Durable Complete Remission and Long-Term Survival in FDG-PET Staged Patients with Stage III Follicular Lymphoma, Treated with Wide-Field Radiation Therapy
Abstract
:1. Introduction
2. Results
2.1. Radiation Therapy Delivery and Acute Toxicity
2.2. PET Response Assessment
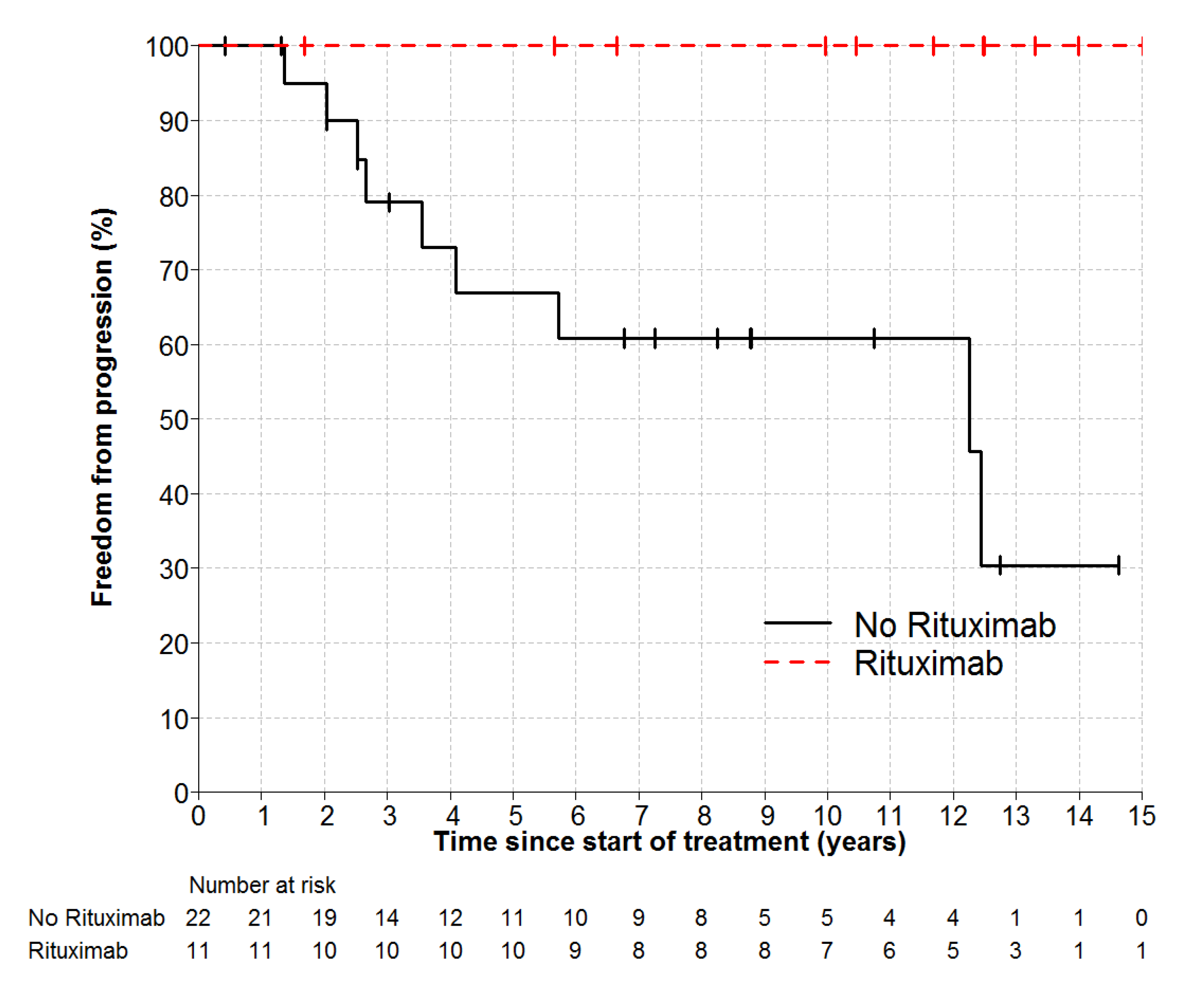
2.3. Overall Survival and Freedom from Progression
2.4. Management after Progression
2.5. Subacute and Late Toxicities of Radiation Therapy
2.6. Second Malignancies
3. Discussion
4. Materials and Methods
4.1. Inclusion Criteria
4.2. Exclusion Criteria
4.3. PET Imaging
4.4. Radiotherapy
4.5. Supradiapragmatic RT
4.6. Infradiaphragmatic RT
4.7. Statistical Considerations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haas, R.L.; Poortmans, P.; de Jong, D.; Aleman, B.M.; Dewit, L.G.; Verheij, M.; Hart, A.A.; van Oers, M.H.; van der Hulst, M.; Baars, J.W.; et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J. Clin. Oncol. 2003, 21, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A. Follicular lymphoma: 2018 update on diagnosis and management. Am. J. Hematol. 2018, 93, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, H.; Schmelter, C.; Leich, E.; Salaverria, I.; Katzenberger, T.; Ott, M.M.; Kalla, J.; Romero, M.; Siebert, R.; Rosenwald, A.; et al. Follicular lymphoma grade 3B is a distinct neoplasm according to cytogenetic and immunohistochemical profiles. Haematologica 2011, 96, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Mac Manus, M.P.; Hoppe, R.T. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J. Clin. Oncol. 1996, 14, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.A.; Voss, N.; Woods, R.; Gascoyne, R.D.; Morris, J.; Pickles, T.; Connors, J.M.; Savage, K.J. Long-term outcomes for patients with limited stage follicular lymphoma: Involved regional radiotherapy versus involved node radiotherapy. Cancer 2010, 116, 3797–3806. [Google Scholar] [CrossRef]
- Heinzelmann, F.; Engelhard, M.; Ottinger, H.; Bamberg, M.; Weinmann, M. Nodal follicular lymphoma: The role of radiotherapy for stages I and II. Strahlenther. Onkol. 2010, 186, 191–196. [Google Scholar] [CrossRef]
- MacManus, M.; Fisher, R.; Roos, D.; O’Brien, P.; Macann, A.; Davis, S.; Tsang, R.; Christie, D.; McClure, B.; Joseph, D.; et al. Randomized Trial of Systemic Therapy After Involved-Field Radiotherapy in Patients With Early-Stage Follicular Lymphoma: TROG 99.03. J. Clin. Oncol. 2018, 36, 2918–2925. [Google Scholar] [CrossRef]
- Seymour, J.F.; Pro, B.; Fuller, L.M.; Manning, J.T.; Hagemeister, F.B.; Romaguera, J.; Rodriguez, M.A.; Ha, C.S.; Smith, T.L.; Ayala, A.; et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin’s lymphoma. J. Clin. Oncol. 2003, 21, 2115–2122. [Google Scholar] [CrossRef]
- Kahl, B.S.; Yang, D.T. Follicular lymphoma: Evolving therapeutic strategies. Blood 2016, 127, 2055–2063. [Google Scholar] [CrossRef] [Green Version]
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; Le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M.; Committee, E.G. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv62–iv71. [Google Scholar] [CrossRef]
