Metabolism and Immune Modulation in Patients with Solid Tumors: Systematic Review of Preclinical and Clinical Evidence
Abstract
:1. Introduction
2. Methods
- Preclinical studies using a tumor model or clinical studies on oncologic patients that evaluate the influence of nutrition/metabolism on the immune system;
- Clinical and/or preclinical studies about the role of specific metabolites and/or gut microbiota in the immune system homeostasis;
- Studies about how specific metabolites could modify ICI efficacy until February 2020.
- No immunomodulation activity endpoint;
- About pediatric or pregnancy patients;
- Reviews;
- Reports;
- Surgical settings;
- Hematological malignancies;
- About carcinogenesis;
- Outcome in healthy people;
- About oncological therapies toxicities;
- About inflammation, infection and cancer prevention.
3. Results
3.1. Selection of Preclinical and Clinical Studies
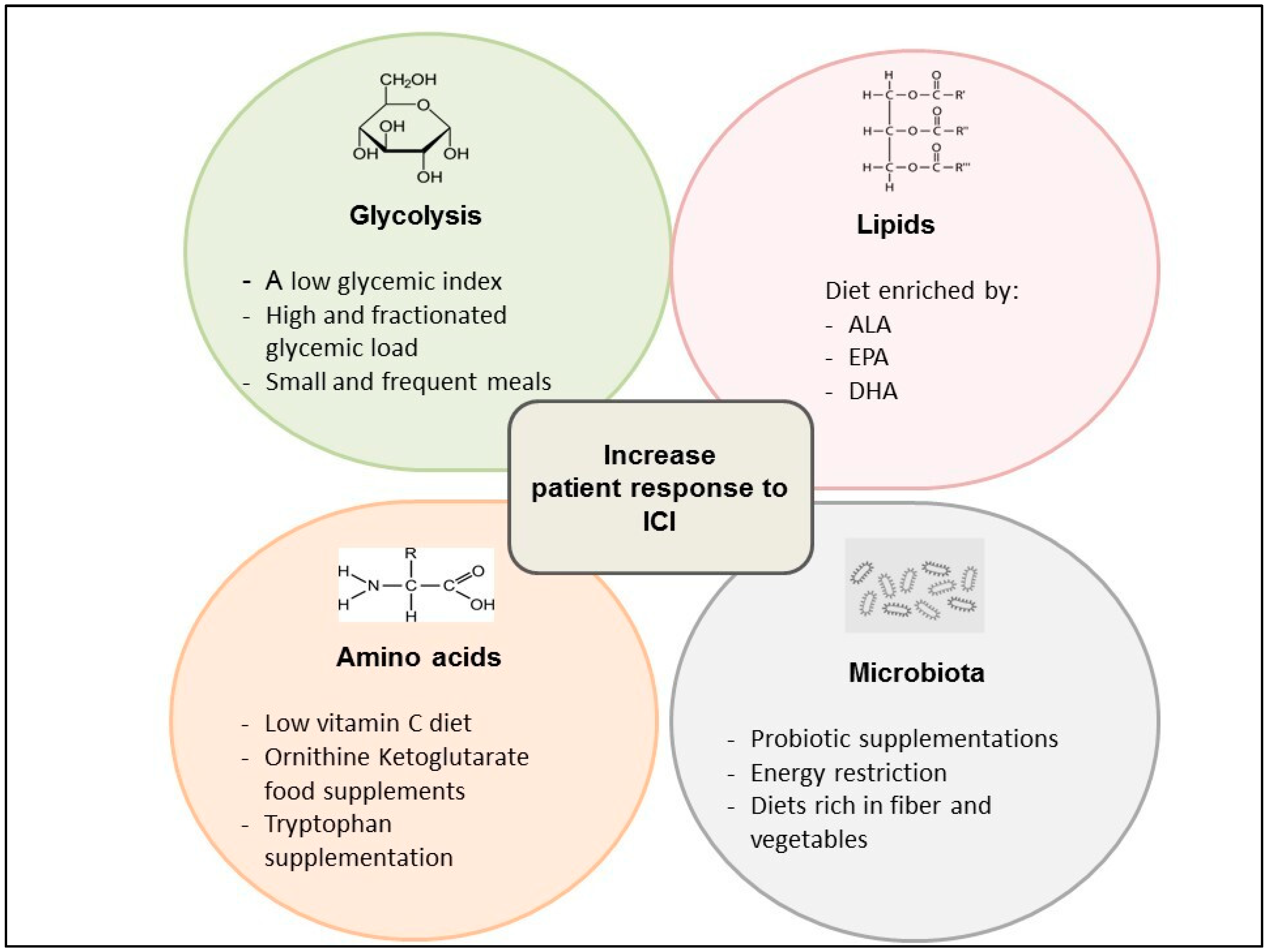
3.2. Glycolysis and Oxidative Metabolism
3.3. Amino Acid Metabolism
3.4. Lipids Metabolism
3.5. Microbiota
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Carella, A.M.; Corradini, P.; Mussetti, A.; Ricardi, U.; Vitolo, U.; Viviani, S. Treatment of classical Hodgkin lymphoma in the era of brentuximab vedotin and immune checkpoint inhibitors. Ann. Hematol. 2018, 97, 1301–1315. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, P.S.; Chamoto, K.; Honjo, T. Combination therapy strategies for improving PD-1 blockade efficacy: A new era in cancer immunotherapy. J. Intern. Med. 2018, 283, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu-Lieskovan, S.; Ribas, A. New combination strategies using PD-1/L1 checkpoint inhibitors as a backbone. Cancer J. Sudbury Mass. 2017, 23, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur J. Clin Nutr. 2002, 56, S73–S76. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Kew, S. The immune system: A target for functional foods? Br. J. Nutr. 2002, 88 (Suppl. 2), S165–S177. [Google Scholar] [CrossRef]
- Grimble, R.F. Effect of antioxidative vitamins on immune function with clinical applications. Int. J. Vitam. Nutr. Res. Int. Z Vitam.bErnahr. J. Int. Vitam. Nutr. 1997, 67, 312–320. [Google Scholar]
- Chandra, R.K.; Kumari, S. Effects of nutrition on the immune system. Nutr. Burbank Los Angel Cty Calif. 1994, 10, 207–210. [Google Scholar]
- Laviano, A.; Di Lazzaro, L.; Koverech, A. Nutrition support and clinical outcome in advanced cancer patients. Proc. Nutr. Soc. 2018, 77, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Tateya, S.; Kim, F.; Tamori, Y. Recent Advances in Obesity-Induced Inflammation and Insulin Resistance. Front. Endocrinol. 2013, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [Green Version]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer. 2018, 18, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; O’Dwyer, P.J.; Clark, J.; Shi, J.G.; Bowman, K.J.; Scherle, P.A.; Newton, R.C.; Schaub, R.; Maleski, J.; Leopold, L.; et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3269–3276. [Google Scholar] [CrossRef] [Green Version]
