Acute Promyelocytic Leukemia during Pregnancy: A Systematic Review of the Literature
Abstract
:1. Introduction
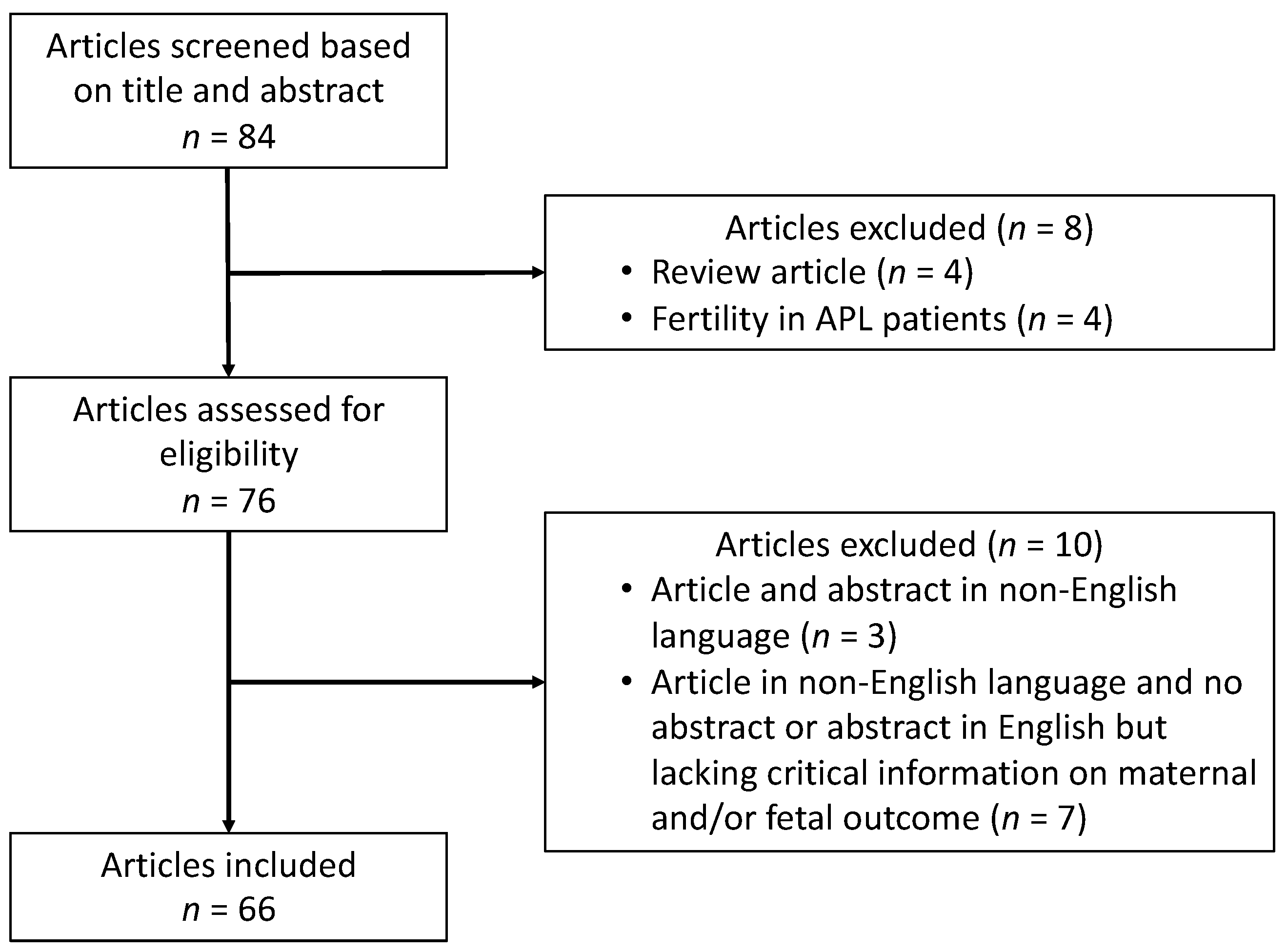
2. Methods
2.1. Search Strategy and Selection of Studies
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Maternal Outcome
3.2. Fetal Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.A.; Montesinos, P.; Casale, M.F.; Díaz-Mediavilla, J.; Jimenez, S.; Fernández, I.; Fernandez, P.; González-Campos, J.; González, J.D.; Herrera, P.; et al. Maternal and fetal outcomes in pregnant women with acute promyelocytic leukemia. Ann. Hematol. 2015, 94, 1357–1361. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, W65–W94. [Google Scholar] [CrossRef]
- Alegre, A.; Chunchurreta, R.; Rodriguez-Alarcon, J.; Cruz, E.; Prada, M. Successful pregnancy in acute promyelocytic leukemia. Cancer 1982, 49, 152–153. [Google Scholar] [CrossRef] [Green Version]
- Simone, M.D.; Stasi, R.; Venditti, A.; Del Poeta, G.; Aronica, G.; Bruno, A.; Masi, M.; Tribalto, M.; Papa, G.; Amadori, S. All-trans retinoic acid (ATRA) administration during pregnancy in relapsed acute promyelocytic leukemia. Leukemia 1995, 9, 1412–1413. [Google Scholar] [PubMed]
- Bhoopathi, B.; Ostapowicz, F.; Bazley, W. Acute promyelocytic leukemia in pregnancy. Obstet. Gynecol. 1973, 41, 275–278. [Google Scholar] [PubMed]
- Sharma, J.B.; Gupta, N.; Vimala, N.; Anand, M.; Deka, D.; Mittal, S. Acute promyelocytic leukemia: An unusual cause of fatal secondary postpartum hemorrhage. Arch. Gynecol. Obstet. 2006, 273, 310–311. [Google Scholar] [CrossRef]
- Naithani, R.; Dayal, N.; Chopra, A.; Sundar, J. Fetal Outcome in Pregnancy with Acute Promyelocytic Leukemia. Indian J. Pediatr. 2016, 83, 752–753. [Google Scholar] [CrossRef]
- Sham, R.L. All-trans retinoic acid-induced labor in a pregnant patient with acute promyelocytic leukemia. Am. J. Hematol. 1996, 53, 145. [Google Scholar] [CrossRef]
- Ewing, P.A.; Whittaker, J.A. Acute leukemia in pregnancy. Obstet. Gynecol. 1973, 42, 245–251. [Google Scholar]
- Bolis, P.F.; Franchi, M.; Salvaneschi, L. Acute leukemia in pregnancy. Clinical problems and description of 4 cases. Minerva Ginecol. 1982, 34, 347–353. [Google Scholar] [PubMed]
- Katagiri, S.; Tsubakio, T.; Minami, G.; Higashimoto, Y.; Yonezawa, T.; Tarui, S.; Hori, S.; Suehara, N.; Taniguchi, N.; Kitani, T. Successful embolization for uterine hemorrhage in a patient with acute promyelocytic leukemia. Acta Hematol. 1983, 70, 119–121. [Google Scholar] [CrossRef]
- Catanzarite, V.A.; Ferguson, J.E. Acute leukemia and pregnancy: A review of management and outcome, 1972–1982. Obstet. Gynecol. Surv. 1984, 39, 663–678. [Google Scholar]
- Fassas, A.; Kartalis, G.; Klearchou, N.; Tsatalas, K.; Sinacos, Z.; Mantalenakis, S. Chemotherapy for acute leukemia during pregnancy. Five case reports. Nouv. Rev. Fr. Hematol. 1984, 26, 19–24. [Google Scholar] [PubMed]
