Respiratory Society research seminar of the Thoracic Oncology Assembly. Eur. Respir. J. 2018, 51, 1702072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudnik, E.; Moskovitz, M.; Daher, S.; Shamai, S.; Hanovich, E.; Grubstein, A.; Shochat, T.; Wollner, M.; Bar, J.; Merimsky, O.; et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer 2017, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, J.; Haiech, J.; Kilhoffer, M.C.; Zeniou, M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016, 2016, 1740936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Xie, S.; Zeng, W.; Fan, G.; Huang, J.; Kang, G.; Geng, Q.; Cheng, B.; Wang, W.; Dong, P. Effect of CXCL12/CXCR4 on increasing the metastatic potential of non-small cell lung cancer in vitro is inhibited through the downregulation of CXCR4 chemokine receptor expression. Oncol. Lett. 2014, 7, 941–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skirecki, T.; Hoser, G.; Kawiak, J.; Dziedzic, D.; Domagała-Kulawik, J. Flow cytometric analysis of CD133- and EpCAM-positive cells in the peripheral blood of patients with lung cancer. Arch. Immunol. Ther. Exp. 2014, 62, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, M.P.; Gray, S.G.; Hoffmann, A.C.; Hilger, R.A.; Thomale, J.; O’Flaherty, J.D.; Fennell, D.A.; Richard, D.; O’Leary, J.J.; O’Byrne, K.J. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS ONE 2013, 8, e54193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine decarboxylase acticity drives non-small cell lung cancer tumorinitiating cells and tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Gao, Q.; Suo, Z.; Munthe, E.; Solberg, S.; Ma, L.; Wang, M.; Westerdaal, N.A.C.; Kvalheim, G.; Gaudernack, G. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS ONE 2013, 8, e57020. [Google Scholar] [CrossRef]
- Badrinath, N.; Yoo, S.Y. Recent Advances in Cancer Stem Cell-Targeted Immunotherapy. Cancers 2019, 11, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogino, S.; Galon, J.; Fuchs, C.S.; Dranoff, G. Cancer immunology—Analysis of host and tumor factors for personalized medicine. Nat. Rev. Clin. Oncol. 2011, 8, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Pereira, E.R.; Padera, T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, J.G.; Hegmans, J.P. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013, 73, 2381–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domagala-Kulawik, J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl. Lung Cancer Res. 2015, 4, 177–190. [Google Scholar] [CrossRef]
- Raniszewska, A.; Polubiec-Kownacka, M.; Rutkowska, E.; Domagała-Kulawik, J. PD-L1 expression on lung cancer stem cells in metastatic lymph nodes aspirates. Stem Cell Rev. 2019, 15, 324–330. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The eighth edition lung cancer stage classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Murthy, V.; Katzman, D.P.; Tsay, J.J.; Bessich, J.L.; Michaud, G.C.; Rafeq, S.; Minehart, J.; Mangalick, K.; de Lafaille, M.A.C.; Goparaju, C.; et al. Tumor-draining lymph nodes demonstrate a suppressive immunophenotype in patients with non-small cell lung cancer assessed by endobronchial ultrasound-guided transbronchial needle aspiration: A pilot study. Lung Cancer 2019, 137, 94–99. [Google Scholar] [CrossRef]
- van de Ven, R.; Niemeijer, A.N.; Stam, A.G.M.; Hashemi, S.M.; Slockers, C.G.; Daniels, J.M.; Thunnissen, E.; Smit, E.F.; de Gruijl, T.D.; de Langen, A.J. High PD-1 expression on regulatory and effector T-cells in lung cancer draining lymph nodes. ERJ Open Res. 2017, 3, 00110–02016. [Google Scholar] [CrossRef] [Green Version]
- Lian, S.; Xi, R.; Ye, Y.; Lu, Y.; Cheng, Y.; Xie, X.; Li, S.; Jia, L. Dual blockage of both PD-L1 and CD47 enhances immunotherapy against circulating tumor cells. Sci. Rep. 2019, 9, 4532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, Y.; Yoshimura, K.; Kurabe, N.; Kahyo, T.; Kawase, A.; Tanahashi, M.; Ogawa, H.; Inui, N.; Funai, K.; Shinmura, K.; et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 2017, 8, 8738–8751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, M.E.; Hadji, A.; Murmann, A.E.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Yang, L.; Li, H.; Li, R.; Yu, J.; Yang, L.; Wei, F.; Yan, C.; Sun, Q.; et al. Anti-CD47 Antibody As a Targeted Therapeutic Agent for Human Lung Cancer and Cancer Stem Cells. Front. Immunol. 2017, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Weiskopf, K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur. J. Cancer 2017, 76, 100–109. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Horrigan, S.K. Reproducibility Project: Cancer B. Replication Study: The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. eLife 2017, 6, e18173. [Google Scholar] [CrossRef]