- Machon, C.; Thezenas, S.; Dupuy, A.M.; Assenat, E.; Michel, F.; Mas, E.; Senesse, P.; Cristol, J.P. Immunonutrition before and during radiochemotherapy: Improvement of inflammatory parameters in head and neck cancer patients. Support Care Cancer 2012, 20, 3129–3135. [Google Scholar] [CrossRef]
- Sunpaweravong, S.; Puttawibul, P.; Ruangsin, S.; Laohawiriyakamol, S.; Sunpaweravong, P.; Sangthawan, D.; Pradutkanchana, J.; Raungkhajorn, P.; Geater, A. Randomized study of antiinflammatory and immune-modulatory effects of enteral immunonutrition during concurrent chemoradiotherapy for esophageal cancer. Nutr. Cancer 2014, 66, 1–5. [Google Scholar] [CrossRef]
- Maruyama, T.; Mimura, K.; Izawa, S.; Shiba, S.; Watanabe, M.; Kawaguchi, Y.; Fujii, H.; Kono, K. Immunonutritional diet modulates natural killer cell activation and Th17 cell distribution in patients with gastric and esophageal cancer. Nutrition 2011, 27, 146–152. [Google Scholar] [CrossRef]
- Talvas, J.; Garrait, G.; Goncalves-Mendes, N.; Rouanet, J.; Vergnaud-Gauduchon, J.; Kwiatkowski, F.; Bachmann, P.; Bouteloup, C.; Bienvenu, J.; Vasson, M.P. Immunonutrition stimulates immune functions and antioxidant defense capacities of leukocytes in radiochemotherapy-treated head & neck and esophageal cancer patients: A double-blind randomized clinical trial. Clin. Nutr. 2015, 34, 810–817. [Google Scholar]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Roller, M.; Clune, Y.; Collins, K.; Rechkemmer, G.; Watzl, B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br. J. Nutr. 2007, 97, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Botticelli, A.; Vernocchi, P.; Marini, F.; Quagliariello, A.; Cerbelli, B.; Reddel, S.; Del Chierico, F.; Di Pietro, F.; Giusti, R.; Tomassini, A.; et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J. Transl. Med. 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, B.A.; Wilson, M.; Moran, U.; Pavlick, A.; Izsak, A.; Wechter, T.; Weber, J.S.; Osman, I.; Ahn, J. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019, 11, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Siska, P.J.; Beckermann, K.E.; Mason, F.M.; Andrejeva, G.; Greenplate, A.R.; Sendor, A.B.; Chiang, Y.J.; Corona, A.L.; Gemta, L.F.; Vincent, B.G.; et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2017, 2, e93411. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Esfahani, A.; Asghari Jafarabadi, M.; Eivazi Ziaei, J.; Movassaghpourakbari, A.; Farrin, N. Effect of Beta glucan on quality of life in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Adv. Pharm. Bull. 2014, 4 (Suppl. 1), 471–477. [Google Scholar] [CrossRef]