- Bartsch, H.H.; Meyer, D.; Teichmann, A.T.; Speer, C.P. Treatment of promyelocytic leukemia during pregnancy. A case report and review of the literature. Blut 1988, 57, 51–54. [Google Scholar] [CrossRef] [PubMed]
- D’Emilio, A.; Dragone, P.; De Negri, G.; Montaldi, A.; Stella, M.; Battista, R. Acute myelogenous leukemia in pregnancy. Hematologica 1989, 74, 601–604. [Google Scholar]
- Wallace, P.J. Complete remission in acute promyelocytic leukemia despite the persistence of the 15;17 translocation. Am. J. Hematol. 1989, 31, 266–268. [Google Scholar] [CrossRef]
- Celo, J.S.; Kim, H.C.; Houlihan, C.; Canavan, B.F.; Manzullo, G.P.; Saidi, P. Acute promyelocytic leukemia in pregnancy: All-trans retinoic acid as a newer therapeutic option. Obstet. Gynecol. 1994, 83, 808–811. [Google Scholar]
- Harrison, P.; Chipping, P.; Fothergill, G.A. Successful use of all-trans retinoic acid in acute promyelocytic leukemia presenting during the second trimester of pregnancy. Br. J. Hematol. 1994, 86, 681–682. [Google Scholar] [CrossRef]
- Tsuda, H.; Doi, H.; Inada, T.; Shirono, K. Successful treatment of acute promyelocytic leukemia in a pregnant woman by using all-trans retinoic acid. Rinsho Ketsueki 1994, 35, 717–719. [Google Scholar]
- Hoffman, M.A.; Wiernik, P.H.; Kleiner, G.J. Acute promyelocytic leukemia and pregnancy. A case report. Cancer 1995, 76, 2237–2241. [Google Scholar] [CrossRef]
- Nakamura, K.; Dan, K.; Iwakiri, R.; Gomi, S.; Nomura, T. Successful treatment of acute promyelocytic leukemia in pregnancy with all-trans retinoic acid. Ann. Hematol. 1995, 71, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Okamoto, S.; Moriki, T.; Kizaki, M.; Kawai, Y.; Ikeda, Y. Treatment of acute promyelocytic leukemia with all-trans retinoic acid during the third trimester of pregnancy. Am. J. Hematol. 1995, 48, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Huang, M.J.; Liu, H.J.; Chang, I.Y.; Tsai, C.H. Successful treatment of acute promyelocytic leukemia in a pregnant Jehovah’s Witness with all-trans retinoic acid, rhG-CSF and erythropoietin. Am. J. Hematol. 1996, 51, 251–252. [Google Scholar] [CrossRef]
- Lipovsky, M.M.; Biesma, D.H.; Christiaens, G.C.; Petersen, E.J. Successful treatment of acute promyelocytic leukemia with all-trans-retinoic-acid during late pregnancy. Br. J. Hematol. 1996, 94, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Incerpi, M.H.; Miller, D.A.; Posen, R.; Byrne, J.D. All-trans retinoic acid for the treatment of acute promyelocytic leukemia in pregnancy. Obstet. Gynecol. 1997, 89, 826–828. [Google Scholar] [CrossRef]
- Terada, Y.; Shindo, T.; Endoh, A.; Watanabe, M.; Fukaya, T.; Yajima, A. Fetal arrhythmia during treatment of pregnancy-associated acute promyelocytic leukemia with all-trans retinoic acid and favorable outcome. Leukemia 1997, 11, 454–455. [Google Scholar] [CrossRef] [Green Version]