- Rezvani, A.R.; Sandmaier, B.M. Allogeneic hematopoietic cell transplantation for indolent non-Hodgkin lymphoma: Indications and outcomes. Curr. Opin. Hematol. 2013, 20, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, V.A.; Peterson, B.A. Combination chemotherapy in the treatment of follicular low-grade lymphoma. Leuk Lymphoma 1993, 10, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Herold, M.; Haas, A.; Srock, S.; Neser, S.; Al-Ali, K.H.; Neubauer, A.; Dolken, G.; Naumann, R.; Knauf, W.; Freund, M.; et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group Hematology and Oncology Study. J. Clin. Oncol. 2007, 25, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Imrie, K.; Belch, A.; Cunningham, D.; Flores, E.; Catalano, J.; Solal-Celigny, P.; Offner, F.; Walewski, J.; Raposo, J.; et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005, 105, 1417–1423. [Google Scholar] [CrossRef]
- Salles, G.; Seymour, J.F.; Offner, F.; Lopez-Guillermo, A.; Belada, D.; Xerri, L.; Feugier, P.; Bouabdallah, R.; Catalano, J.V.; Brice, P.; et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2011, 377, 42–51. [Google Scholar] [CrossRef]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar] [CrossRef]
- Montoto, S.; Matthews, J.; Greaves, P.; Lillington, D.; Anderson, D.; Gribben, J.G.; Lister, T.A. Myeloablative chemotherapy for chemo-sensitive recurrent follicular lymphoma: Potential benefit in second relapse. Haematologica 2013, 98, 620–625. [Google Scholar] [CrossRef]
- Andresen, S.; Brandt, J.; Dietrich, S.; Memmer, M.L.; Ho, A.D.; Witzens-Harig, M. The impact of high-dose chemotherapy, autologous stem cell transplant and conventional chemotherapy on quality of life of long-term survivors with follicular lymphoma. Leuk Lymphoma 2012, 53, 386–393. [Google Scholar] [CrossRef]
- Ladetto, M.; Corradini, P.; Vallet, S.; Benedetti, F.; Vitolo, U.; Martelli, M.; Brugiatelli, M.; Coser, P.; Perrotti, A.; Majolino, I.; et al. High rate of clinical and molecular remissions in follicular lymphoma patients receiving high-dose sequential chemotherapy and autografting at diagnosis: A multicenter, prospective study by the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Blood 2002, 100, 1559–1565. [Google Scholar] [CrossRef] [Green Version]
- Tomas, J.F. The challenge of recurrent follicular lymphoma. Lancet Oncol. 2011, 12, 714–716. [Google Scholar] [CrossRef]
- Sarkozy, C.; Maurer, M.J.; Link, B.K.; Ghesquieres, H.; Nicolas, E.; Thompson, C.A.; Traverse-Glehen, A.; Feldman, A.L.; Allmer, C.; Slager, S.L.; et al. Cause of Death in Follicular Lymphoma in the First Decade of the Rituximab Era: A Pooled Analysis of French and US Cohorts. J. Clin. Oncol. 2019, 37, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, N.P.; Million, R.R. Comprehensive lymphatic irradiation for stage II-III non-Hodgkin’s lymphoma. Am. J. Clin. Oncol. 