- Paixão, E.M.D.S.; Oliveira, A.C.M.; Pizato, N.; Muniz-Junqueira, M.I.; Magalhães, K.G.; Nakano, E.Y.; Ito, M.K. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: A randomized double-blind controlled trial. Nutr. J. 2017, 16, 71. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, C.J.; Hammerman, P.S.; Thompson, C.B. Fuel feeds function: Energy metabolism and the T-cell response. Nat. Rev. Immunol. 2005, 5, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bourgeois, T.; Strauss, L.; Aksoylar, H.I.; Daneshmandi, S.; Seth, P.; Patsoukis, N.; Boussiotis, V.A. Targeting T Cell Metabolism for Improvement of Cancer Immunotherapy. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, A.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; Ochiai, A.; Tomita, M.; et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009, 69, 4918–4925. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Bai, L.; Li, W.; Zeng, T.; Tian, H.; Cui, J. Targeting T cell metabolism in the tumor microenvironment: An anti-cancer therapeutic strategy. J. Exp. Clin. Cancer Res. 2019, 38, 403. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.J.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Doedens, A.L.; Stockmann, C.; Rubinstein, M.P.; Liao, D.; Zhang, N.; DeNardo, D.G.; Coussens, L.M.; Karin, M.; Goldrath, A.W.; Johnson, R.S. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010, 70, 7465–7475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Profiling and Reversing Metabolic Insufficiency in the Tumor Microenvironment in Advanced Melanoma: A Trial of Pembrolizumab and Metformin versus Pembrolizumab Alone in Advanced Melanoma; NCT03311308; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Rubic, T.; Lametschwandtner, G.; Jost, S.; Hinteregger, S.; Kund, J.; Carballido-Perrig, N.; Schwärzler, C.; Junt, T.; Voshol, H.; Meingassner, J.G.; et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol. 2008, 9, 1261–1269. [Google Scholar] [CrossRef]
- Rodríguez-Prados, J.C.; Través, P.G.; Cuenca, J.; Rico, D.; Aragonés, J.; Martín-Sanz, P.; Cascante, M.; Boscá, L. Substrate fate in activated macrophages: A comparison between innate, classic, and alternative activation. J. Immunol. 2010, 185, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Noman, M.Z.; Janji, B.; Hu, S.; Wu, J.C.; Martelli, F.; Bronte, V.; Chouaib, S. Tumor-Promoting Effects of Myeloid-Derived Suppressor Cells Are Potentiated by Hypoxia-Induced Expression of miR-210. Cancer Res. 2015, 75, 3771–3787. [Google Scholar] [CrossRef] [Green Version]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Förster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Maus, M.V.; Grupp, S.A.; Porter, D.L.; June, C.H. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014, 123, 2625–2635. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Using Probiotics to Reactivate Tumor Suppressor Genes in Colon Cancer; NCT03072641; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Siska, P.J.; Rathmell, J.C. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015, 36, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L.; et al. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changes in Intestinal Microbiota in Association with Chemotherapy Treatment; NCT02370277; United States National Library of Medicine: Bethesda, MD, USA, 2015.
