Neuroendocrine Carcinoma of the Uterine Cervix: A Clinicopathologic and Immunohistochemical Study with Focus on Novel Markers (Sst2–Sst5)
Abstract
1. Introduction
2. Results
2.1. Clinical Data
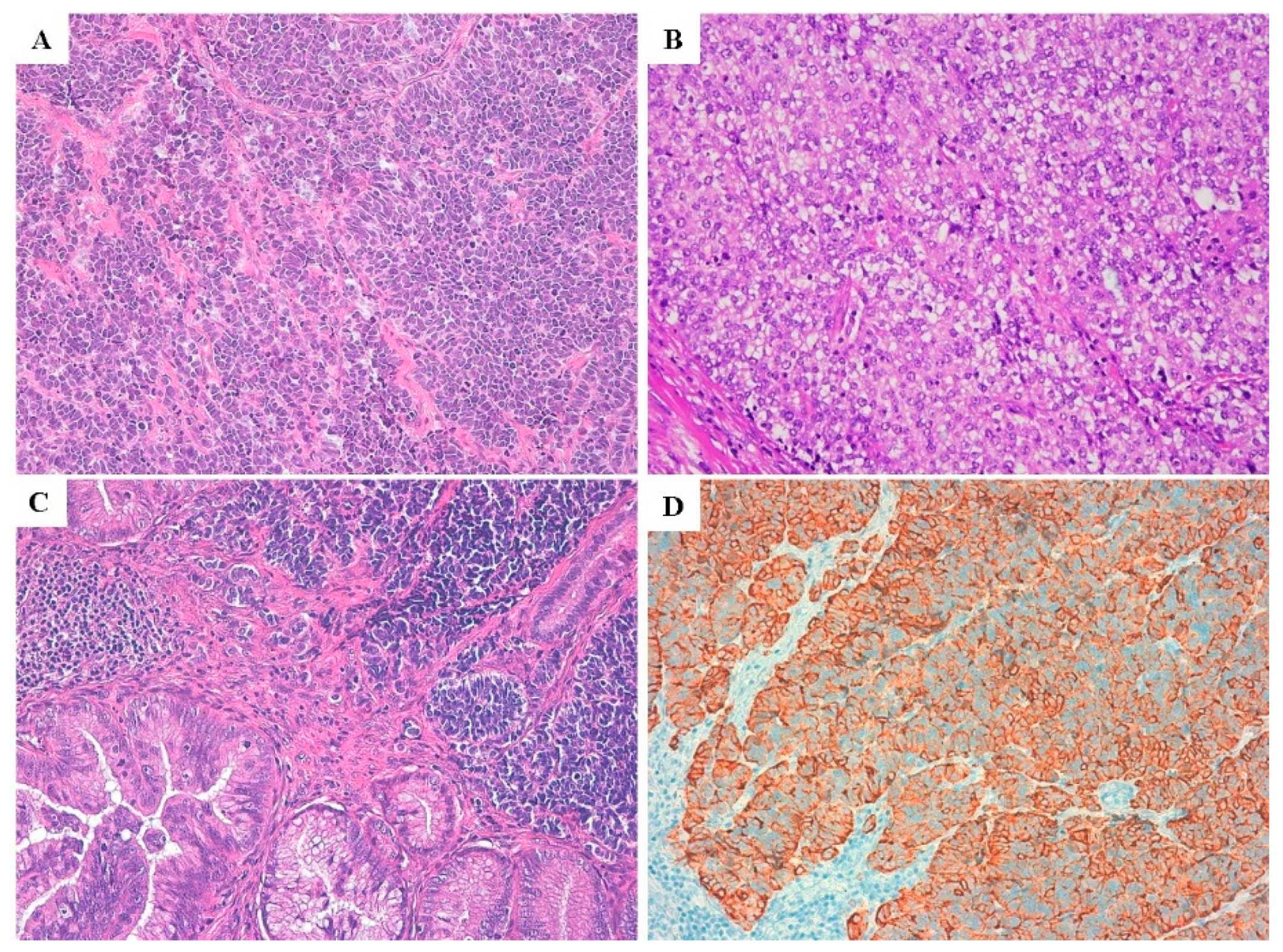
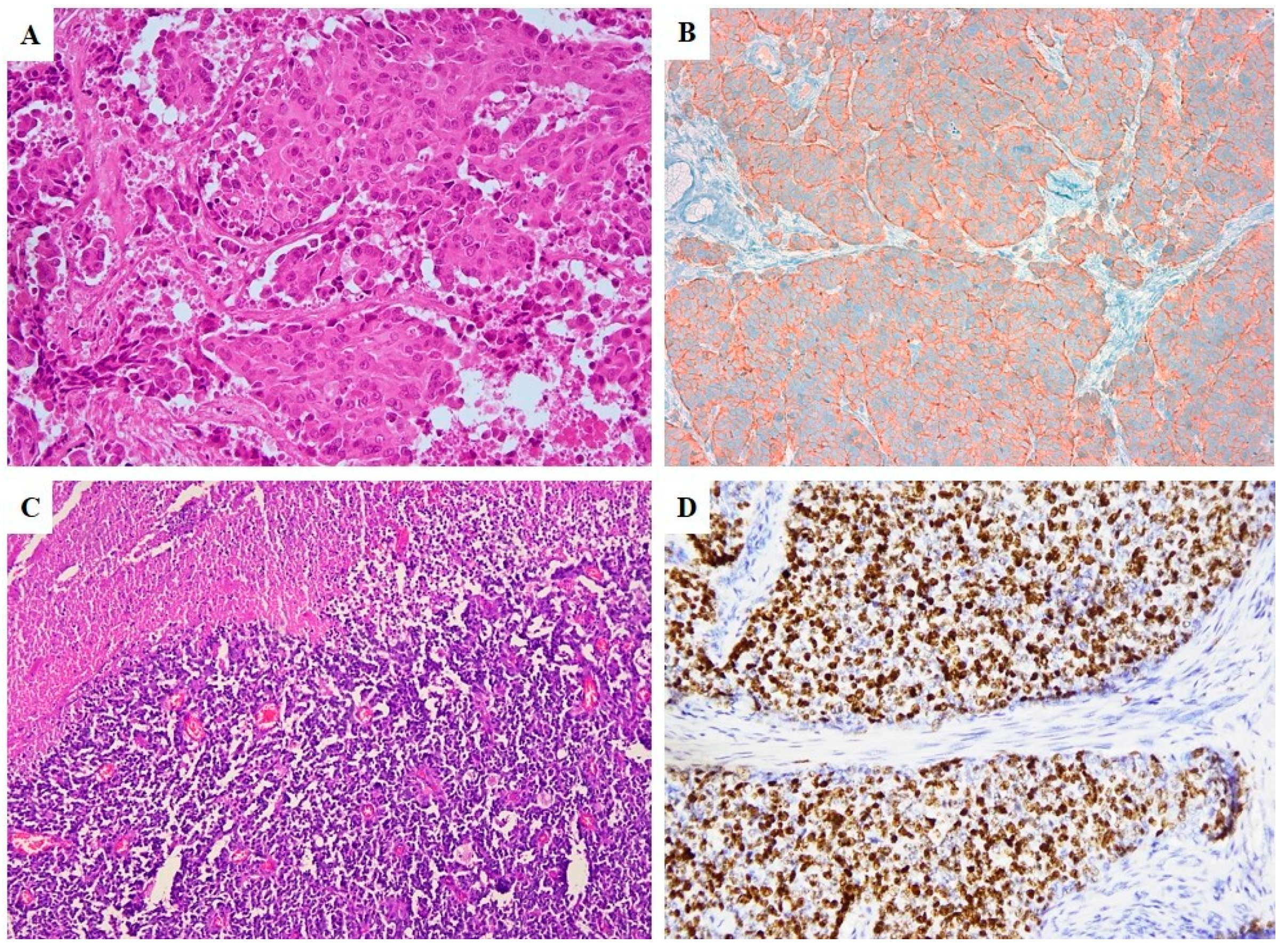
2.2. Pathological Features
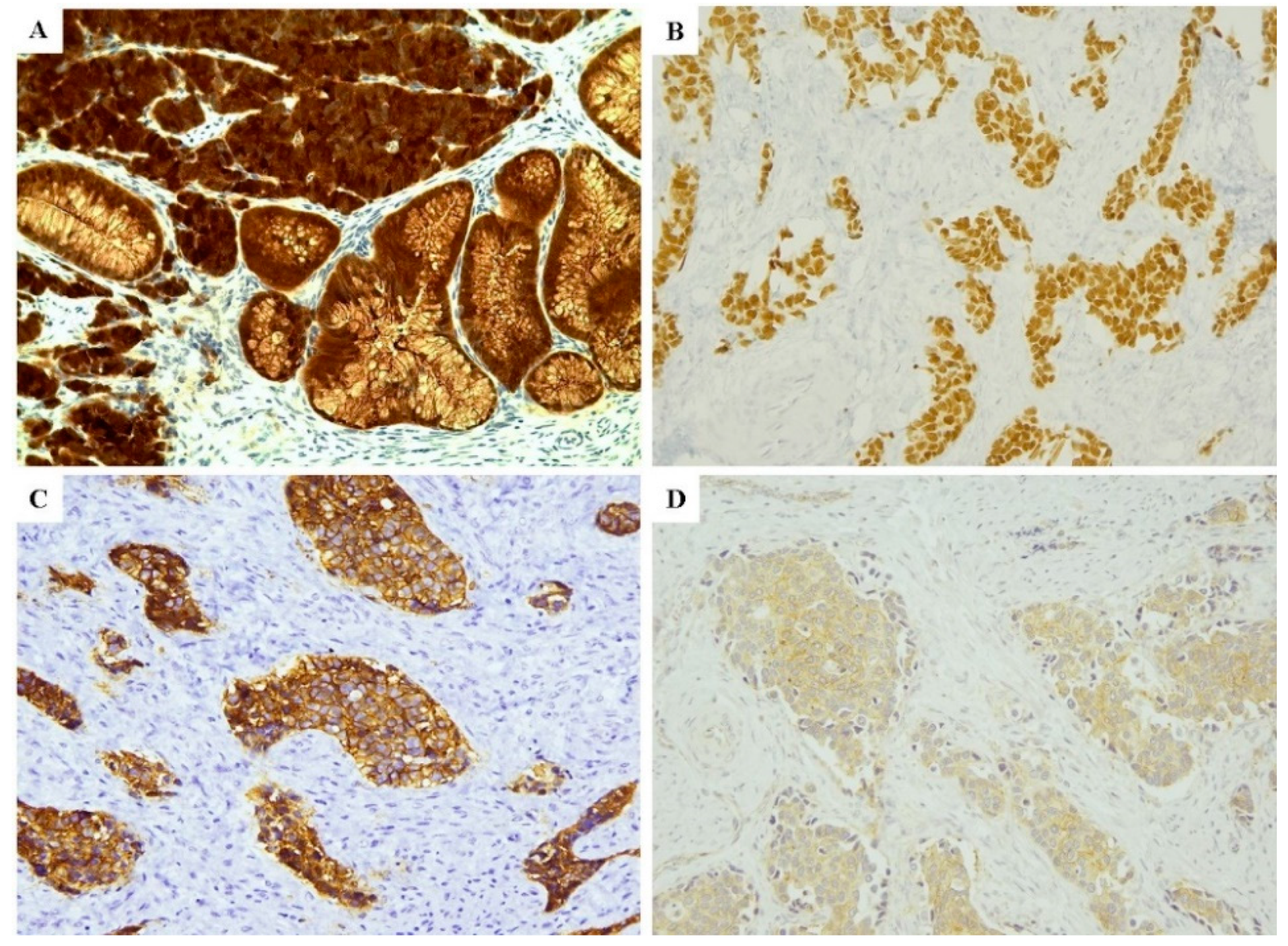
2.3. Immunoprofile
2.4. Statistical Analysis
3. Discussion
4. Methods
4.1. Ethic Statement and Patient Selection
4.2. Pathological Assessment
4.3. Immunohistochemistry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Modlin, I.M.; Drozdov, I.; Kidd, M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS ONE 2013, 8, e63364. [Google Scholar] [CrossRef]
- Inzani, F.; Rindi, G. Classification of neuroendocrine neoplasm. In Radionuclide Imaging and Therapy for Endocrine Tumors; Pacak, K., Taien, D., Eds.; Humana Press: Cham, Switzerland, 2017. [Google Scholar]
- Bellizzi, A.M. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: What can Brown do for you? Hum. Pathol. 2020, 96, 8–33. [Google Scholar] [CrossRef] [PubMed]
- Boyar Cetinkaya, R.; Aagnes, B.; Thiis-Evensen, E.; Tretli, S.; Bergestuen, D.S.; Hansen, S. Trends in incidence of neuroendocrine neoplasms in Norway: A report of 16,075 cases from 1993 through 2010. Neuroendocrinology 2017, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hauso, O.; Gustafsson, B.I.; Kidd, M.; Waldum, H.L.; Drozdov, I.; Chan, A.K.; Modlin, I.M. Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer 2008, 113, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Kelly, P.; McCluggage, W.G. Pathology of neuroendocrine tumours of the female genital tract. Curr. Oncol. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Ganesan, R.; Hirschowitz, L.; Dawson, P.; Askew, S.; Pearmain, P.; Jones, P.W.; Singh, K.; Chan, K.K.; Moss, E.L. Neuroendocrine carcinoma of the cervix: Review of a series of cases and correlation with aoutcome. Int. J. Surg. Pathol. 2016, 24, 490–496. [Google Scholar] [CrossRef]
