Evaluation of Anti-Tumor Effects of Whole-Body Low-Dose Irradiation in Metastatic Mouse Models
Abstract
:1. Introduction
2. Results
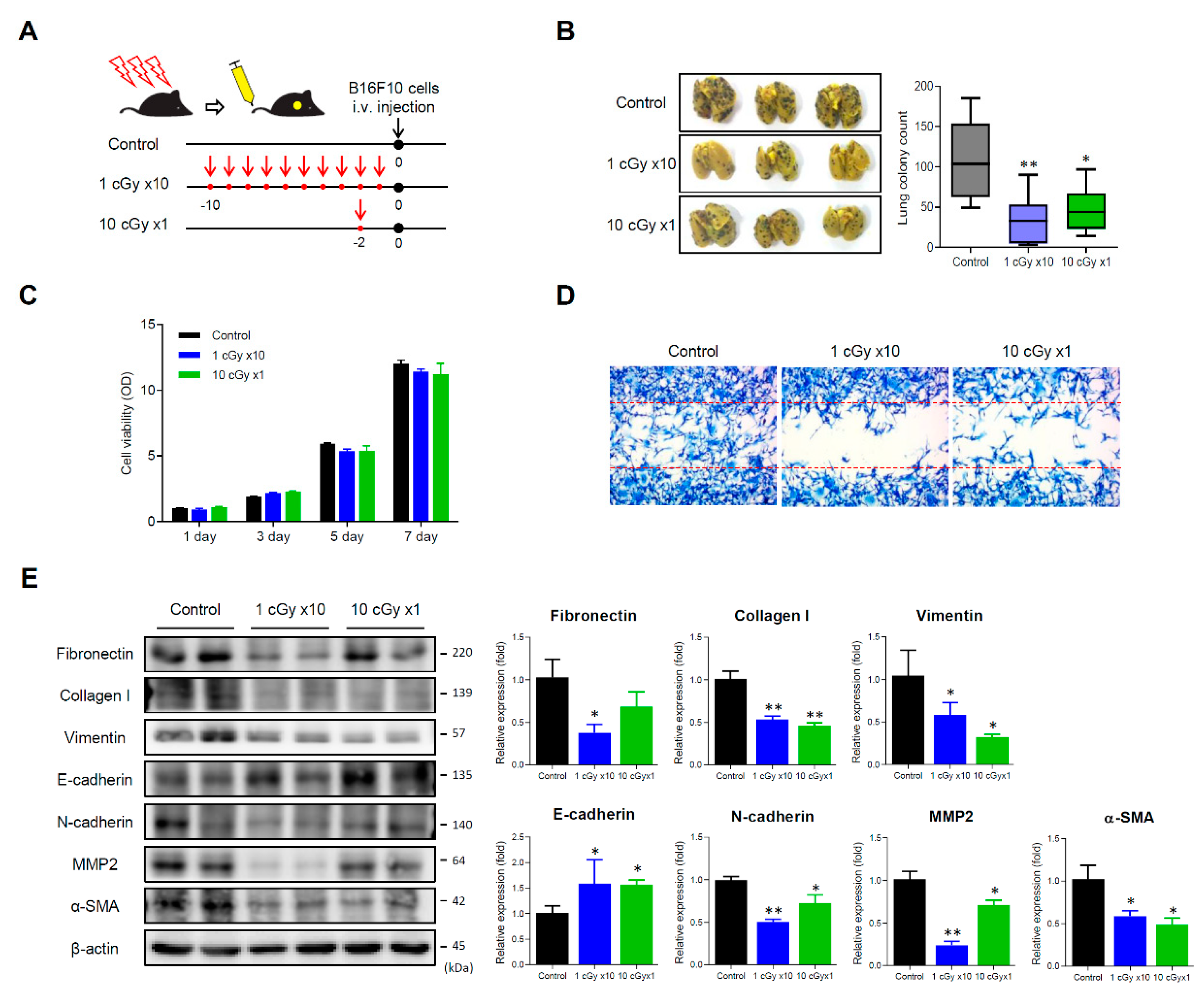
2.1. LDI Inhibits Experimental Lung Metastasis of B16F10 Melanoma
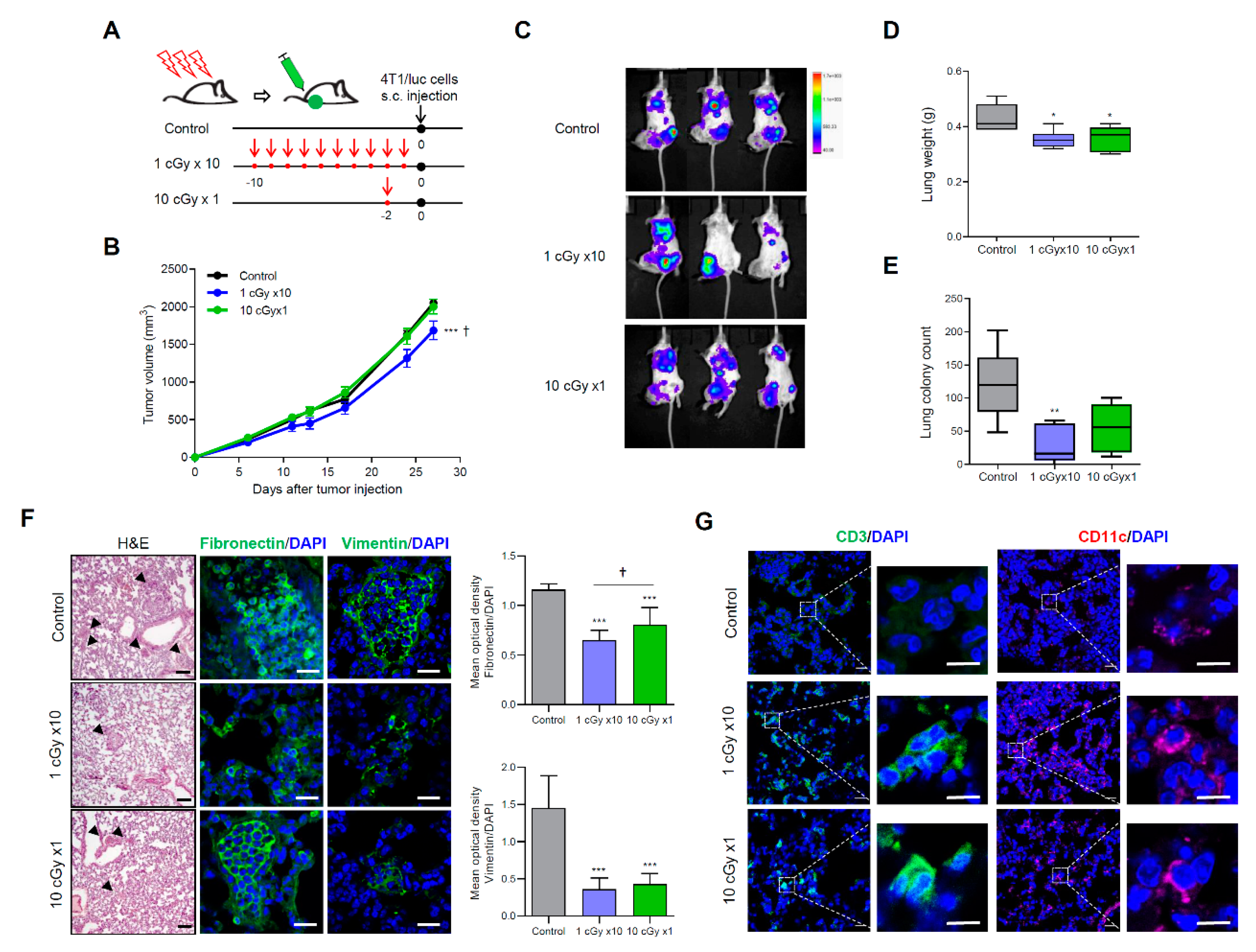
2.2. LDI Suppresses Spontaneous Metastasis of 4T1 Cells
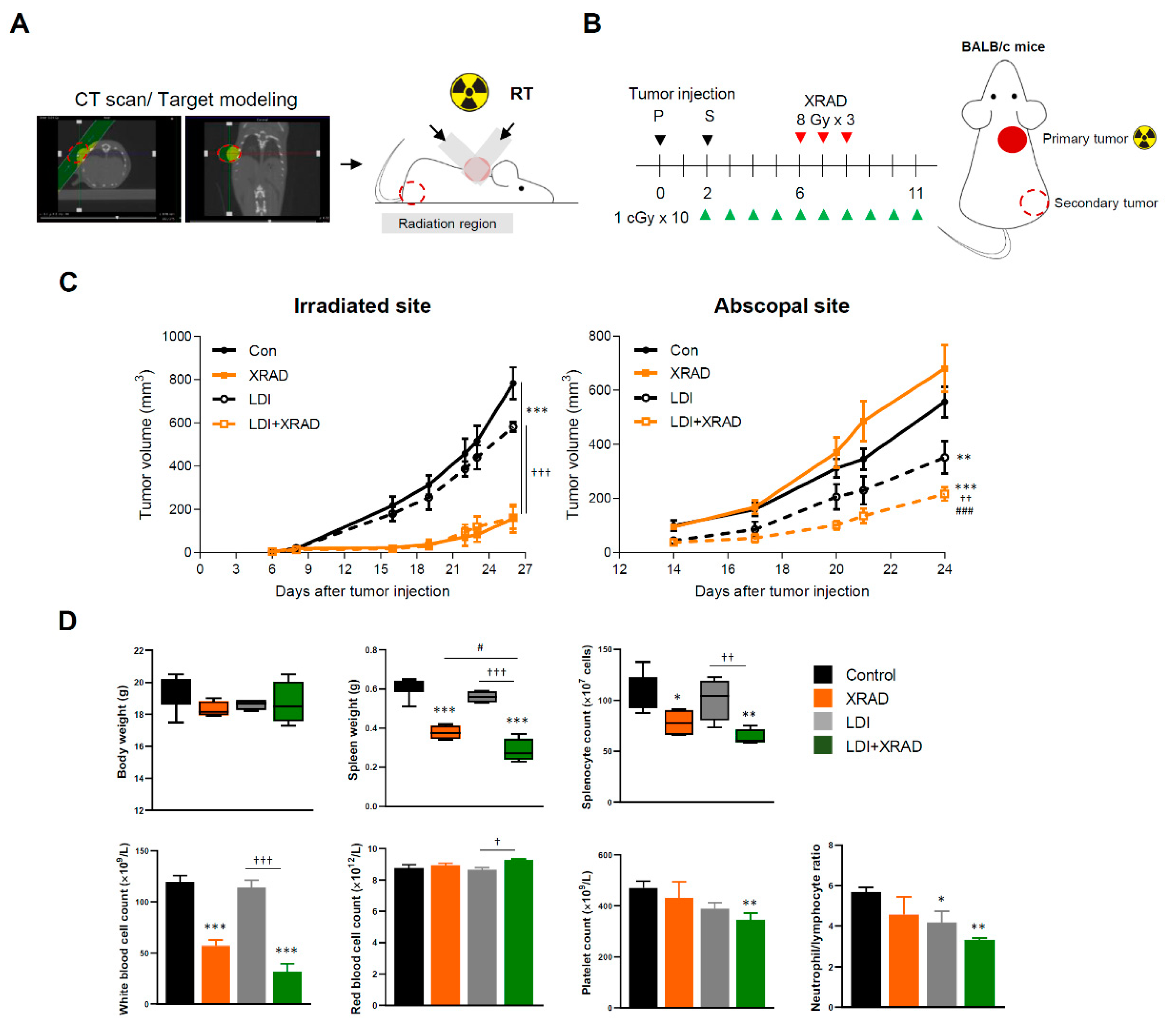
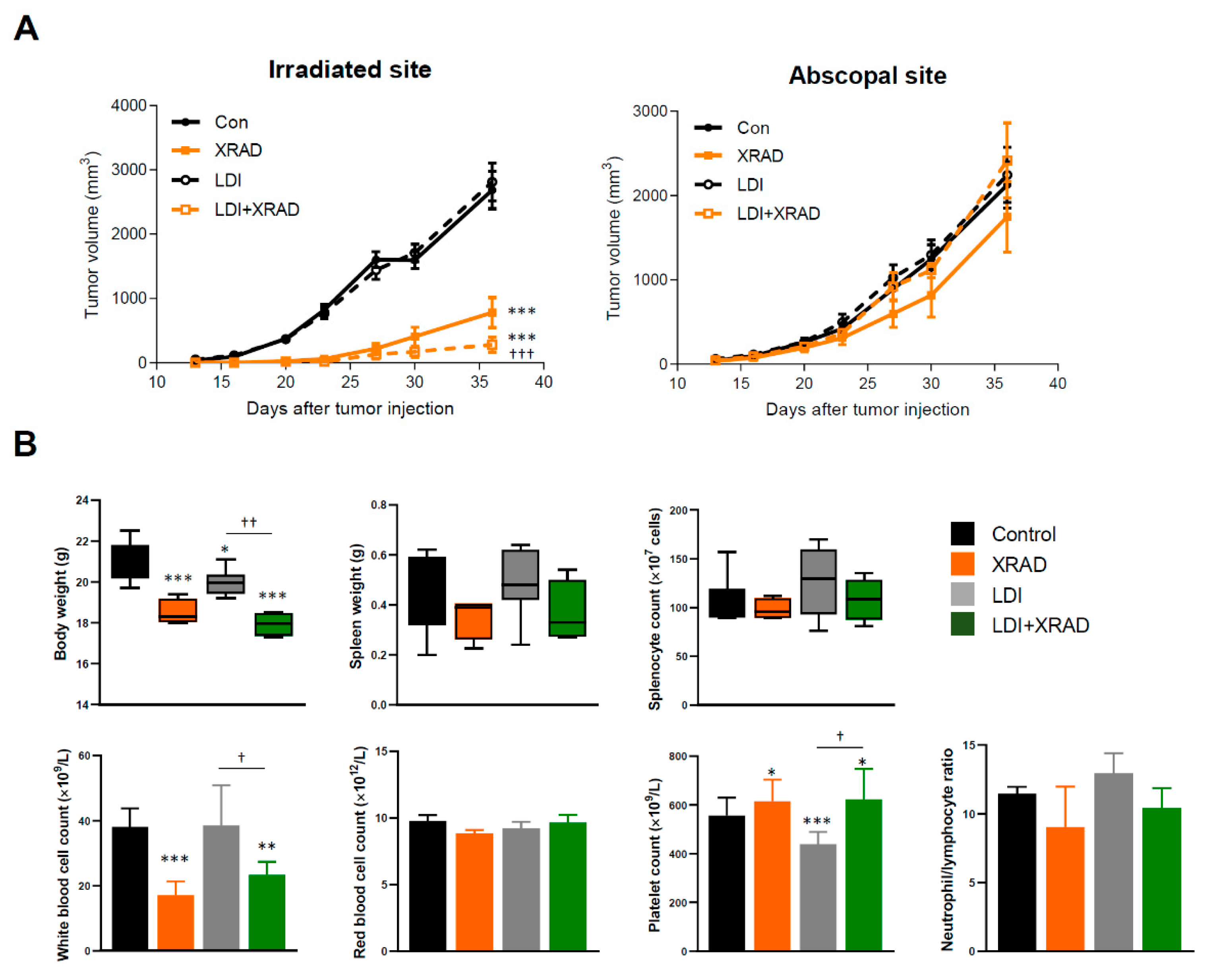
2.3. LDI Induces Abscopal Effects
2.4. LDI Synergizes with RT Via EMT Inhibition
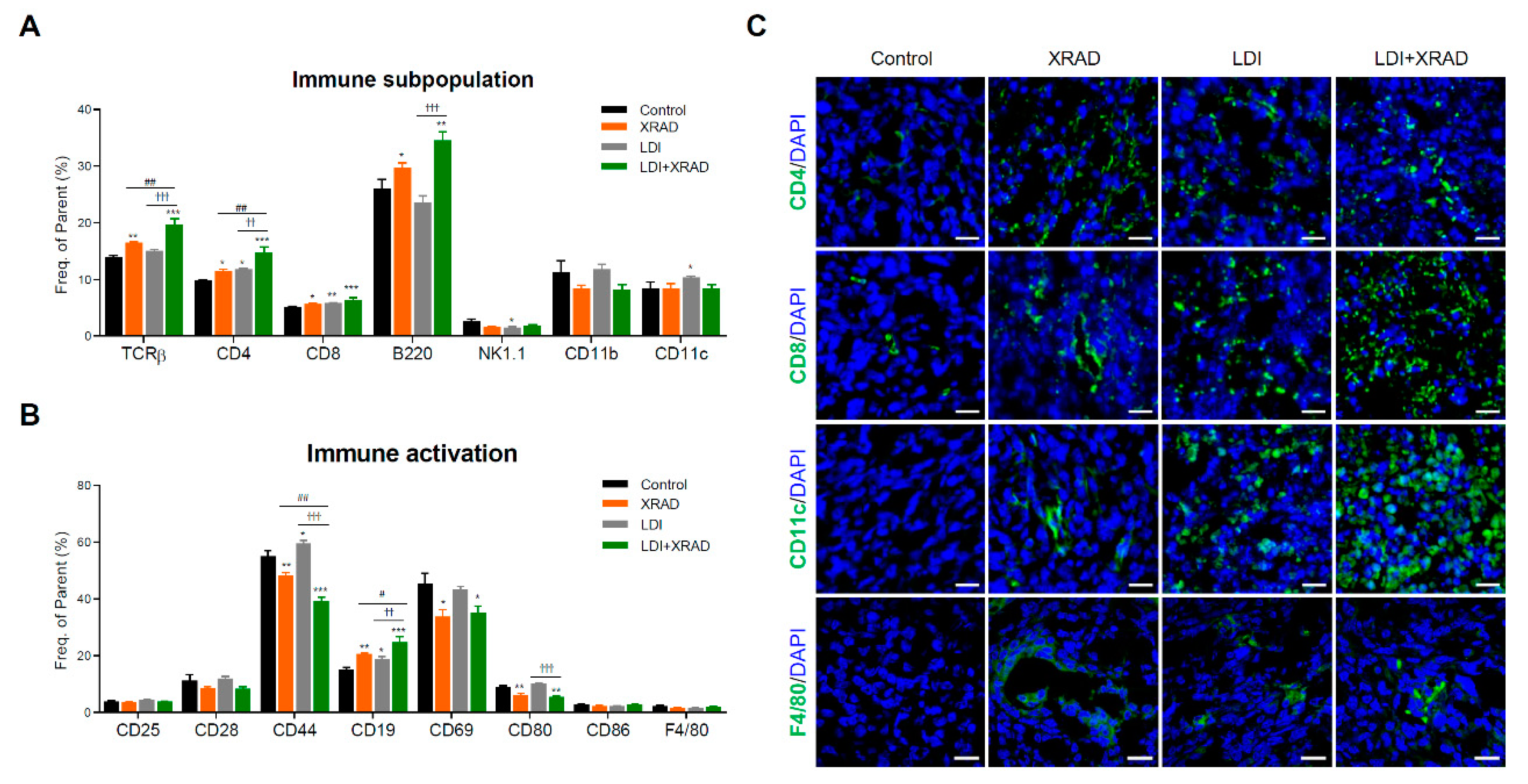
2.5. LDI Induces the Abscopal Effect Via Stimulation of Immune Response
2.6. Abscopal Effect of LDI in Immunodeficient Nude Mice
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Mice
4.3. In Vivo Tumor Challenge and Treatment
4.4. Bioluminescence Imaging
4.5. Hematological Analysis
4.6. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) Assay
4.7. Wound Scratch Assay
4.8. Western Blot Assay
4.9. Phenotypic Analysis of Splenocytes
4.10. Immunofluorescence Staining
4.11. Hematoxylin and Eosin Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gueguen, Y.; Bontemps, A.; Ebrahimian, T.G. Adaptive responses to low doses of radiation or chemicals: Their cellular and molecular mechanisms. Cell. Mol. Life Sci. 2019, 76, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.; Somayaji, R.; Conly, J.; Exner, D.; Rennert-May, E. Timing of device reimplantation and reinfection rates following cardiac implantable electronic device infection: A systematic review and meta-analysis. BMJ Open 2019, 9, e029537. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, J.; Wu, M.; Hu, C.; Yang, J.; Li, D.; Wu, P.; Chen, Y.; Chen, P.; Lin, S.; et al. Low-Dose Total Body Irradiation Can Enhance Systemic Immune Related Response Induced by Hypo-Fractionated Radiation. Front. Immunol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.W.; Tang, C.; de Groot, P.; Naing, A.; Hess, K.R.; Heymach, J.V.; Papadimitrakopoulou, V.A.; Cushman, T.R.; Subbiah, V.; Chang, J.Y.; et al. Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low-Dose Radiation-Related Abscopal Responses. Cancer Immunol. Res. 2019, 7, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Rithidech, K.N. Health Benefits of Exposure to Low-dose Radiation. Health Phys. 2016, 110, 293–295. [Google Scholar] [CrossRef]
- Chambers, D.B. Radiological protection in North American naturally occurring radioactive material industries. Ann. ICRP 2015, 44, 202–213. [Google Scholar] [CrossRef] [Green Version]
- United Nations. Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 2000. [Google Scholar]
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.I.; Cortez, M.A.; Erasmus, J.J.; de Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ruiz, M.E.; Vitale, I.; Harrington, K.J.; Melero, I.; Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 2020, 21, 120–134. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Grass, G.D.; Krishna, N.; Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 2016, 40, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Shankar, B.S.; Sharma, D.; Sainis, K.B. Low dose radiation induced immunomodulation: Effect on macrophages and CD8+ T cells. Int. J. Radiat. Biol. 2005, 81, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Shen, J.; Tomiyama-Miyaji, C.; Watanabe, M.; Kainuma, E.; Inoue, M.; Kuwano, Y.; Abo, T. Augmentation of innate immunity by low-dose irradiation. Cell. Immunol. 2006, 244, 50–56. [Google Scholar] [CrossRef]