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017, 26, 94–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckermann, K.E.; Dudzinski, S.O.; Rathmell, J.C.; Dysfunctional, T. cell metabolism in the tumor microenvironment. Cytokine Growth Factor Rev. 2017, 35, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Salani, B.; Marini, C.; Rio, A.D.; Ravera, S.; Massollo, M.; Orengo, A.M.; Amaro, A.; Passalacqua, M.; Maffioli, S.; Pfeffer, U.; et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci. Rep. 2013, 3, 2070. [Google Scholar] [CrossRef]
- Marini, C.; Salani, B.; Massollo, M.; Amaro, A.; Esposito, A.I.; Orengo, A.M.; Capitanio, S.; Emionite, L.; Riondato, M.; Bottoni, G.; et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013, 12, 3490–3499. [Google Scholar] [CrossRef] [Green Version]
- Laskar, J.; Bhattacharjee, K.; Sengupta, M.; Choudhury, Y. Anti-diabetic drugs: Cure or risk factors for cancer? Pathol. Oncol. Res. 2018, 24, 745–755. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, J.; Prokop, L.J.; Murad, M.H. Pharmacologic therapy of diabetes and overall cancer risk and mortality: A meta-analysis of 265 studies. Sci. Rep. 2015, 5, 10147. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Mercado, R.R.; Shirai, K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J. Immunother. Cancer 2018, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nivolumab and Metformin Hydrochloride in Treating Patients with Stage III-IV Non-small Cell Lung Cancer That Cannot Be Removed by Surgery; NCT03048500; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug-and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [Green Version]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomised double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases-safety, pharmacokinetics and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.S.; Manda, G. Metabolic Pathways of the Warburg Effect in Health and Disease: Perspectives of Choice, Chain or Chance. Int. J. Mol. Sci. 2017, 18, 2755. [Google Scholar] [CrossRef] [Green Version]
- Andrejeva, G.; Rathmell, J.C. Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors. Cell Metab. 2017, 26, 49–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [Green Version]
- de Rosa, V.; Galgani, M.; Porcellini, A.; Colamatteo, A.; Santopaolo, M.; Zuchegna, C.; Romano, A.; de Simone, S.; Procaccini, C.; la Rocca, C.; et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015, 16, 1174–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parallel Proof of Concept Phase 2 Study of Nivolumab and Metformin Combination Treatment in Advanced Non-Small Cell Lung Cancer with and without Prior Treatment with PD-1/PD-L1 Inhibitors; NCT03048500; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postepy. Hig. Med. Dosw. (Online) 2017, 71, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Cheng, W.-C.; Liu, D.; Gaude, E.; Haider, S.; Metcalf, T.; Patel, N.; Teoh, E.J.; Gleeson, F.; Bradley, K.; et al. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metab. 2018, 28, 679–688.e4. [Google Scholar] [CrossRef] [Green Version]
- Alwarawrah, Y.; Kiernan, K.; Maciver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, S.M. Enzymes of arginine metabolism. J. Nutr. 2004, 134, 2743S–2747S. [Google Scholar] [CrossRef]
- Bernard, A.C.; Mistry, S.K.; Morris, S.M., Jr.; O’Brien, W.E.; Tsuei, B.J.; Maley, M.E.; Shirley, L.A.; Kearney, P.A.; Boulanger, B.R.; Ochoa, J.B.; et al. Alterations in arginine metabolic enzymes in trauma. Shock 2001, 15, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Bronte, V.; Serafini, P.; de Santo, C.; Marigo, I.; Tosello, V.; Mazzoni, A.; Segal, D.M.; Staib, C.; Lowel, M.; Sutter, G.; et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 2003, 170, 270–278. [Google Scholar] [CrossRef]
- Mandal, A. Do malnutrition and nutritional supplementation have an effect on the wound healing process? J. Wound Care 2006, 15, 254–257. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raber, P.; Ochoa, A.C.; Rodríguez, P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: Mechanisms of T cell suppression and therapeutic perspectives. Immunol. Investig. 2012, 41, 614–634. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Phase 1/2 Study to Evaluate the Safety, Tolerability, and Efficacy of INCB001158 in Combination with Chemotherapy, in Subjects with Advanced or Metastatic Solid Tumors; NCT03314935; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Safety, Pharmacokinetics, and Pharmacodynamics of Escalating Oral Doses of the Arginase Inhibitor INCB001158 (Formerly Known as CB1158) as a Single Agent and in Combination with Immune Checkpoint Therapy in Patients with Advanced/Metastatic Solid Tumors; NCT02903914; United States National Library of Medicine: Bethesda, MD, USA, 2016.
