Ex Vivo Assessment of Tumor-Targeting Fluorescent Tracers for Image-Guided Surgery
Abstract
:1. Introduction
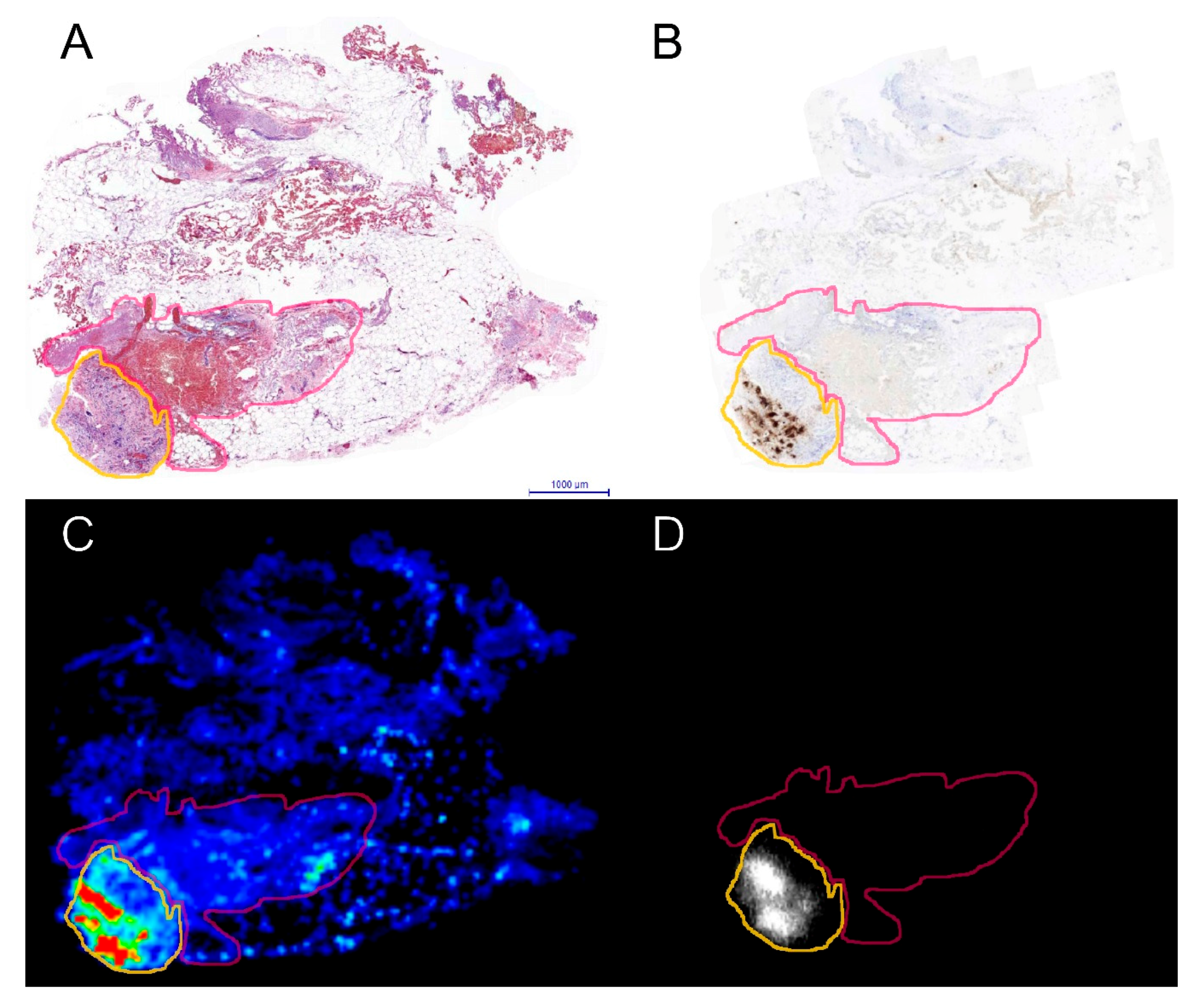
2. Results
3. Discussion
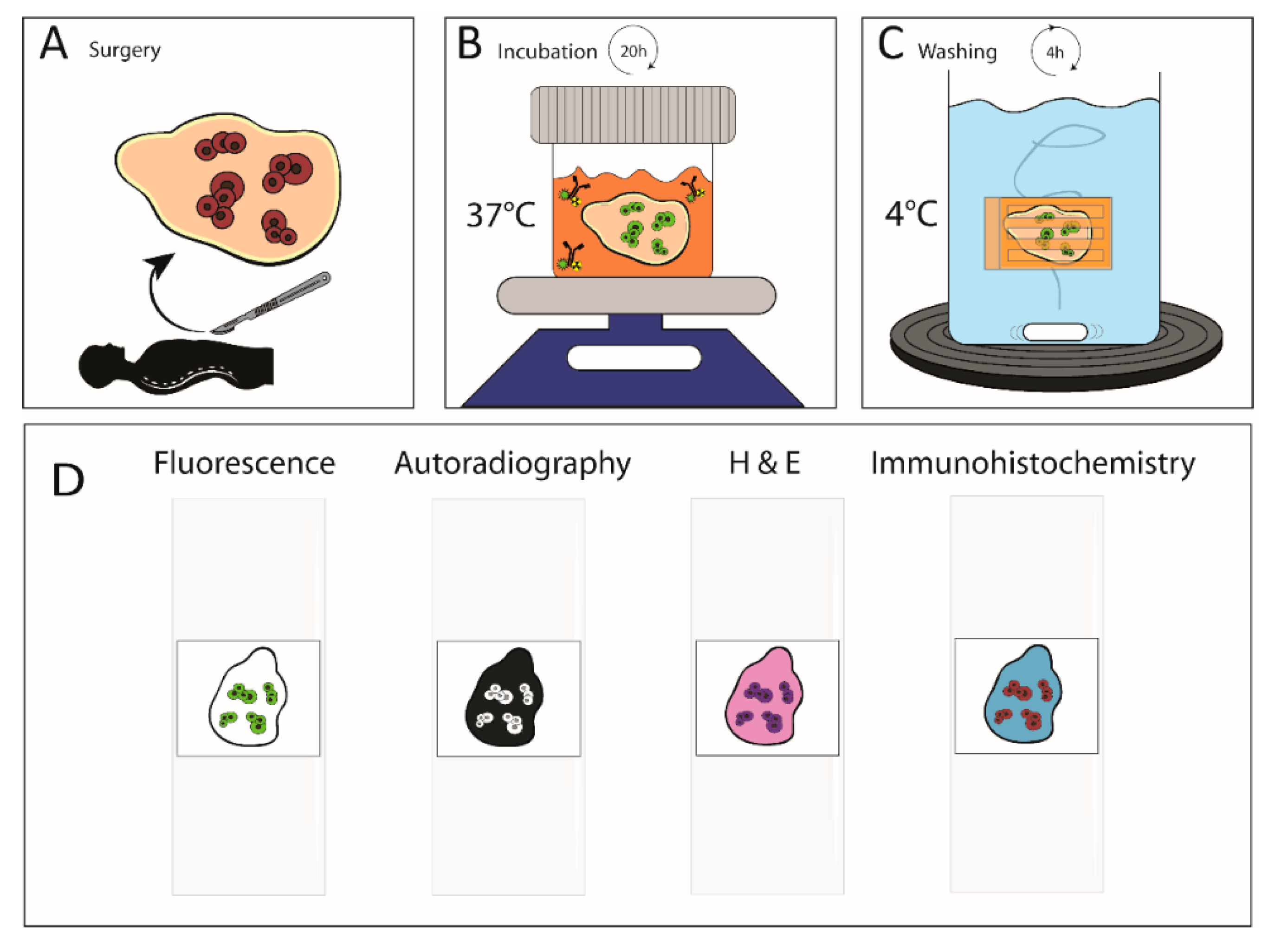
4. Materials and Methods
4.1. Ethical Approval
4.2. Patients and Patient Tissue Samples
4.3. Antibody Preparation
4.4. Radiolabeling of the Antibody Conjugates
4.5. In Vitro Antibody Testing
4.6. Patient Tissue Incubation and Handling
4.7. Immunohistochemistry
4.8. Image Analyses
4.9. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amri, R.; Bordeianou, L.G.; Sylla, P.; Berger, D. Association of Radial Margin Positivity with Colon Cancer. JAMA Surg. 2015, 150, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Ali, S.M.; Darzi, A.; Brown, G.; Tekkis, P. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis. 2012, 14, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Azizi, L.; Macaskill, P.; Irwig, L.; Morrow, M.; Solin, L.J.; Houssami, N. The Association of Surgical Margins and Local Recurrence in Women with Ductal Carcinoma In Situ Treated with Breast-Conserving Therapy: A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3811–3821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osarogiagbon, R.U.; Ray, M.A.; Faris, N.R.; Smeltzer, M.P.; Fehnel, C.; Houston-Harris, C.; Signore, R.S.; McHugh, L.M.; Levy, P.; Wiggins, L.; et al. Prognostic Value of National Comprehensive Cancer Network Lung Cancer Resection Quality Criteria. Ann. Thorac. Surg. 2017, 103, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, T.J.; Krug, J.E.; Yu, J.; Zyromski, N.J.; Schmidt, C.M.; Jacobson, L.E.; Madura, J.A.; Wiebke, E.A.; Lillemoe, K.D. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J. Gastrointest Surg. 2006, 10, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.; Laframboise, S.; Ferguson, S.; Dodge, J.; Bernardini, M.; Murphy, J.; Segev, Y.; Sun, P.; Narod, S.A. The impacts of neoadjuvant chemotherapy and of debulking surgery on survival from advanced ovarian cancer. Gynecol. Oncol. 2014, 134, 462–467. [Google Scholar] [CrossRef]