- Schmidt, C.S.; Mescher, M.F. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J. Immunol. 2002, 168, 5521–5529. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, K.; Ishiwata, Y.; Nakagawa, K.; Yokochi, S.; Taruki, C.; Akuta, T.; Ohtomo, K.; Matsushima, K.; Tamatani, T.; Kanegasaki, S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin. Cancer Res. 2008, 14, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [Green Version]
- Barlesi, F.; Vansteenkiste, J.; Spigel, D.; Ishii, H.; Garassino, M.; de Marinis, F.; Ozguroglu, M.; Szczesna, A.; Polychronis, A.; Uslu, R.; et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 2018, 19, 1468–1479. [Google Scholar] [CrossRef]
- Kelly, K.; Infante, J.R.; Taylor, M.H.; Patel, M.R.; Wong, D.J.; Iannotti, N.; Mehnert, J.M.; Loos, A.H.; Koch, H.; Speit, I.; et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 2018, 124, 2010–2017. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.H.; Kim, M.H.; Kang, S.M.; Jung, S.Y.; Ahn, J.; Woo, H.J.; Nam, S.Y.; Hwang, S.G.; Ryu, S.Y.; Song, J.Y. Analysis of immune cell populations and cytokine profiles in murine splenocytes exposed to whole-body low-dose irradiation. Int. J. Radiat. Biol. 2015, 91, 795–803. [Google Scholar] [CrossRef]
- Song, K.H.; Jung, S.Y.; Kho, S.H.; Hwang, S.G.; Ha, H.; Nam, S.Y.; Song, J.Y. Effects of low-dose irradiation on mice with Escherichia coli-induced sepsis. Toxicol. Appl. Pharmacol. 2017, 333, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Kang, H.; Hong, E.H.; Kim, J.Y.; Nam, S.Y. Transcriptome analysis of low-dose ionizing radiation-impacted genes in CD4(+) T-cells undergoing activation and regulation of their expression of select cytokines. J. Immunotoxicol. 2018, 15, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Xiong, S.; Zhang, L.; Chu, Y. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell. Mol. Immunol. 