- Gardner, G.J.; Reidy-Lagunes, D.; Gehrig, P.A. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011, 122, 190–198. [Google Scholar] [CrossRef]
- Crowder, S.; Tuller, E. Small cell carcinoma of the female genital tract. Semin. Oncol. 2007, 34, 57–63. [Google Scholar] [CrossRef]
- Wang, T.Y.; Chen, B.F.; Yang, Y.C.; Chen, H.; Wang, Y.; Cviko, A.; Quade, B.J.; Sun, D.; Yang, A.; McKeon, F.D.; et al. Histologic and immunophenotypic classification of cervical carcinomas by expression of the p53 homologue p63: A study of 250 cases. Hum. Pathol. 2001, 32, 479–486. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Kennedy, K.; Busam, K.J. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am. J. Surg. Pathol. 2010, 34, 525–532. [Google Scholar] [CrossRef]
- Zhang, C.; Schmidt, L.A.; Hatanaka, K.; Thomas, D.; Lagstein, A.; Myers, J.L. Evaluation of napsin, A.; TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumors. Am. J. Clin. Pathol. 2014, 142, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Tatsumori, T.; Tsuta, K.; Masai, K.; Kinno, T.; Taniyama, T.; Yoshida, A.; Suzuki, K.; Tsuda, H. p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: Comparison with p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, W.; Kwan, M.Y.; Suster, S.; Chan, J.K. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch. Pathol. Lab. Med. 2001, 125, 228–231. [Google Scholar] [PubMed]
- Saqi, A.; Alexis, D.; Remotti, F.; Bhagat, G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am. J. Clin. Pathol. 2005, 123, 394–404. [Google Scholar] [CrossRef]