- Silberstein, J.L.; Eastham, J.A. Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J. Urol. 2014, 30, 423–428. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: A single-centre feasibility study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Denayer, T.; Stöhr, T.; Roy, M.V. Animal models in translational medicine: Validation and prediction. Eur. J. Mol. Clin. Med. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Hammarstrom, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Tiernan, J.P.; Perry, S.L.; Verghese, E.T.; West, N.P.; Yeluri, S.; Jayne, D.G.; Hughes, T.A. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekman, M.; Rijpkema, M.; Bos, D.L.; Oosterwijk, E.; Goldenberg, D.M.; Mulders, P.; Boerman, O.C. Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model. J. Nucl. Med. 2017, 58, 706–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijpkema, M.; Oyen, W.J.; Bos, D.; Franssen, G.M.; Goldenberg, D.M.; Boerman, O.C. SPECT- and fluorescence image-guided surgery using a dual-labeled carcinoembryonic antigen-targeting antibody. J. Nucl. Med. 2014, 55, 1519–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Ehlerding, E.B.; Jiang, D.; Barnhart, T.E.; Cao, T.; Wei, W.; Ferreira, C.A.; Huang, P.; Engle, J.W.; Cai, W. Dual-labeled pertuzumab for multimodality image-guided ovarian tumor resection. Am. J. Cancer Res. 2019, 9, 1454–1468. [Google Scholar] [PubMed]
- Baranski, A.C.; Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendörfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11-Derived Dual-Labeled PSMA Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekman, M.; Boerman, O.C.; Bos, D.L.; Massuger, L.; Weil, S.; Grasso, L.; Rybinski, K.A.; Oosterwijk, E.; Mulders, P.; Rijpkema, M. Improved Intraoperative Detection of Ovarian Cancer by Folate Receptor Alpha Targeted Dual-Modality Imaging. Mol. Pharm. 2017, 14, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Hekman, M.C.; Rijpkema, M.; Muselaers, C.H.; Oosterwijk, E.; Hulsbergen-Van de Kaa, C.A.; Boerman, O.C.; Oyen, W.J.; Langenhuijsen, J.F.; Mulders, P.F. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics 2018, 8, 2161–2170. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using (68)Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- Moore, L.S.; de Boer, E.; Warram, J.M.; Tucker, M.D.; Carroll, W.R.; Korb, M.L.; Brandwein-Gensler, M.S.; van Dam, G.M.; Rosenthal, E.L. Photoimmunotherapy of residual disease after incomplete surgical resection in head and neck cancer models. Cancer Med. 2016, 5, 1526–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, N.R.; Knowles, J.A.; Helman, E.E.; Aldridge, J.C.; Carroll, W.R.; Magnuson, J.S.; Clemons, L.; Ziober, B.; Rosenthal, E.L. Anti-EMMPRIN antibody treatment of head and neck squamous cell carcinoma in an ex-vivo model. Anticancer Drugs 2010, 21, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M.; Qiu, S.Q.; Linssen, M.D.; Jansen, L.; Kelder, W.; de Vries, J.; Kruithof, I.; Zhang, G.J.; Robinson, D.J.; Nagengast, W.B.; et al. Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nat. Commun. 2018, 9, 3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.; Glatz, J.; Kranendonk, M.; Terwisscha van Scheltinga, A.; Jansen, L.; de Vries, J.; Lub-de Hooge, M.N.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [Green Version]
- Boogerd, L.; Hoogstins, C.; Schaap, D.P.; Kusters, M.; Handgraaf, H.; van der Valk, M.; Hilling, D.E.; Holman, F.A.; Peeters, K.; Mieog, J.; et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: A dose-escalation pilot study. Lancet Gastroenterol. Hepatol. 2018, 3, 181–191. [Google Scholar] [CrossRef]
- van Keulen, S.; Nishio, N.; Fakurnejad, S.; van den Berg, N.S.; Lu, G.; Birkeland, A.; Martin, B.A.; Forouzanfar, T.; Colevas, A.D.; Rosenthal, E.L. Intraoperative Tumor Assessment Using Real-Time Molecular Imaging in Head and Neck Cancer Patients. J. Am. Coll. Surg. 2019, 229, 560–567. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Hirakawa, E.; Mori, S.; Hamada, Y.; Kawaguchi, N.; Matsuura, N. Cleavage of carcinoembryonic antigen induces metastatic potential in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2005, 333, 223–229. [Google Scholar] [CrossRef]
- Behr, T.M.; Sharkey, R.M.; Juweid, M.I.; Dunn, R.M.; Ying, Z.; Zhang, C.H.; Siegel, J.A.; Gold, D.V.; Goldenberg, D.M. Factors influencing the pharmacokinetics, dosimetry, and diagnostic accuracy of radioimmunodetection and radioimmunotherapy of carcinoembryonic antigen-expressing tumors. Cancer Res. 1996, 56, 1805–1816. [Google Scholar]
