Aberrant Expression of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in Warthin Tumors
Abstract
:1. Introduction
2. Results
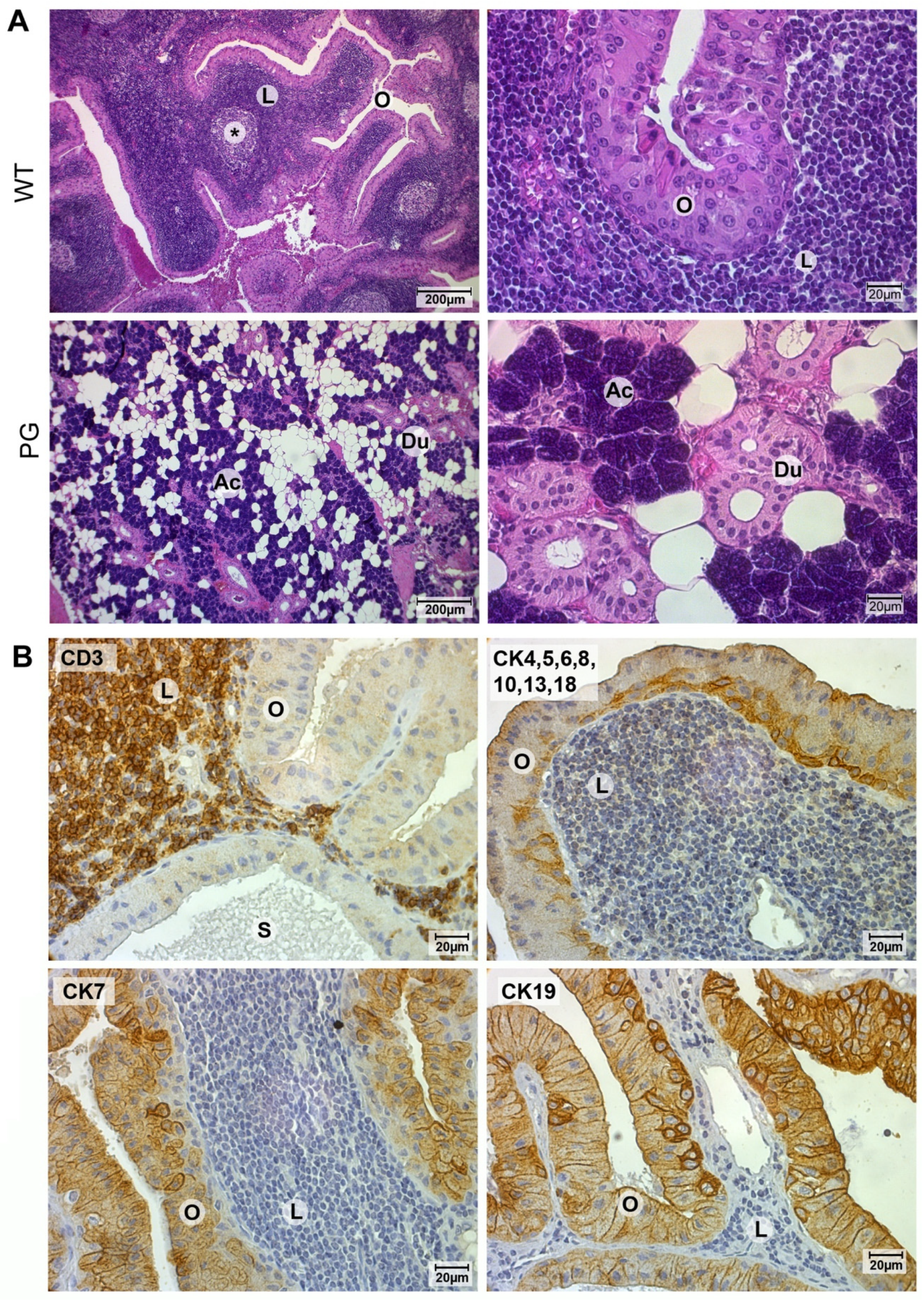
2.1. Validation of Warthin Tumor and Normal Parotid Gland Tissues
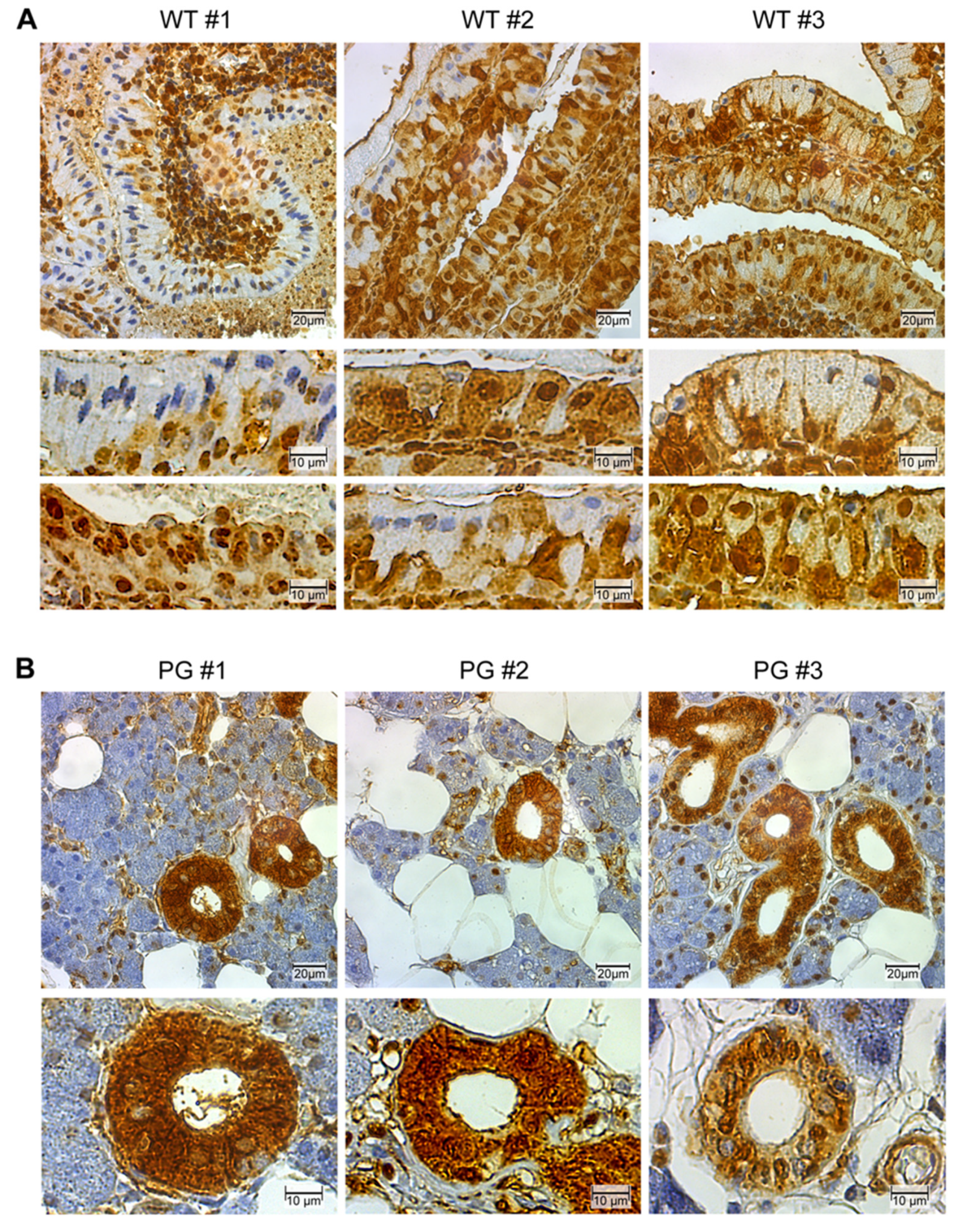
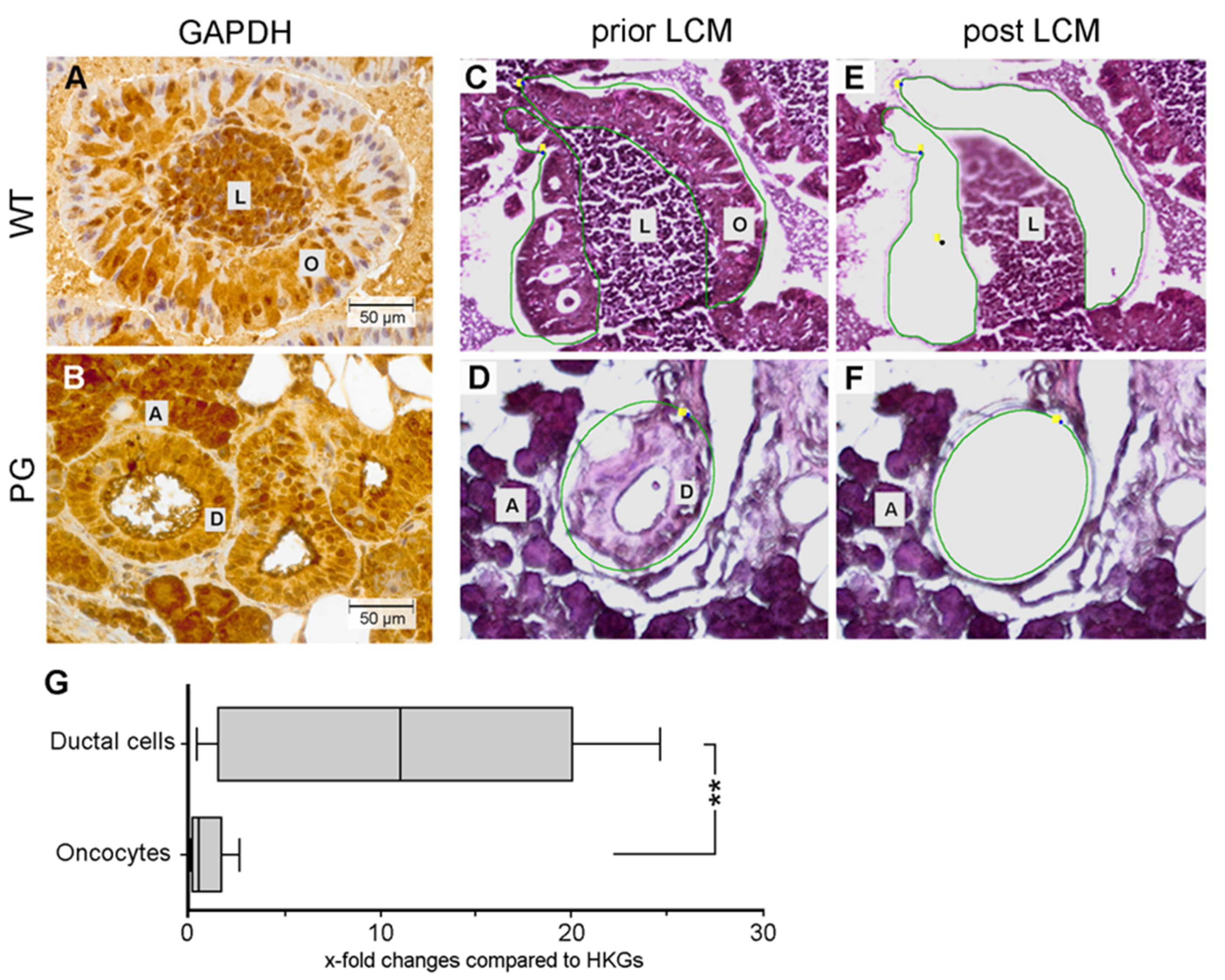
2.2. GAPDH Immunohistochemistry
2.3. Quantification of GAPDH Protein Expression
2.4. Quantification of GAPDH mRNA Expression
2.5. Control Renal Tumor Cohort
3. Discussion
4. Materials and Methods
4.1. Tissues
4.2. Immunohistochemistry
4.3. Cell Sorting, Flow Cytometry and Western Blot Analysis
4.4. Laser-Capture Microdissection (LCM)
4.5. Nucleic Acid Extraction from Cells Isolated by LCM
4.6. Digital Image Analysis with ImageJ/Fiji
4.7. Quantitative RT-PCR
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teymoortash, A.; Krasnewicz, Y.; Werner, J.A. Clinical features of cystadenolymphoma (Warthin’s tumor) of the parotid gland: A retrospective comparative study of 96 cases. Oral Oncol. 2006, 42, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Gabriel, B.; Klussmann, J.P. Risk of facial palsy and severe Frey’s syndrome after conservative parotidectomy for benign disease: Analysis of 610 operations. Acta Otolaryngol. 2006, 126, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Motz, K.M.; Kim, Y.J. Auriculotemporal Syndrome (Frey Syndrome). Otolaryngol. Clin. N. Am. 2016, 49, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadetzki, S.; Oberman, B.; Mandelzweig, L.; Chetrit, A.; Ben-Tal, T.; Jarus-Hakak, A.; Duvdevani, S.; Cardis, E.; Wolf, M. Smoking and risk of parotid gland tumors: A nationwide case-control study. Cancer 2008, 112, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.H.; Eisele, D.W.; Askin, F.B.; Driben, J.S.; Johns, M.E. Warthin’s tumor: A 40-year experience at The Johns Hopkins Hospital. Laryngoscope 1994, 104, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.M.; Kaup Franzen, C.; Guenzel, T.; Lieder, A. Increased incidence of Warthin tumours of the parotid gland: A 42-year evaluation. Eur. Arch. Otorhinolaryngol. 2018, 275, 2593–2598. [Google Scholar] [CrossRef]