2010, 7, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, N.; Kim, M.J.; Kim, R.K.; Kumar Kaushik, N.; Seong, K.M.; Nam, S.Y.; Lee, S.J. Low-dose radiation decreases tumor progression via the inhibition of the JAK1/STAT3 signaling axis in breast cancer cell lines. Sci. Rep. 2017, 7, 43361. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Nakayama, K.; Ishida, H. Low dose gamma-rays activate immune functions via induction of glutathione and delay tumor growth. J. Radiat. Res. 2004, 45, 33–39. [Google Scholar] [CrossRef]
- Bobek, V.; Kolostova, K.; Pinterova, D.; Kacprzak, G.; Adamiak, J.; Kolodziej, J.; Boubelik, M.; Kubecova, M.; Hoffman, R.M. A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Res. 2010, 30, 4799–4803. [Google Scholar]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA 2001, 98, 473–478. [Google Scholar] [CrossRef]
- Brooks, A.L. Evidence for ‘bystander effects’ in vivo. Hum. Exp. Toxicol. 2004, 23, 67–70. [Google Scholar] [CrossRef]
- Ikushima, T.; Aritomi, H.; Morisita, J. Radioadaptive response: Efficient repair of radiation-induced DNA damage in adapted cells. Mutat. Res. 1996, 358, 193–198. [Google Scholar] [CrossRef]
- Rodel, F.; Keilholz, L.; Herrmann, M.; Sauer, R.; Hildebrandt, G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int. J. Radiat. Biol. 2007, 83, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Jeong, H.; Hong, E.H.; Joo, H.M.; Cho, K.S.; Nam, S.Y. Low-dose ionizing radiation alleviates Abeta42-induced cell death via regulating AKT and p38 pathways in Drosophila Alzheimer’s disease models. Biol. Open 2019, 8, bio036657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, L.; Williams, R.O.; Vieira, P.L.; Screaton, G.; Feldmann, M.; Dazzi, F. The therapeutic activity of low-dose irradiation on