- Soliman, B.G.; Karagkounis, G.; Church, J.M.; Plesec, T.; Kalady, M.F. Mucinous Histology Signifies Poor Oncologic Outcome in Young Patients With Colorectal Cancer. Dis. Colon Rectum. 2018, 61, 547–553. [Google Scholar] [CrossRef]
- Hekman, M.C.; Boerman, O.C.; de Weijert, M.; Bos, D.L.; Oosterwijk, E.; Langenhuijsen, J.F.; Mulders, P.F.; Rijpkema, M. Targeted Dual-Modality Imaging in Renal Cell Carcinoma: An Ex Vivo Kidney Perfusion Study. Clin. Cancer Res. 2016, 22, 4634–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, R.M.; Juweid, M.; Shevitz, J.; Behr, T.; Dunn, R.; Swayne, L.C.; Wong, G.Y.; Blumenthal, R.D.; Griffiths, G.L.; Siegel, J.A. Evaluation of a complementarity-determining region-grafted (humanized) anti-carcinoembryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res. 1995, 55, 5935–5945s. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elekonawo, F.M.K.; de Gooyer, J.M.; Bos, D.L.; Goldenberg, D.M.; Boerman, O.C.; Brosens, L.A.A.; Bremers, A.J.A.; de Wilt, J.H.W.; Rijpkema, M. Ex Vivo Assessment of Tumor-Targeting Fluorescent Tracers for Image-Guided Surgery. Cancers 2020, 12, 987. https://doi.org/10.3390/cancers12040987
Elekonawo FMK, de Gooyer JM, Bos DL, Goldenberg DM, Boerman OC, Brosens LAA, Bremers AJA, de Wilt JHW, Rijpkema M. Ex Vivo Assessment of Tumor-Targeting Fluorescent Tracers for Image-Guided Surgery. Cancers. 2020; 12(4):987. https://doi.org/10.3390/cancers12040987
Chicago/Turabian StyleElekonawo, Fortuné M.K., Jan Marie de Gooyer, Desirée L. Bos, David M. Goldenberg, Otto C. Boerman, Lodewijk A.A. Brosens, Andreas J.A. Bremers, Johannes H.W. de Wilt, and Mark Rijpkema. 2020. "Ex Vivo Assessment of Tumor-Targeting Fluorescent Tracers for Image-Guided Surgery" Cancers 12, no. 4: 987. https://doi.org/10.3390/cancers12040987
APA StyleElekonawo, F. M. K., de Gooyer, J. M., Bos, D. L., Goldenberg, D. M., Boerman, O. C., Brosens, L. A. A., Bremers, A. J. A., de Wilt, J. H. W., & Rijpkema, M. (2020). Ex Vivo Assessment of Tumor-Targeting Fluorescent Tracers for Image-Guided Surgery. Cancers, 12(4), 987. https://doi.org/10.3390/cancers12040987