- Orabona, G.D.; Abbate, V.; Piombino, P.; Romano, A.; Schonauer, F.; Iaconetta, G.; Salzano, G.; Farina, F.; Califano, L. Warthin’s tumour: Aetiopathogenesis dilemma, ten years of our experience. J. Craniomaxillofac. Surg. 2015, 43, 427–431. [Google Scholar] [CrossRef]
- Teymoortash, A. Back to the roots of Warthin’s tumor of the parotid gland. Eur. Arch. Otorhinolaryngol. 2013, 270, 2397–2402. [Google Scholar] [CrossRef]
- Teymoortash, A.; Werner, J.A.; Moll, R. Is Warthin’s tumour of the parotid gland a lymph node disease? Histopathology 2011, 59, 143–145. [Google Scholar] [CrossRef]
- Seifert, G. Primary salivary gland tumors in lymph nodes of the parotid gland. Report of 3 cases and review of the literature. Pathologe 1997, 18, 141–146. [Google Scholar] [CrossRef]
- Wakely, P.E., Jr. Oncocytic and oncocyte-like lesions of the head and neck. Ann. Diagn. Pathol. 2008, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kuzenko, Y.V.; Romanuk, A.M.; Dyachenko, O.O.; Hudymenko, O. Pathogenesis of Warthin’s tumors. Interv. Med. Appl. Sci. 2016, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, I.; Basu, A.; Ghosh, N. Oncocytic lesion of parotid gland: A dilemma for cytopathologists. J. Cytol. 2012, 29, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Teymoortash, A.; Werner, J.A. Tissue that has lost its track: Warthin’s tumour. Virchows Arch. 2005, 446, 585–588. [Google Scholar] [CrossRef]
- Seifert, G.; Bull, H.G.; Donath, K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch. A Pathol. Anat. Histol. 1980, 388, 13–38. [Google Scholar] [CrossRef]