- Talia, K.L.; Stewart, C.J.R.; Howitt, B.E.; Nucci, M.R.; McCluggage, W.G. HPV-negative gastric type adenocarcinoma in situ of the cervix: A spectrum of rare lesions exhibiting gastric and intestinal differentiation. Am. J. Surg. Pathol. 2017, 41, 1023–1033. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Shah, R.; Connolly, L.E.; McBride, H.A. Intestinal-type cervical adenocarcinoma in situ and adenocarcinoma exhibit a partial enteric immunophenotype with consistent expression of CDX2. Int. J. Gynecol. Pathol. 2008, 27, 92–100. [Google Scholar] [CrossRef]
- Castle, P.E.; Pierz, A.; Stoler, M.H. A systematic review and meta-analysis on the attribution of human papillomavirus (HPV) in neuroendocrine cancers of the cervix. Gynecol. Oncol. 2018, 148, 422–429. [Google Scholar] [CrossRef]
- Grayson, W.; Rhemtula, H.A.; Taylor, L.F.; Allard, U.; Tiltman, A.J. Detection of human papillomavirus in large cell neuroendocrine carcinoma of the uterine cervix: A study of 12 cases. J. Clin. Pathol. 2002, 55, 108–114. [Google Scholar] [CrossRef]
- Shamir, E.R.; Devine, W.P.; Pekmezci, M.; Umetsu, S.E.; Krings, G.; Federman, S.; Cho, S.J.; Saunders, T.A.; Jen, K.Y.; Bergsland, E.; et al. Identification of high-risk human papillomavirus and Rb/E2F pathway genomic alterations in mutually exclusive subsets of colorectal neuroendocrine carcinoma. Mod. Pathol. 2019, 32, 290–305. [Google Scholar] [CrossRef]
- Beirne, J.P.; McArt, D.G.; James, J.A.; Salto-Tellez, M.; Maxwell, P.; McCluggage, W.G. p16 as a prognostic indicator in ovarian/tubal high-grade serous carcinoma. Histopathology 2016, 68, 615–618. [Google Scholar] [CrossRef]