experimental arthritis depends on the induction of endogenous regulatory T cell activity. Ann. Rheum. Dis. 2010, 69, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Tago, F.; Tsukimoto, M.; Nakatsukasa, H.; Kojima, S. Repeated 0.5-Gy gamma irradiation attenuates autoimmune disease in MRL-lpr/lpr mice with suppression of CD3+CD4-CD8-B220+ T-cell proliferation and with up-regulation of CD4+CD25+Foxp3+ regulatory T cells. Radiat. Res. 2008, 169, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tsukimoto, M.; Nakatsukasa, H.; Sugawara, K.; Yamashita, K.; Kojima, S. Repeated 0.5-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat. Res. 2008, 170, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kojima, S.; Yamaoka, K.; Niki, E. Prevention of type I diabetes by low-dose gamma irradiation in NOD mice. Radiat. Res. 2000, 154, 680–685. [Google Scholar] [CrossRef]
- Hong, E.H.; Song, J.Y.; Lee, S.J.; Park, I.C.; Um, H.D.; Park, J.K.; Lee, K.H.; Nam, S.Y.; Hwang, S.G. Low-dose gamma-radiation inhibits IL-1beta-induced dedifferentiation and inflammation of articular chondrocytes via blockage of catenin signaling. IUBMB Life 2014, 66, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Short, S.C.; Kelly, J.; Mayes, C.R.; Woodcock, M.; Joiner, M.C. Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int. J. Radiat. Biol. 2001, 77, 655–664. [Google Scholar]
- Williams, J.R.; Zhang, Y.; Zhou, H.; Gridley, D.S.; Koch, C.J.; Slater, J.M.; Little, J.B. Overview of radiosensitivity of human tumor cells to low-dose-rate irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 909–917. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Li, H.; Niu, C.; Yu, D.; Yang, G.; Liang, X.; Wen, X.; Li, M.; Cui, J. Validating the pivotal role of the immune system in low-dose radiation-induced tumor inhibition in Lewis lung cancer-bearing mice. Cancer Med. 2018, 7, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Liu, Z.M.; Yu, X.Y.; Song, A.Q.; Liu, N.; Wang, H. Low-dose radiation induces antitumor effects and erythrocyte system hormesis. Asian Pac. J. Cancer Prev. 2013, 14, 4121–4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheda, A.; Wrembel-Wargocka, J.; Lisiak, E.; Nowosielska, E.M.; Marciniak, M.; Janiak, M.K. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat. Res. 2004, 161, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Shirato, H.; Hosokawa, M.; Nishioka, T.; Kuramitsu, Y.; Matushita, K.; Kobayashi, M.; Miyasaka, K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat. Res. 1999, 151, 717–724. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, J.K.; Schiergens, T.S.; Wang, P.X.; Ou, B.C.; Al-Sayegh, R.; Li, M.L.; Lu, A.G.; Yin, S.; Thasler, W.E. Bone marrow-derived mesenchymal stromal cells promote colorectal cancer cell death under low-dose irradiation. Br. J. Cancer 2018, 118, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.K.; Kim, M.J.; Seong, K.M.; Kaushik, N.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Jin, Y.W.; Nam, S.Y.; Lee, S.J. Beneficial effects of low dose radiation in response to the oncogenic KRAS induced cellular transformation. Sci. Rep. 2015, 5, 15809. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. (Berl.) 2016, 94, 509–522. [Google Scholar] [CrossRef]
- Sangaletti, S.; Tripodo, C.; Sandri, S.; Torselli, I.; Vitali, C.; Ratti, C.; Botti, L.; Burocchi, A.; Porcasi, R.; Tomirotti, A.; et al. Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res. 2014, 74, 4706–4719. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. (Berl.) 2013, 91, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Harder, M.J.; Anliker, M.; Hochsmann, B.; Simmet, T.; Huber-Lang, M.; Schrezenmeier, H.; Ricklin, D.; Lambris, J.D.; Barlow, P.N.; Schmidt, C.Q. Comparative Analysis of Novel Complement-Targeted Inhibitors, MiniFH, and the Natural Regulators Factor H and Factor H-like Protein 1 Reveal Functional Determinants of Complement Regulation. J. Immunol. 2016, 196, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Habets, T.H.; Oth, T.; Houben, A.W.; Huijskens, M.J.; Senden-Gijsbers, B.L.; Schnijderberg, M.C.; Brans, B.; Dubois, L.J.; Lambin, P.; De Saint-Hubert, M.; et al. Fractionated Radiotherapy with 3 x 8 Gy Induces Systemic Anti-Tumour Responses and Abscopal Tumour Inhibition without Modulating the Humoral Anti-Tumour Response. PLoS ONE 2016, 11, e0159515. [Google Scholar] [CrossRef]
- Zhang, X.; Niedermann, G. Abscopal Effects With Hypofractionated Schedules Extending Into the Effector Phase of the Tumor-Specific T-Cell Response. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Formenti, S.C.; Demaria, S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology 2017, 6, e1339857. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Gaertner, F.; Massberg, S. Patrolling the vascular borders: Platelets in immunity to infection and cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Demaria, S.; Formenti, S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer 2016, 4, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, C.J.; Smith, J.W.; Soong, S.J.; Urist, M.M.; Maddox, W.A. Neck dissection with and without radiotherapy: Prognostic factors, patterns of recurrence, and survival. Am. J. Surg. 1986, 152, 456–463. [Google Scholar] [CrossRef]
- Suit, H.D. Local control and patient survival. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 653–660. [Google Scholar] [CrossRef]