- Balogh, K., Jr.; Roth, S.I. Histochemical and electron microscopic studies of eosinophilic granular cells (oncocytes) in tumors of the parotid gland. Lab. Invest. 1965, 14, 310–320. [Google Scholar]
- Askew, J.B., Jr.; Bentinck, D.C.; Jenson, A.B.; Fechner, R.E. Epithelial and myoepithelial oncocytes. Ultrastructural study of a salivary gland oncocytoma. Arch. Otolaryngol. 1971, 93, 46–54. [Google Scholar] [CrossRef]
- Hartwick, R.W.; Batsakis, J.G. Non-Warthin’s tumor oncocytic lesions. Ann. Otol. Rhinol. Laryngol. 1990, 99, 674–677. [Google Scholar] [CrossRef]
- Chang, A.; Harawi, S.J. Oncocytes, oncocytosis, and oncocytic tumors. Pathol. Annu. 1992, 27 Pt 1, 263–304. [Google Scholar]
- Capone, R.B.; Ha, P.K.; Westra, W.H.; Pilkington, T.M.; Sciubba, J.J.; Koch, W.M.; Cummings, C.W. Oncocytic neoplasms of the parotid gland: A 16-year institutional review. Otolaryngol. Head Neck Surg. 2002, 126, 657–662. [Google Scholar] [CrossRef]
- Liu, K.E.; Frazier, W.A. Phosphorylation of the BNIP3 C-Terminus inhibits mitochondrial damage and cell death without blocking autophagy. PLoS ONE 2015, 10, e0129667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seirafi, M.; Kozlov, G.; Gehring, K. Parkin structure and function. FEBS J. 2015, 282, 2076–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.; Disatnik, M.H.; Mochly-Rosen, D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol. Med. 2015, 7, 1307–1326. [Google Scholar] [CrossRef]
- Yogalingam, G.; Hwang, S.; Ferreira, J.C.; Mochly-Rosen, D. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) phosphorylation by protein kinase Cdelta (PKCdelta) inhibits mitochondria elimination by lysosomal-like structures following ischemia and reoxygenation-induced injury. J. Biol. Chem. 2013, 288, 18947–18960. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef]
- Mandic, R. Western Blot Analysis; Philipps-Universität Marburg: Marburg, Germany, 2016. [Google Scholar]