- Amiel, E.; Everts, B.; Fritz, D.; Beauchamp, S.; Ge, B.; Pearce, E.L.; Pearce, E.J. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J. Immunol. 2014, 193, 2821–2830. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Everts, B.; Amiel, E.; van der Windt, C.J.W.; Freitas, T.C.; Chott, R.; Yarasheski, K.E.; Pearce, E.L.; Pearce, E.J. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood 2012, 120, 1422–1431. [Google Scholar] [CrossRef] [Green Version]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, M.; Ramirez, M.E.; Sierra, R.A.; Raber, P.; Thevenot, P.; Al-Khami, A.A.; Sanchez-Pino, D.; Hernandez, C.; Wyczechowska, D.D.; Ochoa, A.C.; et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 2015, 75, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Metz, R.; Rust, S.; Duhadaway, J.B.; Mautino, M.R.; Munn, D.H.; Vahanian, N.N.; Link, C.J.; Prendergast, G.C. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012, 1, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, M.; Hofmann, A.D.; Bruns, H.; Haibach, M.; Loschinski, R.; Saul, D.; Mackensen, A.; le Blanc, K.; Jitschin, R.; Mougiakakos, D. Mesenchymal Stromal Cells Disrupt mTOR-Signaling and Aerobic Glycolysis during T-Cell Activation. Stem Cells Dayt. Ohio. 2016, 34, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Jitschin, R.; Braun, M.; Büttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; le Blanc, K.; et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.T.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011, 12, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, W.P.; Feder-Mengus, C.; Chiarugi, A.; Rosenthal, R.; Reschner, A.; Schumacher, R.; Zajac, P.; Misteli, H.; Frey, D.M.; Oertli, D.; et al. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur. J. Immunol. 2006, 36, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; von Knebel Doeberitz, N.; Oezen, I.; Wick, W.; Ochs, K. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front. Immunol. 2014, 5, 673. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Wang, H.-S.; Wang, H.; Zhang, F.; Wang, K.-S.; Guo, Q.; Zhang, G.; Cai, S.-H.; Du, J. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: Focus on macrophage polarization of THP-1 cells. Cell Immunol. 2014, 289, 42–48. [Google Scholar] [CrossRef]
- Combination Therapy with Nivolumab and PD-L1/IDO Peptide Vaccine to Patients with Metastatic Melanoma; NCT03047928; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- A Phase II Study of Epacadostat and Pembrolizumab in Patients with Imatinib Refractory Advanced Gastrointestinal Stromal Tumors; NCT03291054; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- A Phase 1/2 Randomized, Blinded, Placebo Controlled Study of Ipilimumab in Combination with Epacadostat or Placebo in Subjects with Unresectable or Metastatic Melanoma; NCT01604889; United States National Library of Medicine: Bethesda, MD, USA, 2012.
- Komiya, T.; Huang, C.H. Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Muller, A.; Manfredi, M.G.; Zakharia, Y.; Prendergast, G. Inhibiting IDO pathways to treat cancer: Lessons from the ECHO-301 trial and beyond. Semin. Immunopathol. 2019, 41, 41–48. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.M.; Wang, Z.; Ma, J. Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell. 2018, 175, 1780–1795.e19. [Google Scholar] [CrossRef] [PubMed]
- Phase I/II Study of CB-839 and Capecitabine in Patients with Advanced Solid Tumors and Fluoropyrimidine Resistant PIK3CA Mutant Colorectal Cancer; NCT02861300; United States National Library of Medicine: Bethesda, MD, USA, 2016.
- A Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial Comparing CB-839 in Combination with Cabozantinib (CB-Cabo) vs. Placebo with Cabozantinib (Pbo-Cabo) in Patients with Advanced or Metastatic Renal Cell Carcinoma (RCC); NCT03428217; United States National Library of Medicine: Bethesda, MD, USA, 2018.
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domblides, C.; Lartigue, L.; Faustin, B. Control of the Antitumor Immune Response by Cancer Metabolism. Cells 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Phase 1/2 Study of the Safety, Pharmacokinetics, and Pharmacodynamics of the Glutaminase Inhibitor CB-839 in Combination with Nivolumab in Patients with Advanced/Metastatic Melanoma, Renal Cell Carcinoma and Non-Small Cell Lung Cancer; NCT02771626; United States National Library of Medicine: Bethesda, MD, USA, 2016.