- Vicini, F.A.; Kestin, L.; Huang, R.; Martinez, A. Does local recurrence affect the rate of distant metastases and survival in patients with early-stage breast carcinoma treated with breast-conserving therapy? Cancer 2003, 97, 910–919. [Google Scholar] [CrossRef]
- Kim, M.H.; Jung, S.Y.; Song, K.H.; Park, J.I.; Ahn, J.; Kim, E.H.; Park, J.K.; Hwang, S.G.; Woo, H.J.; Song, J.Y. A new FGFR inhibitor disrupts the TGF-beta1-induced fibrotic process. J. Cell. Mol. Med. 2020, 24, 830–840. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.-H.; Jung, S.-Y.; Park, J.-I.; Ahn, J.; Park, J.-K.; Hwang, S.-G.; Kim, E.-H.; Nam, S.Y.; Park, S.; Ha, H.; et al. Evaluation of Anti-Tumor Effects of Whole-Body Low-Dose Irradiation in Metastatic Mouse Models. Cancers 2020, 12, 1126. https://doi.org/10.3390/cancers12051126
Song K-H, Jung S-Y, Park J-I, Ahn J, Park J-K, Hwang S-G, Kim E-H, Nam SY, Park S, Ha H, et al. Evaluation of Anti-Tumor Effects of Whole-Body Low-Dose Irradiation in Metastatic Mouse Models. Cancers. 2020; 12(5):1126. https://doi.org/10.3390/cancers12051126
Chicago/Turabian StyleSong, Kyung-Hee, Seung-Youn Jung, Jeong-In Park, Jiyeon Ahn, Jong-Kuk Park, Sang-Gu Hwang, Eun-Ho Kim, Seon Young Nam, Seungwoo Park, Hunjoo Ha, and et al. 2020. "Evaluation of Anti-Tumor Effects of Whole-Body Low-Dose Irradiation in Metastatic Mouse Models" Cancers 12, no. 5: 1126. https://doi.org/10.3390/cancers12051126
APA StyleSong, K.-H., Jung, S.-Y., Park, J.-I., Ahn, J., Park, J.-K., Hwang, S.-G., Kim, E.-H., Nam, S. Y., Park, S., Ha, H., & Song, J.-Y. (2020). Evaluation of Anti-Tumor Effects of Whole-Body Low-Dose Irradiation in Metastatic Mouse Models. Cancers, 12(5), 1126. https://doi.org/10.3390/cancers12051126