- Daguci, L.; Stepan, A.; Mercut, V.; Daguci, C.; Bataiosu, M.; Florescu, A. Immunohistochemical expression of CK7, CK5/6, CK19, and p63 in Warthin tumor. Rom. J. Morphol. Embryol. 2012, 53, 603–607. [Google Scholar]
- Mandic, R.; Bette, M. Immunohistochemistry; Philipps-Universität Marburg: Marburg, Germany, 2019. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Agaimy, A.; Stoehr, C.G. Tissue Microarray Analysis; Friedrich-Alexander-University Erlangen-Nürnberg: Erlangen, Germany, 2020. [Google Scholar]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Kataoka, R.; Hyo, Y.; Hoshiya, T.; Miyahara, H.; Matsunaga, T. Ultrastructural study of mitochondria in oncocytes. Ultrastruct. Pathol. 1991, 15, 231–239. [Google Scholar] [CrossRef]
- Li, L.; Yu, G.; Gao, H. Functional evaluation of the mitochondria from Warthin tumor. Zhonghua Kou Qiang Yi Xue Za Zhi 1996, 31, 370–371. [Google Scholar]
- Lewis, P.D.; Baxter, P.; Paul Griffiths, A.; Parry, J.M.; Skibinski, D.O. Detection of damage to the mitochondrial genome in the oncocytic cells of Warthin’s tumour. J. Pathol. 2000, 191, 274–281. [Google Scholar] [CrossRef]
- Gasparre, G.; Hervouet, E.; de Laplanche, E.; Demont, J.; Pennisi, L.F.; Colombel, M.; Mege-Lechevallier, F.; Scoazec, J.Y.; Bonora, E.; Smeets, R.; et al. Clonal expansion of mutated mitochondrial DNA is associated with tumor formation and complex I deficiency in the benign renal oncocytoma. Hum. Mol. Genet. 2008, 17, 986–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, D.; Luna, M.A.; Weber, R.S.; Kaye, F.J.; El-Naggar, A.K. CRTC1/MAML2 fusion transcript in Warthin’s tumor and mucoepidermoid carcinoma: Evidence for a common genetic association. Genes Chromosomes Cancer 2008, 47, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.; Roser, K.; Belge, G.; Loning, T.; Bullerdiek, J. A closer look at Warthin tumors and the t(11;19). Cancer Genet. Cytogenet. 