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017, 3, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Yaqoob, P. Glutamine and the immune system. Amino Acids 1999, 17, 227–241. [Google Scholar] [CrossRef]
- Combet, E.; Paterson, S.; Iijima, K.; Winter, J.; Mullen, W.; Crozier, A.; Preston, T.; McColl, K.E.L. Fat transforms ascorbic acid from inhibiting to promoting acid-catalysed N-nitrosation. Gut 2007, 56, 1678–1684. [Google Scholar] [CrossRef]
- Berod, L.; Friedrich, C.; Nandan, A.; Freitag, J.; Hagemann, S.; Harmrolfs, K.; Sandouk, A.; Hesse, C.; Castro, C.N.; Bähre, H.; et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef]
- van der Windt, G.J.W.; O’Sullivan, D.; Everts, B.; Huang, S.C.-C.; Buck, M.D.; Curtis, J.D.; Chang, C.-H.; Smith, A.M.; Ai, T.; Faubert, B.; et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. USA 2013, 110, 14336–14341. [Google Scholar] [CrossRef] [Green Version]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131 (Suppl. 9), 2515S–2522S; [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Jung, M.; Ören, B.; Mora, J.; Mertens, C.; Dziumbla, S.; Popp, R.; Weigert, A.; Grossmann, N.; Fleming, I.; Brüne, B. Lipocalin 2 from macrophages stimulated by tumor cell-derived sphingosine 1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci. Signal. 2016, 9, ra64. [Google Scholar] [CrossRef]
- Khatib, S.A.; Rossi, E.L.; Bowers, L.W.; Hursting, S.D. Reducing the burden of obesity-associated cancers with anti-inflammatory long-chain omega-3 polyunsaturated fatty acids. Prostaglandins Lipid Mediat. 2016, 125, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Chen, L.; Lian, G.; Yang, J.; Li, F.; Zou, Y.; Lu, F.; Yin, Y. Antitumor mechanisms of bifidobacteria. Oncol. Lett. 2018, 16, 3–8. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Paulino, D.S.; Brambilla, S.R.; Camargo, J.A.; Persinoti, G.F.; Carvalheira, J.B.C. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 2018, 24, 1995–2008. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.-H.; Gao, P.; Song, Y.-X.; Xu, Y.; Sun, J.-X.; Chen, X.-W.; Zhao, J.-H.; Wang, Z.-N. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: A pooled analysis of 2740 cancer patients. Oncoimmunology 2019, 8, e1665973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkrief, A.; El Raichani, L.; Richard, C.; Messaoudene, M.; Belkaid, W.; Malo, J.; Belanger, K.; Miller, W.; Jamal, R.; Letarte, N.; et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1568812. [Google Scholar] [CrossRef]
- PDL-1 Targeting in Resectable Oesophageal Cancer: A Phase II Feasibility Study of Atezolizumab and Chemoradiation; NCT03087864; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- The Role of Microbiome in Cancer Therapy; NCT02960282; United States National Library of Medicine: Bethesda, MD, USA, 2016.
- A Multicenter Phase 1b Randomized, Placebo-controlled, Blinded Study to Evaluate the Safety, Tolerability and Efficacy of Microbiome Study Intervention Administration in Combination with Anti-PD-1 Therapy in Adult Patients with Unresectable or Metastatic Melanoma; NCT03817125; United States National Library of Medicine: Bethesda, MD, USA, 2019.
- Gut Microbiota in Patients with HCC; NCT02599909; United States National Library of Medicine: Bethesda, MD, USA, 2015.
- Characterization of Microbiota (Intestinal, From Lungs, and Upper Airways) in Patients with Non-Small Cell Lung Carcinoma: Exploratory Study; NCT03068663; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Gut Microbiota Prediction of Metachronous Colorectal Neoplasms in Patients with Colorectal Cancer; NCT03383159; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Characteristics of the Intestinal Microbiota in Patients with Cancer (Catalogue-Onco); NCT03196609; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Hibberd, A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [Green Version]
- Altering the Gut Microbiota of Melanoma Patients Who Failed Immunotherapy Using Fecal Microbiota Transplantation (FMT) From Responding Patients; NCT03353402; United States National Library of Medicine: Bethesda, MD, USA, 2017.