- Alos, L.; Hakim, S.; Larque, A.B.; de la Oliva, J.; Rodriguez-Carunchio, L.; Caballero, M.; Nadal, A.; Marti, C.; Guimera, N.; Fernandez-Figueras, M.T. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: Potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016, 469, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Švajdler, M.; Mezencev, R.; Ondič, O.; Šašková, B.; Mukenšnábl, P.; Michal, M. P16 is a useful supplemental diagnostic marker of pulmonary small cell carcinoma in small biopsies and cytology specimens. Ann. Diagn. Pathol. 2018, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Grönberg, M.; Federspiel, B.; Scoazec, J.Y.; Hjortland, G.O.; Grønbæk, H.; Ladekarl, M.; Langer, S.W.; Welin, S.; Vestermark, L.W. Expression of p53 protein in high-grade gastroenteropancreatic neuroendocrine carcinoma. PLoS ONE 2017, 12, e0187667. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, H.; Hirabayashi, K.; Miyazawa, M.; Nakamura, N.; Hirasawa, T.; Muramatsu, T.; Mikami, M.; Yasuda, M.; Osamura, R.Y. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine carcinoma of uterine cervix. Arch. Gynecol. Obstet. 2009, 279, 521–525. [Google Scholar] [CrossRef]
- Damian, A.; Lago, G.; Rossi, S.; Alonso, O.; Hengler, H. Early detection of bone metastasis in small cell neuroendocrine carcinoma of the cervix by 68Ga-DOTATATE PET/CT imaging. Clin. Nucl. Med. 2017, 42, 216–217. [Google Scholar] [CrossRef]
- Mizutani, G.; Nakanishi, Y.; Watanabe, N.; Honma, T.; Obana, Y.; Seki, T.; Ohni, S.; Nemoto, N. Expression of Somatostatin Receptor (SSTR) Subtypes (SSTR-1, 2A, 3, 4 and 5) in neuroendocrine tumors using real-time RT-PCR method and immunohistochemistry. Acta Histochem. Cytochem. 2012, 45, 167–176. [Google Scholar] [CrossRef]
- Albertelli, M.; Arvigo, M.; Boschetti, M.; Ferone, D.; Gatto, F.; Minuto, F. Somatostatin receptor pathophysiology in the neuroendocrine system. Expert Rev. Endocrinol. Metab. 2013, 8, 149–157. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of the Female Reproductive Organs; IARC Press: Lyon, France, 2014. [Google Scholar]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Köbel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of P53 immunohistochemistry in endometrial carcinomas: Toward increased reproducibility. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S123–S131. [Google Scholar] [CrossRef]
- Volante, M.; Brizzi, M.P.; Faggiano, A.; La Rosa, S.; Rapa, I.; Ferrero, A.; Mansueto, G.; Righi, L.; Garancini, S.; Capella, C.; et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. 2007, 20, 1172–1182. [Google Scholar] [CrossRef]
| Cases | Age (ys) | Surgical Procedure | Histotype | FIGO Stage | Associated Neoplastic non-NE Component | Prognosis | Recurrent/Metastatic Disease (Site) | Follow-Up (Months) | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | HA | SCNEC | IA2 | - | DOD | Pancreas | 36 | Cysplatin + Etoposide (7 cycles) + BRT |
| 2 | 36 | HA | SCNEC | IA2 | - | A | 36 | Cysplatin + Etoposide (6 cycles) + BRT | |
| 3 | 45 | HA | LCNEC | IB1 | HSIL | A | 24 | Cysplatin + Etoposide (6 cycles) + BRT | |
| 4 | 41 | HA | LCNEC + IA | IB1 | - | AWD | Lung, bone, liver, pelvic limph nodes | 30 | NAD: Cysplatin + Etoposide (6 cycles) AD: Cysplatin (1st line); Topotecan + Etoposide (2nd line) + BRT |
| 5 | 30 | HA | LCNEC | IB1 | HSIL, AIS | A | 24 | Cysplatin + Etoposide (5 cycles) + BRT | |
| 6 | 81 | HA | LCNEC + IA | IB1 | - | DOD | Peritoneal carcinosis, liver metastases | 28 | Cysplatin + Etoposide (6 cycles) + BRT |
| 7 | 44 | HA | SCNEC + IA | IB2 | - | A | 46 | Cysplatin + Etoposide (6 cycles) + BRT | |
| 8 | 57 | HA | SCNEC | IIA2 | - | DOD | Lung, lombo-aortic lymph nodes | 6 | Untreated |
| 9 | 33 | HA | SCNEC | IIB | - | AWD | Subcutaneous and pelvic recurrences | 28 | 1st line: Carboplatin + Etoposide (7 cycles) + BRT 2nd line: Topotecan + Etoposide |