- Wang, J.H.; Huang, S.T.; Zhang, L.; Liu, Z.G.; Liang, R.X.; Jiang, S.W.; Jiang, Y.N.; Yu, X.J.; Jiang, Y.C.; Li, X.Z.; et al. Combined prognostic value of the cancer stem cell markers CD47 and CD133 in esophageal squamous cell carcinoma. Cancer Med. 2019, 8, 1315–1325. [Google Scholar] [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Sek, K.; Mølck, C.; Stewart, G.D.; Kats, L.; Darcy, P.K.; Beavis, P.A. Targeting Adenosine Receptor Signaling in Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3837. [Google Scholar] [CrossRef] [Green Version]
- Lupia, M.; Angiolini, F.; Bertalot, G.; Freddi, S.; Sachsenmeier, K.F.; Chisci, E.; Kutryb-Zajac, B.; Confalonieri, S.; Smolenski, R.T.; Giovannoni, R.; et al. CD73 Regulates Stemness and Epithelial-Mesenchymal Transition in Ovarian Cancer-Initiating Cells. Stem Cell Rep. 2018, 10, 1412–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzarella, L.; Duso, B.A.; Trapani, D.; Belli, C.; D’Amico, P.; Ferraro, E.; Viale, G.; Curigliano, G. The evolving landscape of ‘next-generation’ immune checkpoint Inhibitor: A Review. Eur. J. Cancer 2019, 117, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, K.P.; Jiang, D.; Zhao, J.; Ge, J.F.; Zheng, S.Y. Relationship of Fas, FasL, p53 and bcl-2 expression in human non-small cell lung carcinomas. Int. J. Clin. Exp. Pathol. 2015, 8, 13978–13986. [Google Scholar]
- Zheng, H.; Li, W.; Wang, Y.; Xie, T.; Cai, Y.; Wang, Z.; Jiang, B. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis 2014, 35, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceppi, P.; Hadji, A.; Kohlhapp, F.J.; Pattanayak, A.; Hau, A.; Liu, X.; Liu, H.; Murmann, A.E.; Peter, M.E. CD95 and CD95L promote and protect cancer stem cells. Nat. Commun. 2014, 5, 5238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domagala-Kulawik, J.; Hoser, G.; Dabrowska, M.; Chazan, R. Increased proportion of Fas positive CD8+ cells in peripheral blood of patients with COPD. Respir. Med. 2007, 101, 1338–1343. [Google Scholar] [CrossRef] [Green Version]
- Hoser, G.; Wasilewska, D.; Domagala-Kulawik, J. Expression of Fas receptor on peripheral blood lymphocytes from patients with non-small cell lung cancer. Folia Histochem. Cytobiol. 2004, 42, 249–252. [Google Scholar]
- Prado-Garcia, H.; Romero-Garcia, S.; Aguilar-Cazares, D.; Meneses-Flores, M.; Lopez-Gonzalez, J.S. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin. Dev. Immunol. 2012, 2012, 741741. [Google Scholar] [CrossRef] [Green Version]
- Raniszewska, A.; Vroman, H.; Dumoulin, D.; Domagala-Kulawik, J.; Aerts, J.G.J.V. Immunomodulating properties of PD-L1 positive cancer stem cells in metastatic lymph nodes. Eur. Respir. J. 2019, 54, OA1910. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 863–870. [Google Scholar] [CrossRef]
- Bugalho, A.; Martins, C.; Dias, S.S.; Nunes, G.; Silva, Z.; Correia, M.; Gomes, M.J.M.; Videira, P.A. Cytokeratin 19, carcinoembryonic antigen, and epithelial cell adhesion molecule detect lung cancer lymph node metastasis in endobronchial ultrasound-guided transbronchial aspiration samples. Clin. Lung Cancer 2013, 14, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Gwóźdź, P.; Pasieka-Lis, M.; Kołodziej, K. Prognosis of patients with stages I and II non-small cell lung Cancer with nodal micrometastases. Ann. Thorac. Surg. 2018, 105, 1551–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raniszewska, A.; Kwiecień, I.; Sokołowski, R.; Rutkowska, E.; Domagała-Kulawik, J. Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates. Cancers 2020, 12, 838. https://doi.org/10.3390/cancers12040838
Raniszewska A, Kwiecień I, Sokołowski R, Rutkowska E, Domagała-Kulawik J. Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates. Cancers. 2020; 12(4):838. https://doi.org/10.3390/cancers12040838
Chicago/Turabian StyleRaniszewska, Agata, Iwona Kwiecień, Rafał Sokołowski, Elżbieta Rutkowska, and Joanna Domagała-Kulawik. 2020. "Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates" Cancers 12, no. 4: 838. https://doi.org/10.3390/cancers12040838