- Maeda, M.; Tyugu, H.; Okubo, T.; Yamamoto, M.; Nakamura, K.; Dan, K. A neonate born to a mother with acute promyelocytic leukemia treated by all-trans retinoic acid. Rinsho Ketsueki 1997, 38, 770–775. [Google Scholar]
- García, L.; Valcárcel, M.; Santiago-Borrero, P.J. Chemotherapy during pregnancy and its effects on the fetus—Neonatal myelosuppression: Two case reports. J. Perinatol. 1999, 19, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Giagounidis, A.A.; Beckmann, M.W.; Giagounidis, A.S.; Aivado, M.; Emde, T.; Germing, U.; Riehs, T.; Heyll, A.; Aul, C. Acute promyelocytic leukemia and pregnancy. Eur. J. Hematol. 2000, 64, 267–271. [Google Scholar] [CrossRef]
- Leong, K.W.; Teh, A.; Bosco, J.J. Tretinoin in pregnancy complicated with acute promyelocytic leukemia. Med. J. Malays. 2000, 55, 277–279. [Google Scholar]
- Fadilah, S.A.; Hatta, A.Z.; Keng, C.S.; Jamil, M.A.; Singh, S. Successful treatment of acute promyelocytic leukemia in pregnancy with all-trans retinoic acid. Leukemia 2001, 15, 1665–1666. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Cimino, G.; Alimena, G.; De Carolis, S.; Lo-Coco, F.; Mandelli, F. AIDA treatment for high-risk acute promyelocytic leukemia in a pregnant woman at 21 weeks of gestation. Hematologica 2002, 87, ELT12. [Google Scholar]
- Carradice, D.; Austin, N.; Bayston, K.; Ganly, P.S. Successful treatment of acute promyelocytic leukemia during pregnancy. Clin. Lab. Hematol. 2002, 24, 307–311. [Google Scholar] [CrossRef]
- Lorenzo Marcos, E.; Fernández Corona, A.; De las Heras Rodríquez, N.; Fernández Ferrero, S.; Sandoval Guerra, V.; Hernández Rodríguez, J.L. Acute promyelocytic leukemia during pregnancy. Toko-Ginecol. Pract. 2002, 61, 427–430. [Google Scholar]
- Siu, B.L.; Alonzo, M.R.; Vargo, T.A.; Fenrich, A.L. Transient dilated cardiomyopathy in a newborn exposed to idarubicin and all-trans-retinoic acid (ATRA) early in the second trimester of pregnancy. Int. J. Gynecol. Cancer 2002, 12, 399–402. [Google Scholar] [CrossRef]
- Itoh, M.; Takao, S.; Yago, K.; Shimada, H. Successful treatment of acute promyelocytic leukemia in a pregnant patient with all-trans retinoic acid and chemotherapy resulting in a safe delivery. Rinsho Ketsueki 2003, 44, 401–403. [Google Scholar]
- Lee, D.D.; Park, T.S.; Lee, D.S.; Lee, E.Y. Acute promyelocytic leukemia in late pregnancy with unusual secondary chromosomal change and its prognostic importance. Cancer Genet. Cytogenet. 2005, 157, 92–93. [Google Scholar] [CrossRef]
- Dilek, I.; Topcu, N.; Demir, C.; Bay, A.; Uzun, K.; Gul, A.; Faik Oner, A.; Ugras, S. Hematological malignancy and pregnancy: A single-institution experience of 21 cases. Clin. Lab. Hematol. 2006, 28, 170–176. [Google Scholar] [CrossRef]
- Valappil, S.; Kurkar, M.; Howell, R. Outcome of pregnancy in women treated with all-trans retinoic acid; a case report and review of literature. Hematology 2007, 12, 415–418. [Google Scholar] [CrossRef]