| 10 | 50 | HA | SCNEC | IIB | - | DOD | Liver, pelvic lymph nodes | 48 | Cysplatin + Etoposide (6 cycles) + BRT |
| 11 | 50 | HA | SCNEC | IIB | - | DOD | Lung, liver, pelvic lymph nodes | 26 | Cysplatin + Etoposide (4 cycles) + BRT |
| 12 | 62 | HA | LCNEC | IVA | - | DOD | Liver | 10 | Cysplatin + Etoposide (6 cycles) + BRT |
| 13 | 62 | HA | SCNEC | IVA | - | A | 60 | Cysplatin + Etoposide (4 cycles) + BRT | |
| 14 | 66 | HA | LCNEC + IA | IVB | - | DOD | Peritoneal carcinosis, lung metastases | 18 | Cysplatin + Etoposide (3 cycles) + BRT; After tumoral progression: Taxol + Bevacizumab + Gemcitabin |
| 15 | 61 | B | SCNEC | ND | - | DOD | Bone, lombo-aortic lymph nodes | 24 | Cysplatin + Etoposide (6 cycles) + BRT |
| 16 | 47 | B | SCNEC | ND | AIS | A | 36 | NAD: Cysplatin + Etoposide (3 cycles) AD Cysplatin+Etoposide (7 cycles) + BRT |
| Cases | FIGO Stage | CgA | Syn | CD56 | TTF1 | CDX2 | p16 | p53 | p40 | p63 | SSTR2A | SSTR5 | Ki67/Mib1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IA2 | 3 | 3 | 3 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 90% |
| 2 | IA2 | 2 | 3 | 3 | 2 | 2 | 3 | 1 | 0 | 2 | 2 | 2 | 98% |
| 3 | IB1 | 3 | 3 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 70% |
| 4 | IB1 | 3 | 3 | 2 | 1 | 3 | 3 | 1 | 0 | 0 | 0 | 2 | 60% |
| 5 | IB1 | 3 | 3 | 3 | 0 | 3 | 3 | 1 | 0 | 0 | 3 | 2 | 95% |
| 6 | IB1 | 2 | 3 | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 45% |
| 7 | IB2 | 3 | 3 | 0 | 0 | 3 | 3 | 1 | 0 | 0 | 2 | 2 | 80% |
| 8 | IIA2 | 2 | 3 | 2 | 0 | 0 | 3 | 3 | 0 | 0 | 2 | 1 | 90% |
| 9 | IIB | 3 | 3 | 3 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 0 | 95% |
| 10 | IIB | 3 | 3 | 3 | 2 | 2 | 3 | 1 | 0 | 0 | 3 | 2 | 80% |
| 11 | IIB | 2 | 2 | 2 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 90% |
| 12 | IVA | 3 | 2 | 3 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 75% |