1989, 12, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Murray, K.J.; Schultz, C.J.; Wilson, J.F.; Goswitz, M.S.; Stevens, C.W.; Cox, J.D. Central lymphatic irradiation for stage III nodular malignant lymphoma: Long-term results. J. Clin. Oncol. 1993, 11, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Murtha, A.D.; Rupnow, B.A.; Hansosn, J.; Knox, S.J.; Hoppe, R. Long-term follow-up of patients with Stage III follicular lymphoma treated with primary radiotherapy at Stanford University. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 3–15. [Google Scholar] [CrossRef]
- Guckenberger, M.; Alexandrow, N.; Flentje, M. Radiotherapy alone for stage I-III low grade follicular lymphoma: Long-term outcome and comparison of extended field and total nodal irradiation. Radiat. Oncol. 2012, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Paryani, S.B.; Hoppe, R.T.; Cox, R.S.; Colby, T.V.; Kaplan, H.S. The role of radiation therapy in the management of stage III follicular lymphomas. J. Clin. Oncol. 1984, 2, 841–848. [Google Scholar] [CrossRef]
- McLaughlin, P.; Fuller, L.M.; Velasquez, W.S.; Butler, J.J.; Hagemeister, F.B.; Sullivan-Halley, J.A.; Dixon, D.O. Stage III follicular lymphoma: Durable remissions with a combined chemotherapy-radiotherapy regimen. J. Clin. Oncol. 1987, 5, 867–874. [Google Scholar] [CrossRef]
- Mahe, M.; Bourdin, S.; Le Pourhiet-Le Mevel, A.; Moreau, P.; Campion, L.; Hamidou, M.; Milpied, N.; Moreau, A.; Gaillard, F.; Harousseau, J.L.; et al. Salvage extended-field irradiation in follicular non-Hodgkin’s lymphoma after failure of chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 735–738. [Google Scholar] [CrossRef]
- Ha, C.S.; Kong, J.S.; Tucker, S.L.; McLaughlin, P.; Wilder, R.B.; Hess, M.A.; Cabanillas, F.; Cox, J.D. Central lymphatic irradiation for stage I-III follicular lymphoma: Report from a single-institutional prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 316–320. [Google Scholar] [CrossRef]
- De Los Santos, J.F.; Mendenhall, N.P.; Lynch, J.W., Jr. Is comprehensive lymphatic irradiation for low-grade non-Hodgkin’s lymphoma curative therapy? Long-term experience at a single institution. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 3–8. [Google Scholar] [CrossRef]
- Campbell, J.K.; Matthews, J.P.; Seymour, J.F.; Wolf, M.M.; Juneja, S.K. Australasian Leukaemia Lymphoma, G. Optimum trephine length in the assessment of bone marrow involvement in patients with diffuse large cell lymphoma. Ann. Oncol. 2003, 14, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Foo, M.; Seymour, J.F.; Macmanus, M.P.; Hicks, R.J. Impact of [18f] fluorodeoxyglucose positron emission tomography on staging and management of early-stage follicular non-hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.L.; Binkley, M.S.; Hajj, C.; Chelius, M.; Chau, K.; Balogh, A.; Levis, M.; Filippi, A.R.; Jones, M.; Mac Manus, M.; et al. Definitive radiotherapy for localized follicular lymphoma staged by (18)F-FDG PET-CT: A collaborative study by ILROG. Blood 2019, 133, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.W.D.; Rule, G.; Colvin, K.; Calvente, L.; Hodgson, D.; Bell, S.; Dunduru, C.; Gallo, J.; Tsang, E.S.; Tan, X.; et al. Outcomes of stage I/II follicular lymphoma in the PET era: An international study from the Australian Lymphoma Alliance. Blood Adv. 2019, 3, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Follicular lymphoma: Watch and wait is watch and worry. Lancet Oncol. 2014, 15, 368–369. [Google Scholar] [CrossRef]