- Nakashima, H.; Norimichi, H.; Saito, B.; Yanagisawa, K.; Nakamaki, T.; Tomoyasu, S. Acute promyelocytic leukemia in the first trimester of pregnancy. J. Showa Med. Assoc. 2007, 67, 92–97. [Google Scholar]
- Park, T.S.; Lee, S.T.; Kim, J.S.; Song, J.; Lee, K.-A.; Kim, S.J.; Seok, Y.-M.; Lee, H.-J.; Han, J.-H.; Kim, J.-K.; et al. Battista, R. Acute promyelocytic leukemia in early pregnancy with translocation t(15;17) and variant PML/RARA fusion transcripts. Cancer Genet. Cytogenet. 2009, 188, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Ganzitti, L.; Fachechi, G.; Driul, L.; Marchesoni, D. Acute promyelocytic leukemia during pregnancy. Fertil. Steril. 2010, 94, 2330.e5–2330.e6. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Cho, E.H.; Cho, S.Y.; Shin, S.Y.; Park, J.C.; Yang, Y.J.; Oh, S.H.; Marschalek, R.; Meyer, C.; Park, T.S. A novel PML-ADAMTS17-RARA gene rearrangement in a patient with pregnancy-related acute promyelocytic leukemia. Leuk. Res. 2011, 35, e106–e110. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Yoneda, N.; Yoneda, S.; Miyazono, T.; Sugiyama, T.; Saito, S. Massive postpartum hemorrhage after chemotherapy in a patient with acute promyelocytic leukemia. J. Obstet. Gynaecol. Res. 2011, 37, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- López Sánchez, J.M.; Fernández Hinojosa, E.; Contreras Virves, M.; Bautista Lorite, A. Acute promyelocytic leukemia in pregnancy. Prog. Obstet. Ginecol. 2011, 54, 428–430. [Google Scholar] [CrossRef]
- Oehler, A.; Shah, S. Myopericarditis in a pregnant woman with acute promyelocytic leukemia. J. Cardiol. Cases 2014, 10, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Li, M. Pregnancy-induced hypertension caused by all-trans retinoic acid treatment in acute promyelocytic leukemia. Oncol. Lett. 2015, 10, 364–366. [Google Scholar] [CrossRef] [Green Version]
- Biscoe, A.; Kidson-Gerber, G. ‘Avoidable’ death of a pregnant Jehovah’s Witness with acute promyelocytic leukemia: Ethical considerations and the internal conflicts and challenges encountered by practitioners. Intern. Med. J. 2015, 45, 461–462. [Google Scholar] [CrossRef]
- Agarwal, K.; Patel, M.; Agarwal, V. A Complicated Case of Acute Promyelocytic Leukemia in the Second Trimester of Pregnancy Successfully Treated with All-trans-Retinoic Acid. Case Rep. Hematol. 2015, 2015, 634252. [Google Scholar] [CrossRef]
- Maruyama, S.; Sato, Y.; Moriuchi, K.; Kanbayashi, S.; Ri, Y.; Taga, A.; Emoto, I.; Kim, T. Fetal death following idarubicin treatment for acute promyelocytic leukemia in pregnancy-A case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 140. [Google Scholar] [CrossRef] [PubMed]
- Nellessen, C.M.; Janzen, V.; Mayer, K.; Giovannini, G.; Gembruch, U.; Brossart, P.; Merz, W.M. Successful treatment of acute promyelocytic leukemia in pregnancy with single-agent all-trans retinoic acid. Arch. Gynecol. Obstet. 2018, 297, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, E.N.; Miklin, D.N.; Kornyaeva, E.P. Successful treatment of newly diagnosed acute promyelocytic leukemia in a pregnant woman. Gematol. Transfusiologiya 2017, 62, 105–108. [Google Scholar]