2008, 180, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Mateo, F.; Serratosa, J.; Salaet, I.; Carujo, S.; Bachs, O.; Pujol, M.J. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase is regulated by acetylation. Int. J. Biochem. Cell Biol. 2010, 42, 1672–1680. [Google Scholar] [CrossRef]
- Huang, Q.; Lan, F.; Zheng, Z.; Xie, F.; Han, J.; Dong, L.; Xie, Y.; Zheng, F. Akt2 kinase suppresses glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis in ovarian cancer cells via phosphorylating GAPDH at threonine 237 and decreasing its nuclear translocation. J. Biol. Chem. 2011, 286, 42211–42220. [Google Scholar] [CrossRef] [Green Version]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Hirano, Y.; Inomata, A.; Yokota, S.; Miyachi, K.; Kaneda, M.; Umeda, M.; Furukawa, K.; Omata, S.; Horigome, T. Participation of a fusogenic protein, glyceraldehyde-3-phosphate dehydrogenase, in nuclear membrane assembly. J. Biol. Chem. 2003, 278, 20395–20404. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Yasunaga, R.; Higashimura, Y.; Yamaji, R.; Fujimoto, K.; Moss, J.; Inui, H.; Nakano, Y. Glyceraldehyde-3-phosphate dehydrogenase enhances transcriptional activity of androgen receptor in prostate cancer cells. J. Biol. Chem. 2007, 282, 22651–22661. [Google Scholar] [CrossRef] [Green Version]
- Carujo, S.; Estanyol, J.M.; Ejarque, A.; Agell, N.; Bachs, O.; Pujol, M.J. Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene 2006, 25, 4033–4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangima Zannat, M.; Bhattacharjee, R.B.; Bag, J. In the absence of cellular poly (A) binding protein, the glycolytic enzyme GAPDH translocated to the cell nucleus and activated the GAPDH mediated apoptotic pathway by enhancing acetylation and serine 46 phosphorylation of p53. Biochem. Biophys. Res. Commun. 