- Pettengell, R.; Schmitz, N.; Gisselbrecht, C.; Smith, G.; Patton, W.N.; Metzner, B.; Caballero, D.; Tilly, H.; Walewski, J.A.; Bence-Bruckler, I.; et al. Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: A prospective randomized trial from the lymphoma working party of the European group for blood and marrow transplantation. J. Clin. Oncol. 2013, 31, 1624–1630. [Google Scholar] [CrossRef]
- Mikhaeel, N.G.; Milgrom, S.A.; Terezakis, S.; Berthelsen, A.K.; Hodgson, D.; Eich, H.T.; Dieckmann, K.; Qi, S.N.; Yahalom, J.; Specht, L. The Optimal Use of Imaging in Radiation Therapy for Lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Herfarth, K.; Borchmann, P.; Schnaidt, S.; Hohloch, K.; Budach, V.; Engelhard, M.; Viardot, A.; Engenhart-Cabillic, R.; Keller, U.; Reinartz, G.; et al. Rituximab With Involved Field Irradiation for Early-stage Nodal Follicular Lymphoma: Results of the MIR Study. Hemasphere 2018, 2, e160. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Kirkwood, A.A.; Popova, B.; Smith, P.; Robinson, M.; Gallop-Evans, E.; Coltart, S.; Illidge, T.; Madhavan, K.; Brammer, C.; et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): A randomised phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Mac Manus, M.P.H.R.; Matthews, J.P.; McKenzie, A.; Rischin, D.; Salminen, E.K.; Ball, D.L. Positron Emission Tomography is superior to CT scanning for response-assessment after radical radiotherapy/chemoradiotherapy in patients with non-small cell lung cancer. J. Clin. Oncol. 2003, 21, 1285–1292. [Google Scholar] [CrossRef]
- Mac Manus, M.P.; Hicks, R.J.; Matthews, J.P.; Wirth, A.; Rischin, D.; Ball, D.L. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer 2005, 49, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, J.; Mauch, P. The involved field is back: Issues in delineating the radiation field in Hodgkin’s disease. Ann Oncol. 2002, 13 (Suppl. 1), 79–83. [Google Scholar] [CrossRef] [PubMed]





| Variable | Result |
|---|---|
| Age | |
| Mean (SD) | 50 (9) |
| Median (range) | 49 (2–71) |
| Interquartile range | 44–56 |
| Histological grade | |
| 1–2 | 32 (97%) |
| 3a | 1 (3%) |
| Number of involved Ann Arbor Sites | |
| 2 | 7 (21%) |
| 3 | 6 (18%) |
| 4 | 9 (27%) |
| 5 | 7 (21%) |
| ≥6 | 4 (12%) |
| Prescribed RT dose | |
| 24Gy | 4 (12%) |
| 30Gy | 29 (88%) |
| Maximum nodal diameter (cm) | |
| Mean (SD) | 3 (2) |
| Median (range) | 2.5 (1–8.5) |
| Interquartile range | 2–3 |
| Maximum diameter | |
| ≤5 cm | 28 (85%) |
| >5 cm | 5 (15%) |
| Treatment | |
| RT alone | 18 (55%) |
| RT + systemic therapy | 15 (45%) |
| Rituximab | |
| No Rituximab | 22 (67%) |
| Rituximab | 11 (33%) |