- Šajn, M.; Zver, S.; Lučovnik, M. Cases of leukemia in pregnancy in Slovenia during the period from 2006 to 2016—How they were treated and literature review. Zdr. Vestn. 2018, 87, 429–438. [Google Scholar]
- Zhang, L.; Tomsula, J.; Garcia, A.; Wahed, A.; Nguyen, N.; Chen, L. Fatal Intracranial Hemorrhage in a Young Pregnant Patient with Acute Promyelocytic Leukemia. Ann. Clin. Lab. Sci. 2019, 49, 94–96. [Google Scholar] [PubMed]
- Gstöttner, M.; Frisch, H.; Dienstl, F. Delivery of a normal child after chemotherapy of acute promyelocytic leukemia during pregnancy (author’s transl). Blut 1978, 36, 171–174. [Google Scholar] [CrossRef]
- Fei, F.; Faye-Petersen, O.M.; Vachhani, P.; Jamy, O.; Reddy, V.V. Acute promyelocytic leukemia during pregnancy: A case report and 10-year institutional review of hematologic malignancies during pregnancy. Pathol. Res. Pract. 2019, 215, 152672. [Google Scholar] [CrossRef]
- Takatsuki, H.; Abe, Y.; Goto, T.; Sadamura, S.; Taguchi, F.; Muta, K.; Miyoshi, T.; Katsuno, M.; Umemura, T.; Nishimura, J. Two cases of acute promyelocytic leukemia in pregnancy and the effect of anthracyclines on fetal development. Rinsho Ketsueki 1992, 33, 1736–1740. [Google Scholar]
- Stentoft, J.; Nielsen, J.L.; Hvidman, L.E. All-trans retinoic acid in acute promyelocytic leukemia in late pregnancy. Leukemia 1994, 8, 1585–1588. [Google Scholar]
- Requena, A.; Velasco, J.G.; Pinilla, J.; Gonzalez-Gonzalez, A. Acute leukemia during pregnancy: Obstetric management and perinatal outcome of two cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 63, 139–141. [Google Scholar] [CrossRef]
- Delgado-Lamas, J.L.; Garcés-Ruiz, O.M. Malignancy: Case Report: Acute Promyelocytic Leukemia in Late Pregnancy. Successful Treatment with All-Trans-Retinoic Acid (ATRA) and Chemotherapy. Hematology 2000, 4, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Ozkalemkaş, F.; Ozçelik, T.; Ozkocaman, V.; Ozan, U.; Kimya, Y.; Tunali, A. Maternal and fetal outcomes in pregnancy complicated with acute leukemia: A single institutional experience with 10 pregnancies at 16 years. Leuk. Res. 2003, 27, 381–385. [Google Scholar] [CrossRef]
- Li, H.; Han, C.; Li, K.; Li, J.; Wang, Y.; Xue, F. New onset acute promyelocytic Leukemia during pregnancy: Report of 2 cases. Cancer Biol. Ther. 2019, 20, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Hattori, Y.; Ito, S.; Ohshima, R.; Kuwabara, H.; Machida, S.; Shirasugi, Y.; Miyazaki, K.; Sakai, R.; Tomita, N.; et al. Acute leukemia during pregnancy: An investigative survey of the past 11 years. Int. J. Lab. Hematol. 