- Kim, J. Regulation of Immune Cell Functions by Metabolic Reprogramming. J. Immunol. Res. 2018, 2018, 8605471. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ahn, E.; Kissick, H.T.; Ahmed, R. Reinvigorating Exhausted T Cells by Blockade of the PD-1 Pathway. Forum Immunopathol. Dis. Ther. 2015, 6, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Delgoffe, G.M.; Powell, J.D. Feeding an army: The metabolism of T cells in activation, anergy, and exhaustion. Mol. Immunol. 2015, 68, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef] [Green Version]
- Finlay, D.K.; Rosenzweig, E.; Sinclair, L.V.; Feijoo-Carnero, C.; Hukelmann, J.L.; Rolf, J.; Panteleyev, A.A.; Okkenhaug, K.; Cantrell, D.A. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 2012, 209, 2441–2453. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.P.; Loftus, R.M.; Keating, S.E.; Liou, K.T.; Biron, C.A.; Gardiner, C.M.; Finlay, D.K. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol. 2014, 193, 4477–4484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévesque, S.; le Naour, J.; Pietrocola, F.; Paillet, J.; Kremer, M.; Castoldi, F.; Baracco, E.E.; Wang, Y.; Vacchelli, E.; Stoll, G.; et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology 2019, 8, e1657375. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Mechanisms | Studies n = 66 | |
|---|---|---|
| 49 Preclinical | 17 Clinical | |
| Glycolysis and Oxidative | 15 (22.7%) | 2(3%) |
| Arginine, Tryptophan and Glutamine | 18 (27.3%) | 1 (1.5%) |
| Lipids | 3 (4.5%) | 1 (1.5%) |
| Microbiota | 3 (4.5%) | 11 (16.6%) |
| Mixed | 10 (15%) | 4 (6%) |
| Trial | Type of Tumor | Number of Patients | Topics | Evidences | Analyzed Parametres | Results |
|---|---|---|---|---|---|---|
| Beatty et al [24] | Colorectal/Melanoma | 52 | IDO1 inhibitor | Phase 1 | Toxicity Objective responses | Well tolerated. No objective responses. SD lasting ≥ 16 weeks in 7/52 patients. |
| Machon et al. [25] | Head and neck | 31 | Aminoacids, vitamins, fatty acids, ribonucleic acids, antioxidants | Observational | Inflammatory/oxidative stress | Decreased hs-CRP (9.8 vs. 3.2, p = 0.002) and α-1 acid glycoprotein (1.2 vs. 1.0, p = 0.020) |
| Sunpaweravong et al. [26] | Esophageal | 71 | Arginine, EPA, DHA and nucleotides | Randomized | Immune cells | Decreased CRP (p = 0.001) and TNF (p = 0.014) |
| Maruyama et al. [27] | Gastric and esophageal cancer | 22 | Arginine, fatty acids and nucleotides | Randomized | Immune cells | Increased Th17 (9.0 ± 2.2 vs. 14.4 ± 3.5%) |
| Talvas et al. [28] | Head and neck and esophageal | 28 | Arginine, fatty acids and glutamine | Double blind | Immune cells | Maintained LT4/LT8 counts ratio (2.47 ± 0.31 vs. 1.95 ± 0.20); Decreased PGE2 (66 ± 16 vs. 107 ± 16, p < 0.05); Increased IFNγ (10.3 ± 3.4 vs. 4.4 ± 1.4, p < 0.05), IL12/IL10 (2.39 vs. 3.4 p = 0.1) and IL2 (1.3 ± 0.42 vs. 0.6 ± 0.3) |
| Derosa et al. [29] | NSCLC and RCC | 64 | Microbiome | Observational | Outcome (OS and PFS) | ATB vs. no ATB in RCC: increased risk of PD (75% versus 22%, p < 0.01), shorter PFS [median 1.9 vs. 7.4 mos, HR 3.1, 95% CI 1.4–6.9, p < 0.01], and shorter OS (median 17.3 vs. 30.6 mos, HR 3.5, 95% CI 1.1–10.8, p = 0.03). NSCLC: PD (52% versus 43%, p = 0.26) but decreased PFS (median 1.9 vs. 3.8 mos, HR 1.5, 95% CI 1.0–2.2, p = 0.03) and OS (median 7.9 vs. 24.6 mos, HR 4.4, 95% CI 2.6–7.7, p < 0.01). |
| Rolleret al. [30] | Colon cancer | 37 | Microbiome | Double blind | Immune cells | Increased mean IL-2 (221 ng/L vs. 132 ng/L) and IFNγ (1071 vs. 712 ng/L) |
| Botticelli et al. [31] | NSCLC | 11 | Microbiome | Observational | Immune cells | Tridecane and 2-pentanone associated to early progression (respectively p = 0.032 and p = 0.016). Fatty acids, lysine and nicotinic acids associated to long term beneficial effects of therapies (respectively p = 0.016, p = 0.032 and p = 0.016), |
| Routy et al. [32] | NSCLC and RCC | 100 | Microbiome | Observational | Immune cells | Increased PFS in presence of CD4+ and CD8+ against A. muciniphila and E. Hirae (p = 0.031 and p = 0.044 respectively) |