| 13 | IVA | 0 | 2 | 3 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 80% |
| 14 | IVB | 3 | 3 | 2 | 0 | 2 | 3 | 1 | 0 | 0 | 3 | 3 | 85% |
| 15 | ND | 2 | 3 | 3 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 95% |
| 16 | ND | 3 | 3 | 3 | 2 | 0 | 3 | 1 | 0 | 0 | 2 | 1 | 95% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inzani, F.; Santoro, A.; Angelico, G.; Feraco, A.; Spadola, S.; Arciuolo, D.; Valente, M.; Carlino, A.; Piermattei, A.; Scaglione, G.; et al. Neuroendocrine Carcinoma of the Uterine Cervix: A Clinicopathologic and Immunohistochemical Study with Focus on Novel Markers (Sst2–Sst5). Cancers 2020, 12, 1211. https://doi.org/10.3390/cancers12051211
Inzani F, Santoro A, Angelico G, Feraco A, Spadola S, Arciuolo D, Valente M, Carlino A, Piermattei A, Scaglione G, et al. Neuroendocrine Carcinoma of the Uterine Cervix: A Clinicopathologic and Immunohistochemical Study with Focus on Novel Markers (Sst2–Sst5). Cancers. 2020; 12(5):1211. https://doi.org/10.3390/cancers12051211
Chicago/Turabian StyleInzani, Frediano, Angela Santoro, Giuseppe Angelico, Angela Feraco, Saveria Spadola, Damiano Arciuolo, Michele Valente, Angela Carlino, Alessia Piermattei, Giulia Scaglione, and et al. 2020. "Neuroendocrine Carcinoma of the Uterine Cervix: A Clinicopathologic and Immunohistochemical Study with Focus on Novel Markers (Sst2–Sst5)" Cancers 12, no. 5: 1211. https://doi.org/10.3390/cancers12051211
APA StyleInzani, F., Santoro, A., Angelico, G., Feraco, A., Spadola, S., Arciuolo, D., Valente, M., Carlino, A., Piermattei, A., Scaglione, G., Scambia, G., Rindi, G., & Zannoni, G. F. (2020). Neuroendocrine Carcinoma of the Uterine Cervix: A Clinicopathologic and Immunohistochemical Study with Focus on Novel Markers (Sst2–Sst5). Cancers, 12(5), 1211. https://doi.org/10.3390/cancers12051211