2015, 37, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Greenlund, L.J.; Letendre, L.; Tefferi, A. Acute leukemia during pregnancy: A single institutional experience with 17 cases. Leuk. Lymphoma 2001, 41, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Consoli, U.; Figuera, A.; Milone, G.; Meli, C.R.; Guido, G.; Indelicato, F.; Moschetti, G.; Leotta, S.; Tornello, A.; Poidomani, M.; et al. Acute promyelocytic leukemia during pregnancy: Report of 3 cases. Int. J. Hematol. 2004, 79, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Takitani, K.; Hino, N.; Terada, Y.; Kurosawa, Y.; Koh, M.; Inoue, A.; Kawakami, C.; Kuno, T.; Tamai, H. Plasma all-trans retinoic acid level in neonates of mothers with acute promyelocytic leukemia. Acta Hematol. 2005, 114, 167–169. [Google Scholar] [CrossRef]
- de van Chelghoum, Y.; Vey, N.; Raffoux, E.; Huguet, F.; Pigneux, A.; Witz, B.; Pautas, C.; de Botton, S.; Guyotat, D.; Lioure, B.; et al. Acute leukemia during pregnancy: A report on 37 patients and a review of the literature. Cancer 2005, 104, 110–117. [Google Scholar] [CrossRef]
- Verma, V.; Giri, S.; Manandhar, S.; Pathak, R.; Bhatt, V.R. Acute promyelocytic leukemia during pregnancy: A systematic analysis of outcome. Leuk. Lymphoma 2016, 57, 616–622. [Google Scholar] [CrossRef]
- Kondo, H.; Hoshida, M.; Kawamura, A. Renal cortical necrosis in a pregnant patient with acute promyelocytic leukemia. Rinsho Ketsueki 1972, 13, 844–849. [Google Scholar]
- Beller, F.K.; Wagner, H.; Buchner, T. Peripheral microthrombotic purpura associated with acute promyelocytic leukemia in pregnancy. A light and electron microscopic study. Klin. Wochenschr. 1978, 56, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Coser, P.; Prinoth, O.; Fabris, P.; Colombetti, V.; Mengarda, G.; Delucca, A. Successful pregnancy in acute promyelocytic leukemia (author’s transl). Hematologica 1979, 64, 356–361. [Google Scholar]
- Tono, A.; Shimokawa, H.; Uchino, H.; Miyamoto, S.; Kadomatsu, K.; Nakano, H.; Sato, S.; Noma, M.; Yamamoto, Y.; Niho, Y. Pregnancy complicated with acute promyelocytic leukemia case report. Nippon Sanka Fujinka Gakkai Zasshi 1986, 38, 131–134. [Google Scholar] [PubMed]
- Aragona, M.; Asmundo, A. 2 fatal cases of acute myeloid leukemia (M3, M4) during pregnancy. Pathologica 1995, 87, 125–134. [Google Scholar]
- Ebert, U.; Löffler, H.; Kirch, W. Cytotoxic therapy and pregnancy. Pharmacol. Ther. 1997, 74, 207–220. [Google Scholar] [CrossRef]
- Simionescu, A.A.; Berbec, N. Misinterpretation of hematological changes in pregnancy and postpartum hemorrhage due to leukemia and pregnancy: A case report. J. Reprod. Med. 2017, 62, 463–465. [Google Scholar]
- Yang, R.; Qian, S.X.; Chen, C. Treatment of acute promyelocytic leukemia during pregnancy. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 439–442. [Google Scholar] [CrossRef]
- Kardaszewicz, E.; Bujak, M.; Spychałowicz, W.; Siudyka, A.; Harbut-Gryłka, A. 2 cases of acute disseminated intravascular coagulation in normal pregnancy and as the first symptom of acute promyelocytic leukemia. Pol. Tyg. Lek. 1990, 45, 257–259. [Google Scholar]