| Peters et al. [33] | Melanoma | 27 | Microbiome | Observational | Immune cells | Longer PFS (HR 95% CI) = 0.97 (0.95, 1.00), p = 0.02; number of shotgun subspecies: HR (95% CI) = 0.89 (0.79, 0.99), p = 0.03) |
| Gopalakrishnan et al. [34] | Melanoma | 43 | Microbiome | Observational Prospectic | Immune cells | PFS (HR = 2.95, 95% C.I. = 1.31–7.29, p = 0.03). |
| Matson et al. [35] | Melanoma | 42 | Microbiome | Observational Prospectic | Immune cells | Role of Microbial composition in R versus NR for this subset (p < 0.01) |
| Chaput et al. [36] | Melanoma | 26 | Microbiome | Observational Prospectic | Immune cells | Longer PFS (p = 0.0039) and overall survival (p = 0.051 |
| Frankel et al. [37] | Melanoma | 39 | Microbiome | Observational Prospectic | Immune cells | Higher ICT responder if microbiomes is enriched with B. caccae (p = 0.032) and Streptococcus parasanguinis (p = 0.048) |
| Siska et al. [38] | RCC | 54 | Glycolysis | Observational | Immune cells | Higher PD-1highCD8+ T cells with hyperpolarized mitochondria and increased mitochondrial ROS and MTG staining (p < 0.05) and decreased PBMC PD-1lowCD8+ T cells cytoplasmic ROS (p < 0.05). |
| Ostadrahimi et al. [39] | Breast | 30 | Beta-glucano | Randomized, double blind, placebo controlled | Immune cells | Increased Global health status/QoL (p = 0.023) |
| Paixãoet al. [40] | Breast | 45 | n-3 fatty acids | Double blind randomized | Immune cells | Stable hsCRP in FG (initial median 0.1 (IQR 0.1–0.5), final median 0.3 (IQR 0.0–0.7), p = 0.510) vs. increased hsCRP in PG (initial median 0.1 (IQR 0.0–0.2), final median 0.2 (IQR 0.1–0.3), p = 0.024). |
| Study Number | Target | Treatment | Evidence |
|---|---|---|---|
| NCT03072641 | Colon Cancer | Probiotics | Randomized |
| NCT03048500 | NSCLC | Metformin Hydrocloride + Nivolumab | Phase 2 |
| NCT03311308 | Melanoma | Metformin + Pembrolizumab vs. Pembrolizumab | Randomized double blind |
| NCT03048500 | NSCLC | Metformin + Nivolumab | Randomized, Phase 2 |
| NCT03314935 | Advanced or Metastatic solid tumors | INCB001158 (Arginase inhibitors) + chemotherapy | Phase 1/2 |
| NCT02903914 | Advanced or Metastatic solid tumors | INCB001158 (Arginase inhibitors) +/− immune checkpoint therapy | Phase 1 |
| NCT03047928 | Melanoma | PDL1/IDO Vaccine + Nivolumab | Phase 1/2 |
| NCT03291054 | GIST | Epacadostat + Pembrolizumab | Phase 2 |
| NCT01604889 | Melanoma | Epacadostat + Ipilimumab | Phase 1/2 randomized, blinded |
| NCT02861300 | Colon Cancer | CB-839 (oral glutaminase inhibitor) + Capecitabine | Phase 1/2 |
| NCT03428217 | Renal cell carcinoma | CB-839 (oral glutaminase inhibitor) + Cabozantinib vs. Cabozantinib | Phase 2, double blind randomized |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirabile, A.; Rivoltini, L.; Daveri, E.; Vernieri, C.; Mele, R.; Porcu, L.; Lazzari, C.; Bulotta, A.; Viganò, M.G.; Cascinu, S.; et al. Metabolism and Immune Modulation in Patients with Solid Tumors: Systematic Review of Preclinical and Clinical Evidence. Cancers 2020, 12, 1153. https://doi.org/10.3390/cancers12051153
Mirabile A, Rivoltini L, Daveri E, Vernieri C, Mele R, Porcu L, Lazzari C, Bulotta A, Viganò MG, Cascinu S, et al. Metabolism and Immune Modulation in Patients with Solid Tumors: Systematic Review of Preclinical and Clinical Evidence. Cancers. 2020; 12(5):1153. https://doi.org/10.3390/cancers12051153
Chicago/Turabian StyleMirabile, Aurora, Licia Rivoltini, Elena Daveri, Claudio Vernieri, Roberto Mele, Luca Porcu, Chiara Lazzari, Alessandra Bulotta, Maria Grazia Viganò, Stefano Cascinu, and et al. 2020. "Metabolism and Immune Modulation in Patients with Solid Tumors: Systematic Review of Preclinical and Clinical Evidence" Cancers 12, no. 5: 1153. https://doi.org/10.3390/cancers12051153
APA StyleMirabile, A., Rivoltini, L., Daveri, E., Vernieri, C., Mele, R., Porcu, L., Lazzari, C., Bulotta, A., Viganò, M. G., Cascinu, S., & Gregorc, V. (2020). Metabolism and Immune Modulation in Patients with Solid Tumors: Systematic Review of Preclinical and Clinical Evidence. Cancers, 12(5), 1153. https://doi.org/10.3390/cancers12051153