| Patient ID Number | Stem Cell Harvest Mobilization | Stem Cell Harvest Purge | Systemic Therapy Pre-RT | Systemic Therapy Post RT |
|---|---|---|---|---|
| Patient | Cyclophosphamide 1.5 g/m2 | Rituximab | ||
| 1 | Yes | No | ||
| 2 | Yes | No | ||
| 3 | Yes | No | R-CHOP × 6 | |
| 4 | No | No | CVP × 6 | |
| 5 | Yes | Yes | Rituximab × 4 | |
| 6 | Yes | Yes | ||
| 7 | Yes | Yes | ||
| 8 | Yes | No | ||
| 9 | Yes | Yes | ||
| 10 | No | No | R-CVP × 2 | R-CVP × 3 |
| 11 | No | Yes | Rituximab | |
| 12 | No | Yes | ||
| 13 | No | Yes | ||
| 14 | No | No | R-CHOP × 3 | |
| 15 | No | Yes | Rituximab × 4 |
| Toxicity | Hemoglobin | Platelets | Neutrophils |
|---|---|---|---|
| Nadir blood count Median Range | 10.6 g/dL (7.4–13.8) | 46 (11–238) | 0.87 (0.3–3.17) |
| Patients with any toxicity ≥3 | 2/33 (6%) | 16/33 (48%) | 17/33 (52%) |
| Duration Gd ≥3 toxicity Median Range | 4 days (1–8) | 13 days (7–115) | 12 days (1–44) |
| Hematological Support given | 5/33 (15%) Red cell transfusion | 1/33 (3%) Platelet transfusion | 5/33 (15%) (G-CSF injection) |
| Residual Toxicity after 1y | 3/33 Grade 1 | 3/33 grade1 1/33 grade 2 * | 0 |
| Variable | Level | Number of Cases | 5 years FFP (95% CI) | HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Any systemic therapy | No | 18 | 57% (36–91) | 1 | 0.002 |
| Yes | 15 | 100% | 0.1 (0.01–0.5) | ||
| Any Rituximab | No | 22 | 67% (48–93) | 1 | 0.025 |
| Yes | 11 | 100% | Not estimable | ||
| Maximum tumour diameter | ≤5 cm | 28 | 82% (68–100) | 1 | 0.057 |
| >5 cm | 5 | 60% (29–100) | 4.8 (1.1–21.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacManus, M.P.; Hicks, R.J.; Bressel, M.; Campbell, B.A.; Wirth, A.; Ryan, G.; Prince, H.M.; Wolf, M.; Brown, R.; Seymour, J.F. Durable Complete Remission and Long-Term Survival in FDG-PET Staged Patients with Stage III Follicular Lymphoma, Treated with Wide-Field Radiation Therapy. Cancers 2020, 12, 991. https://doi.org/10.3390/cancers12040991
MacManus MP, Hicks RJ, Bressel M, Campbell BA, Wirth A, Ryan G, Prince HM, Wolf M, Brown R, Seymour JF. Durable Complete Remission and Long-Term Survival in FDG-PET Staged Patients with Stage III Follicular Lymphoma, Treated with Wide-Field Radiation Therapy. Cancers. 2020; 12(4):991. https://doi.org/10.3390/cancers12040991
Chicago/Turabian StyleMacManus, Michael P., Rodney J. Hicks, Mathias Bressel, Belinda A. Campbell, Andrew Wirth, Gail Ryan, H. Miles Prince, Max Wolf, Rachel Brown, and John F. Seymour. 2020. "Durable Complete Remission and Long-Term Survival in FDG-PET Staged Patients with Stage III Follicular Lymphoma, Treated with Wide-Field Radiation Therapy" Cancers 12, no. 4: 991. https://doi.org/10.3390/cancers12040991
APA StyleMacManus, M. P., Hicks, R. J., Bressel, M., Campbell, B. A., Wirth, A., Ryan, G., Prince, H. M., Wolf, M., Brown, R., & Seymour, J. F. (2020). Durable Complete Remission and Long-Term Survival in FDG-PET Staged Patients with Stage III Follicular Lymphoma, Treated with Wide-Field Radiation Therapy. Cancers, 12(4), 991. https://doi.org/10.3390/cancers12040991