- Troitskaia, V.V.; Parovichnikova, E.N.; Sokolov, A.N.; Kokhno, A.V.; Makhinia, S.A.; Galstian, G.M.; Konstantinova, T.S.; Mazurok, L.A.; Goriachok, I.G.; Korobkin, A.V.; et al. Treatment for acute promyelocytic leukemia during pregnancy. Ter. Arkh. 2013, 85, 56–63. [Google Scholar]
- Culligan, D.J.; Merriman, L.; Kell, J.; Parker, J.; Jovanovic, J.V.; Smith, N.; Grimwade, D. The Management of Acute Promyelocytic Leukemia Presenting During Pregnancy. Clin. Leuk. 2007, 1, 183–191. [Google Scholar] [CrossRef]
- Sanz, M.A.; Grimwade, D.; Tallman, M.S.; Löwenberg, B.; Fenaux, P.; Estey, E.H.; Naoe, T.; Lengfelder, E.; Büchner, T.; Döhner, H.; et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009, 113, 1875–1891. [Google Scholar] [CrossRef] [PubMed]
| No. of Patients Reported by Article | No. of Articles | No. of Patients | References |
|---|---|---|---|
| One | 53 | 53 | [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
| Two | 7 | 14 | [57,58,59,60,61,62,63] |
| Three | 4 | 11 * | [64,65,66,67] |
| Four | 1 | 4 | [68] |
| More than four | 1 | 14 | [2] |
| Characteristic | Median (Range) | No. (%) | |
|---|---|---|---|
| Overall | 96 (100) | ||
| Age, year | 30 (16–41) | ||
| 16–20 | 8 (8) | ||
| 21–30 | 44 (46) | ||
| 31–40 | 41 (43) | ||
| 41 | 3 (3) | ||
| Gestational age at diagnosis (n = 95) | 25 (1–42) | ||
| First trimester | 16 (17) | ||
| Second trimester | 46 (48) | ||
| Third trimester | 29 (31) | ||
| After delivery | 4 (4) | ||
| WBC count, ×109/L (n = 57) | 1.8 (0.4–295) | ||
| Less than 5 | 44 (77) | ||
| 5–10 | 5 (9) | ||
| 10–50 | 4 (7) | ||
| 50 or higher | 4 (7) | ||
| Platelet count, ×109/L (n = 57) | 22 (1.5–131) | ||
| Less than 40 | 48 (84) | ||
| 40 or higher | 9 (16) | ||
| Hemoglobin, g/dL (n = 40) | 8.3 (3.2–12) | ||
| Less than 10 | 36 (90) | ||
| 10 or higher | 4 (10) | ||
| Risk score (n = 56) | |||
| Low | 9 (16) | ||
| Intermediate | 40 (71) | ||
| High | 7 (12) | ||
| Coagulopathy (n = 53) | |||
| No | 10 (19) | ||
| Yes | 43 (81) | ||
| Genetic diagnosis (n = 90) | |||
| No | 22 (24) | ||
| Yes | 68 (76) | ||
| Induction Therapy. | No. Patients (%) | CR/No. patients (%) | ||||
|---|---|---|---|---|---|---|
| First Trimester | Second Trimester | Third Trimester | Total * | |||
| Total | 92 (100) | 16/16 (100) | 37/44 (84) | 25/28 (89) | 78/88 (89) | |
| Chemotherapy alone | 20 (22) | 5/5 (100) | 8/9 (89) | 3/4 + (75) | 16/18 (89) | |
| ATRA alone | 29 (32) | 2/2 (100) | 10/14 (71) | 10/11 (91) | 22/27 (81) | |
| ATRA + Ida/Dauno | 31 (34) | 6/6 (100) | 13/14 (93) | 11/11 (100) | 30/31 (97) | |
| ATRA + Chemotherapy | 9 (10) | 3/3 (100) | 5/6 (83) | - | 8/9 (89) | |
| ATO ± ATRA ± Chemotherapy | 3 (3) | - | 1/1 (100) | 1/2 (50) | 2/3 (67) | |
| Pregnancy Outcome | Overall | First Trimester | Second Trimester | Third Trimester | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients (%) | Gestational Age at Diagnosis, wks | Gestational Age at Delivery/Abortion, wks | No. of Patients (%) | Gestational Age at Diagnosis, wks | Gestational Age at Delivery/Abortion, wks | No. of Patients (%) | Gestational Age at Diagnosis, wks | Gestational Age at Delivery/Abortion, wks | |||