2011, 409, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pareja, F.; Brandes, A.H.; Basili, T.; Selenica, P.; Geyer, F.C.; Fan, D.; Da Cruz Paula, A.; Kumar, R.; Brown, D.N.; Gularte-Merida, R.; et al. Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nat. Commun. 2018, 9, 3533. [Google Scholar] [CrossRef] [PubMed]
- Teymoortash, A.; Wiegand, S.; Borkeloh, M.; Bette, M.; Ramaswamy, A.; Steinbach-Hundt, S.; Neff, A.; Werner, J.A.; Mandic, R. Variations in the expression and distribution pattern of AQP5 in acinar cells of patients with sialadenosis. In Vivo 2012, 26, 951–955. [Google Scholar] [PubMed]
- Rodepeter, F.R.; Wiegand, S.; Luers, H.G.; Bonaterra, G.A.; Lowe, A.W.; Bette, M.; Jacob, R.; Mandic, R. Indication for differential sorting of the rat v-SNARE splice isoforms VAMP-1a and -1b. Biochem. Cell Biol. 2017, 95, 500–509. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandic, R.; Agaimy, A.; Pinto-Quintero, D.; Roth, K.; Teymoortash, A.; Schwarzbach, H.; Stoehr, C.G.; Rodepeter, F.R.; Stuck, B.A.; Bette, M. Aberrant Expression of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in Warthin Tumors. Cancers 2020, 12, 1112. https://doi.org/10.3390/cancers12051112
Mandic R, Agaimy A, Pinto-Quintero D, Roth K, Teymoortash A, Schwarzbach H, Stoehr CG, Rodepeter FR, Stuck BA, Bette M. Aberrant Expression of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in Warthin Tumors. Cancers. 2020; 12(5):1112. https://doi.org/10.3390/cancers12051112
Chicago/Turabian StyleMandic, Robert, Abbas Agaimy, Daniel Pinto-Quintero, Katrin Roth, Afshin Teymoortash, Hans Schwarzbach, Christine G. Stoehr, Fiona R. Rodepeter, Boris A. Stuck, and Michael Bette. 2020. "Aberrant Expression of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in Warthin Tumors" Cancers 12, no. 5: 1112. https://doi.org/10.3390/cancers12051112