| Delivery | 65 (68) | 2 (12) | 32 (70) | 31 (94) | |||||||
| Cesarean | 37 (58) | 1 (50) | 4 | 32 | 19 (59) | 24 (13–28) | 31 (25–40) | 17 (55) | 33 (29–38) | 33 (32–39) | |
| Vaginal | 26 (39) | 1 (50) | 8 | 39 | 12 (38) | 23 (13–28) | 32 (26–37) | 13 (42) | 38 (29–42) | 38 (29–42) | |
| Unknown | 2 (3) | 1 (3) | 25 | 28 | 1 (3) | 29 | 32 | ||||
| Abortion | 31 (32) | 14 (88) | 14 (30) | 2 (6) | |||||||
| Spontaneous * | 8 (26) | 5 (36) | 7 (4–9) | 7 (6–12) | 2 (14) | 14, 19 | 19, 19 | ||||
| Therapeutic | 12 (38) | 9 (64) | 9 (3–11) | 9 (5–18) | 3 (21) | 13 (13–14) | 15 (13–17) | ||||
| Late stillbirth | 8 (26) | 7 (50) | 26 (23–28) | 26 (25–30) | 1 (50) | 29 | 29 | ||||
| Maternal death during pregnancy | 3 (10) | 2 (14) | 25, 28 | 25, 28 | 1 (50) | 29 | 29 | ||||
| Trimester of Pregnancy | Weight at Birth, g | Apgar Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preterm | At Term | 1 min | 5 min | |||||||||
| n | Median | Range | n | Median | Range | n | Median | Range | n | Median | Range | |
| First trimester | 1 | 1820 | 1 | 3050 | 0 | - | - | |||||
| Second trimester | 23 | 1975 | 857–2950 | 0 | - | 12 | 6 | 2–9 | 12 | 7 | 4–10 | |
| Third trimester | 9 | 2045 | 1634–3200 | 8 | 3124 | 2450–4000 | 11 | 8 | 6–10 | 10 | 10 | 7–10 |
| Overall | 33 | 2200 | 857–3200 | 9 | 3124 | 2450–4000 | 23 | 6 | 2–10 | 22 | 9 | 4–10 |
| Induction therapy | No. Patients Who Achieved CR | Miscarriage/Stillbirth * | |||
|---|---|---|---|---|---|
| First Trimester | Second Trimester | Third Trimester | Total | ||
| Total | 78 | 5/0 | 0/4 | 0/2 | 5/6 |
| Chemotherapy alone | 16 | 1/0 | - | 0/1 | 1/1 |
| ATRA alone | 22 | - | 0/1 | 0/1 | 0/2 |
| ATRA + Ida/Dauno | 30 | 2/0 | 0/2 | - | 2/2 |
| ATRA + Chemotherapy | 8 | 2/0 | - | - | 2/0 |
| ATO ± ATRA ± Chemotherapy | 2 | - | 0/1 + | - | 0/1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santolaria, A.; Perales, A.; Montesinos, P.; Sanz, M.A. Acute Promyelocytic Leukemia during Pregnancy: A Systematic Review of the Literature. Cancers 2020, 12, 968. https://doi.org/10.3390/cancers12040968
Santolaria A, Perales A, Montesinos P, Sanz MA. Acute Promyelocytic Leukemia during Pregnancy: A Systematic Review of the Literature. Cancers. 2020; 12(4):968. https://doi.org/10.3390/cancers12040968
Chicago/Turabian StyleSantolaria, Andrea, Alfredo Perales, Pau Montesinos, and Miguel A. Sanz. 2020. "Acute Promyelocytic Leukemia during Pregnancy: A Systematic Review of the Literature" Cancers 12, no. 4: 968. https://doi.org/10.3390/cancers12040968
APA StyleSantolaria, A., Perales, A., Montesinos, P., & Sanz, M. A. (2020). Acute Promyelocytic Leukemia during Pregnancy: A Systematic Review of the Literature. Cancers, 12(4), 968. https://doi.org/10.3390/cancers12040968






